ABSTRACT
Human papillomaviruses (HPV) cause precancerous lesions and cancers of the cervix. In Côte d'Ivoire, cervical cancer screening program based on visual inspection is the gold standard. This study aimed to detect high risk (HR) HPV DNA on women attending cervical cancer screening program based on visual inspection after application of acid acetic and Lugol. From March to December 2015, cervical samples from women attending cervical screening were tested for some HR to HPV. HPV DNA was amplified using PGMY09 /11 primers which generated 450 base pairs at the L1 region in which the samples harboring HPV DNA were genotyped using the multiplex PCR with HPV 16, 18, 31, 33, 35, 45 and 51 primers. The mean age of this population was 32 years and of about 339 women enrolled on visual inspection, 6.19% were positive. HPV DNA was obtained in 9.73 of the population in which 31 of 33 samples (93.93.%) of HPV DNA+ were genotyped using multiplex PCR testing for HPV 16,18, 31, 33, 35, 45 and 51, of those women with HPV DNA+; 28.57% had a single infection while 71.43% had a multiple infection. HPV genotypes prevalence followed: HPV 16 (30.00%), HPV 18 (25.00%), HPV35 (20.00%), HPV 45 (20.00%), HPV 51 (3.30%) and HPV 33 (1.60%), by using PCR as gold standard while VIA sensitivity and specificity was 16.12 and 95.45%, respectively. HPV prophylactic vaccine would prevent 33.33% of HR HPV infection with 2v, 33.33% with the 4v and 66.66% with the 9v vaccines, respectively. In Cote d'Ivoire screening for cervical cancer with HR HPV testing and triaging for treatment with visual inspection would represent a very efficient prevention of cervical cancer program.
Key words: Human papillomaviruses (HPV), genotypes, acetic acid (IVA), Côte d’Ivoire.
Human Papillomaviruses (HPV) are small virus with non-enveloped double-stranded circular DNA responsible for
genital warts papillomas, precancerous lesions and cancers (cervix, vulva) (zur Hausen H, 2009).Cytology has now become the most widely used for invasive cervical cancer screening method in the world. Cytology-based invasive cervical cancer screening programs initiated in many industrialized countries in decades have resulted in a decrease in the incidence and mortality associated with cancer in these populations (Adami et al., 1994; Miller et al., 1990). There are many limitations to cytological screening of cervical invasive cancers in resource-limited settings.
The technique is relatively expensive and requires trained pathologists and technicians. As a result, the diagnostic performance (sensitivity and specificity) and the reproducibility of cytology evaluated under real conditions proved to be poor in a number of resource-constrained countries (Cervical cancer project, 1999; Arbyn et al., 2008; Lazcano et al., 1997). All of these limitations may explain the failure to organize cytology-based organized cervical screening in a context of limited resources (Sankaranarayanan et al., 2001; Miller et al., 2003).
Many of these countries do not have the laboratories and resources to consider organized cytology-based screening in the general population. Alternative and adapted solutions have been developed such as visual inspection methods with application of dyes such as acetic acid (IVA) and Lugol (IVL).
These methods rely on direct visualization of the cervix during a gynecological examination of the speculum. The IVA consists of applying a solution of acetic acid titrated between 4 and 5% with an impregnated cotton. The application of acetic acid to a healthy neck causes only a slight coagulation in the superficial cellular layer, because the cellular activity is weak.
Cote d’Ivoire has a population of 6.37 million women ages 15 years and older who are at risk of developing cervical cancer. Current estimates indicate that every year 1346 women are diagnosed with cervical cancer and 866 die from the disease. Cervical cancer ranks as the 2nd most frequent cancer among women in Cote d’Ivoire and the 2nd most frequent cancer among women between 15 and 44 years of age. Data is not yet available on the HPV burden in the general population of Cote d’Ivoire.
However, in Western Africa, the region Cote d’Ivoire, about 4.3% of women in the general population are estimated to harbor cervical HPV- 16/18 infection at a given time, and 56.7% of invasive cervical cancers are attributed to HPVs 16 or 18 (Globocan cancer fact sheets, 2012; GLOBOCAN, 2008). In the country, IVA strategy is just been introduced but virological screening is not widely available. The aim of this work was to investigate the presence of HPV DNA in the genital secretions of women consulting for screening for precancerous lesions of the cervix by the visual inspection technique with acetic acid and Lugol solute (Jaquet et al., 2010; Sankaranarayanan and Wesley, 2003).
Patient recruitment
This study was carried out at the Gynecology Department of Abobo South General Hospital in the North of Abidjan in which concerned women of age and volunteers who were attending precancerous lesions screening by visual inspection by acetic acid from March to December 2015 were recruited. After women’s consent, they were administered a questionnaire assessing socio-demographic characteristics and sexual habits that is, age, lifetime number of sexual partners, date of first sex, sex habits, parity and number of pregnancies, place of living and tobacco use. For each women included for this study, visual inspection by acetic acid (VIA) and iodine of Lugol (IVL) is done and samples for HPV DNA detection by PCR were taken.
Visual inspection by acetic acid (VIA) and iodine of Lugol (IVL)
During visual inspection, the woman is placed in a gynecological position and non-lubricated speculum is placed to make visible the cervix with the aid of an ordinary light. Before applying acetic acid, samples are taken in the cervix and vagina and stored in virus medium transport (VTM).
The samples were stored at -20°C and then at -80°C at the biobank of Pasteur Institute of Cote d’Ivoire before using for the PCR. Then a solution of glacial acetic acid diluted to 3 to 5% was used, though it is preferred to use a 5% dilute acetic acid solution because acid bleaching (acidophilic reaction) appears more rapidly and more clearly than with a 3 to 4% solution. In some cases white table vinegar was used, also, it should be known that this usually contains 5% acetic acid (Sankaranarayanan et al., 2003; Globocan Cancer Fact sheets, 2012).
The acetic acid neck is generously washed with a cotton pad; with care the surface of the entire neck, including the external orifice is covered. Dabbing the cervix several times with a cotton pad or other sufficiently wide applicator promotes coagulation and mucus removal, which in turn facilitates the penetration of acetic acid into the epithelium. This step requires patience because the bleaching reaction with the acid develops gradually in the space of 60 s and disappears soon after. It may therefore be necessary to repeat the application of acetic acid every 2 to 3 min for the duration of the examination. The appearance or not of the bleaching was noted.
The procedure is the same for Lugol solute. The normal epithelium (original or mature metaplastic) contains glycogen stocks which are the source of the brown mahogany or almost black coloration that appears when applying an iodinated solution such as Lugol's solution. On the other hand, the cylindrical epithelium, which does not contain glycogen, does not take iodine staining. It is iodo-negative. In the same way, immature metaplastic squamous epithelium, the epithelium of inflammatory or regenerative zones, and the congenital remodeling zone, contain very little or almost no glycogen, and therefore do not take iodine color if only very partially. Thus, there is a range of staining ranging from the partial brown mahogany to the mustard yellow color, which reflects the range of CIN lesions (Sankaranarayanan et al., 2003; Globocan Cancer Fact sheets, 2012)
PCR detection of HPV
The PCR detection of HPV was performed at molecular biology plate forms of Pasteur Institute of Côte d’Ivoire. PCR detection of HPV was performed according to the procedure of Ausubel et al. (1999) in which this procedure is based on the chloroform phenol extraction method which consists of treating the cell lysate (obtained after enzymatic digestion with proteinase K) with a mixture of phenol/chloroform/isoamyl alcohol. Phenol is a deproteinizing agent that the nucleic acids are not soluble and also chloroform is capable of causing protein denaturation. The anti-foaming activity of isoamyl alcohol will promote the separation of the deproteinized aqueous phase. Recovery of the genomic DNA is achieved following precipitation steps with ethanol and centrifugation steps.
HPV DNA extraction protocol
Endo-cervical cell samples of 500 μL were centrifuged at 14000 rpm for 15 min. The pellet (endocervical cells) obtained after centrifugation is subjected to lysis at 65°C for 1 h by adding 400 μL of cell lysis buffer (200 μL of Tris-HCl pH 8 (0.1 M), 0.8 ΜL of EDTA (0.5 M), 20 μL of SDS (10%), 1.5 μL of RNAse (4 mg / mL) and 10 μL of proteinase K (10 mg / mL). After enzymatic digestion, the mixture was centrifuged at 14000 rpm for 5 min, then later, two volumes of phenol / chloroform / isoamyl alcohol (25/24/1) was added to the lysate. The mixture thus obtained was stirred for 2 min in a vortex and then centrifuged for 5 min at 12000 rpm. The DNA contained in the aqueous phase was supplemented with two volumes of absolute ethanol, 1/10 volume of 3 M NaCl, precipitated at -20°C overnight and then centrifuged for 10 min at 14000 rpm. The pellet obtained was washed in 500 μL of 70% ethanol at 14000 rpm for 10 min. The precipitate containing the viral DNA is dried with DNA SpeedVac and taken up in 60 μL of elution buffer. The DNA extract was subsequently stored at -20°C for further use or at 4°C for immediate use.
HPV detection by PGMY11/09 PCR
Consensus primers (primarily targeting the HPV L1 region) were used to detect the viral presence. The PCR was carried out in a reaction medium of 50 μL containing 5 μL of 5X colored buffer, 5 μL of uncoloured 5 × buffer, 3 μL of MgCl2 (25 mM), 0.5 μL of dNTPs (10 μm), 1 μL of each primer (10 μM), 0.4 μL of GoTaq polymerase and 10 μL of DNA. The DNA was amplified in a thermal cycler under the following conditions: initial denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, hybridization at 53°C for 30 s (35 cycles), elongation at 72°C for 30 s, final elongation at 72°C for 7 min. For each serial of cases tested, a negative control containing no matrix DNA was carried out in parallel in order to check the absence of any contamination of the reagents used. A positive control is also tested in parallel with each set of cases. The consensus primers used for HPV detection targeting the conserved L1 region and producing 450 bp amplicons (Gravitt et al., 2000) are: Sequence (5 '3') of the forward primer PGMY11 A: GCACAGGGACATAACAATGG; Sequence (5 '3') of the reverse primer PGMY09 F: CGTCCCAAAGGAAACTGATC (Figure 1).

Separation of DNA fragments by agarose gel electrophoresis
The electrophoresis apparatus was formed of a Plexiglas plate placed horizontally on a flat support. A comb for forming the wells is aligned parallel to the top of the plate. The assembly is connected by electrodes to a current generator. Agarose was prepared at a concentration of 1.5% with 1X TBE buffer. The cooled microwave-fused agarose solution was added with 10 μL of BET. The solution was then poured onto the plate. After curing the gel, the comb was removed and the cured gel coated with 1 × 15 μL TBE buffer of each DNA sample was deposited in each well. In parallel with the samples, a molecular weight marker is deposited in a well. The migration lasts 20 to 35 min and is carried out under a voltage of 110V.
HPV genotyping
A method of HPV genotyping by designing seven type-specific primer sets that allow the amplification of strictly conserved regions of L1 gene in order to identify the HPV genotypes 16, 18, 45, 35, 33, 51, and 31 with the use of human β-actin gene as internal control was used. The method was established by designing nine type-specific primer sets that target conserved regions of the L1 gene (Tsakiogiannis et al., 2012) (Figure 2).
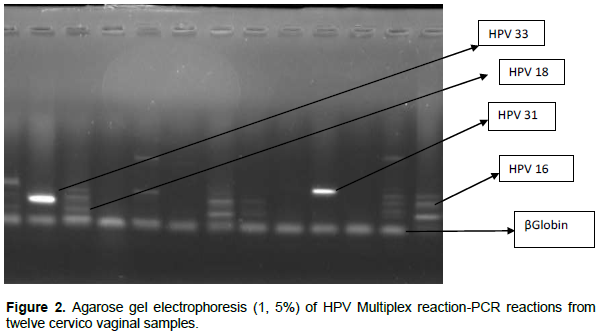
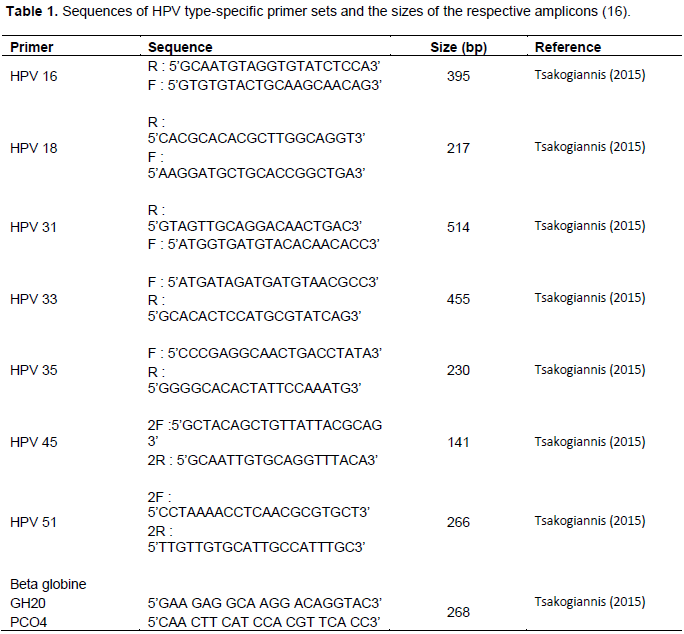
In the present study, method of HPV genotyping was developed by designing nine type-specific primer sets that allow the amplification of strictly conserved regions of L1 gene in order to identify the HPV genotypes 16, 18, 31, 33, 35, 45, and 5 with the use of the human β-actin gene as internal control. In particular, two distinct primer mixtures were arranged, each containing three L1 type-specific primer sets (Table
1). Moreover, the primer set b-actinF/b-actinR, which allows the amplification of the human β-actin gene was included in each multiplex PCR primer mixture as an internal control. Specifically, primer mixture I contained primers specific for HPV 16, 31 and 33; primer mixture II contained primers specific for HPV 18, 35, 45 and 51.
Each multiplex PCR primer mixture contained 1 μL of each L1 type-specific primer set and 1 μmol of the primer pair b-actinF/b-actinR. Each multiplex PCR assay was performed in a final volume of 50 μL, containing 40 μL of the corresponding multiplex PCR primer mixture, 5 µl Buffer A, 2 mm MgCl2, 1.2 mm dNTPs and 0.5 U of thermostable DNA polymerase. PCR cycling conditions were as follows: an initial denaturation step at 95°C for 2 min and then 40 cycles of 95°C for 30 s, 58°C for 50 sand 72°C for 10 s. The PCR reaction ended with a 1 min incubation step at 72°C.
The general characteristics of the study population are; the minimum age is 18 years and the maximum age is 64 years with an average age of 34 years. At the matrimonial level, the population consisted of 31.9% single women, 1.5% divorced women, 59.7% married women, 3.7% common-law women and 3.3% widowed women in which majority of the population came from the city of Abidjan and in particular from the quarter of Abobo at 65.2% and that of Cocody at 19.8%.
The population was educated at 66.3% with 21.6% at the primary and secondary level and 23.1% at the higher level respectively. At the professional level, the population is made up of partly liberal women (69.7%) followed by female employees (19.3%) and finally students (10.9%) in which 87.4% of women are below five pregnancies and 12.6% above five pregnancies also about 77.12% of women have below five children and 22.88% above five children and 84.4% of the population does not use oral contraceptives.
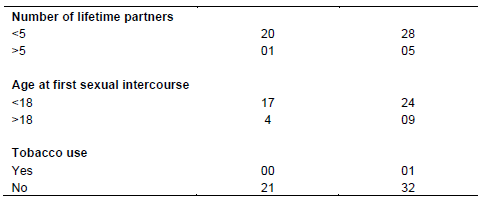
Among those using contraceptives, 13.2% used the intrauterine device IUD, 28.3% used injections and 58.5% used oral contraceptives in which 1.2% of the population consumed tobacco, 51.8% of the population used condoms in their lives compared with 48.2% who never used condoms. Of those who used condoms, 4.2% admitted using it every day, 12.1% rarely and 35.5% sometimes (Globocan Cancer Fact sheets, 2012) in which 83.4% of women had fewer than five partners during their lifetime, 16.6% experienced more while 80.82% of the women in this study had their first sexual intercourse before the age of 18 years (Tables 2 to 4).
Of the 339 women who agreed to participate to this study, 6.2% were positive at visual inspection with acetic acid and Lugol. HPV DNA was obtained in 33 (9.37%) of the population and a total of 31 (93.93%) specimens harboring HPV DNA were genotypes using multiplex PCR versus 14.3%, which were not genotyped using HPV 16,18, 31, 33, 35, 45 and 51 by multiplex PCR.
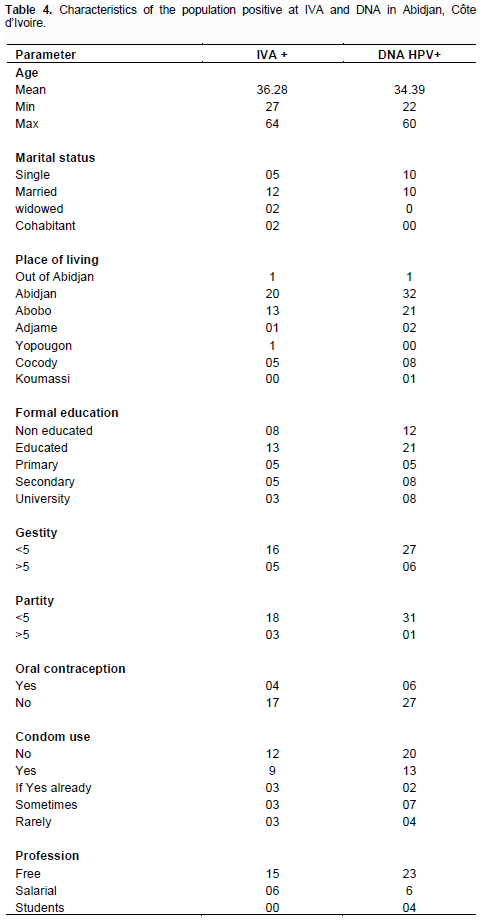
These 30 strains permit us to identify 60 strains of HPV on whom we have 28.57% with single infection while 71.43% with a multiple infection. Among the multiple infection 55% had double HPV infection, 40% had triple HPV infection and 5% had four HPV infections. HPV genotypes prevalence followed: HPV 16 (30.0%),HPV 18 (25.0%), HPV35 (20.0%), HPV 45(20.0%), HPV 51 (3.3%) and HPV 33 (1.6%).
No case of HPV genotype 31 was found. By using PCR as gold standard VIA sensibility were 16.12% and the specificity 95.45%. Among the samples, only 3 (0.81%) were both positive to the two techniques used. By using PCR as gold standard, VIA sensitivity was 16.12% and specificity 95.45% (Table 5).
The aim of this work was to determine the presence of Papillomavirus DNA in the genital samples of women who were consulted for cervical cancer screening using the Visual Inspection with Acetic Acid or Lugol iodide. This work permits us to find the rate of 6.2% of women carrying precancerous lesions by visual inspection by acetic acid. This rate is substantially equal to that of Jacquet et al. (2010) and lowers than that of Horo et al. (2012), with both studies realized in Cote d’Ivoire.
The difference of prevalence with that obtained by Horo et al. (2012) could be explained by several facts. Firstly Horo et al. (2012) worked on a fairly large workforce of 2998 people versus 339 in this study. In addition, Horo et al. (2012) have worked on a population of HIV-infected women and therefore when it is known that HIV infection is a factor in the development of cervical cancer. Elsewhere in Africa, notably in Mali west Africa, Teguete et al. (2012) and Moussavou et al. (2016) in a similar study in Gabon found in Central Africa have found a high prevalence of precancerous lesions by VIA but in Tanzania, Ngoma et al. (2010) found a lower prevalence of precancerous lesions.
This study found the presence of Papillomavirus DNA in 9.37% of the population. This rate is higher than the presence of precancerous lesions observed in the same study. This difference of frequency would originate in the execution of the tests themselves. Indeed visual inspection in its design is a visual examination and depends in part on the one who does this analysis and therefore it requires a fairly high level of training for a better interpretation of the results mainly as a function of the density of the whiteness (Sankaranarayanan and Wesley, 2003). However, the high prevalence of the presence of HPV DNA could be explained by the fact that, unlike the visual inspection with acid, the virus may be present in women but may not yet initiate its carcinogenesis in which case it cannot be seen by the VIA.
Nevertheless, this prevalence of Papillomavirus remains low compared to that obtained by a study done in the same country (Jaquet et al., 2010). This must be added to the fact that the transport medium used for samples containing 0.5% phenol red, tryptose-phosphate, and an association of antibiotics (penicillin, streptomycin and gentamycin), would have increased the survival of Papillomaviruses. For this study the medium was simply virus medium transport (VTM).
A total of 31 (93.93%) specimens harboring HPV DNA were genotypes using multiplex PCR versus 14.3%, which were not genotyped using HPV 16,18, 31, 33, 35, 45 and 51 by multiplex PCR. These 30 strains permit us to identify 60 strains of HPV on whom we have 28.57% with single infection while 71.43% with a multiple infection. Among the multiple infection, 55% had double HPV infection, 40% had triple HPV infection and 5% had four HPV infections. HPV genotypes prevalence were the following: HPV 16 (30.0%), HPV 18 (25.0%), HPV35 (20.0%), HPV 45 (20.0%), HPV 51 (3.3%) and HPV 33 (1.6%). No case of HPV genotype 31 was found.
Our results demonstrate a large burden of HPV among our study population with a notably high prevalence of multiple concurrent infections. Several multiple concurrent HPV infections has been reported by numerous studies in particular to Cote d'Ivoire (Jaquet et al., 2010), Benin (Piras et al., 2011), Gabon (Moussavou et al., 2016) and Greece but with rates slightly lower than that obtained in our study. The prevalence of HPV genotypes obtained in our study have also been found by numerous authors in Cote d’Ivoire (Jaquet et al., 2010), within chronological order who obtained HPV 35, HPV 16 and HPV 18.
In Nigeria, Okolo et al. (2010) demonstrated the presence of HPV 35 followed by HPV 16 and HPV 18. On the other hand, Piras et al. (2011) have identified the rate of 33.2% prevalence of HPV with a presence of HPV 59 followed by HPV 35 and finally HPV 16, HPV 18 and HPV 58 as the most predominant in the female population in Benin (Piras et al., 2011). At least Moussavou et al. (2016) isolated HPV 16 followed by HPV 18 and HPV 33 in Gabonese women. However, the most worldwide commonly detected types in Invasive Cervical Cancer were HPV 16 and HPV 18.
These data on HPV type epidemiology of HPV infection in this area are very similar to those in Benin, Nigeria and Burkina Faso (Djigma et al., 2011). The prevalence of HPV genotypes in cervical cytological samples varies greatly in different geographical regions (Piras et al., 2011). HPV-16 is the most common type of HPV-18 in Europe, Central and South America, HPV-52 and HPV-58 in Asia, HPV-53 and HPV-52 in North America, while HPV-31 and HPV-35 in Nigeria (Okolo et al., 2010).
In Burkina Faso, HPV-52, -35 and -58 were detected as the most prevalent types in high-risk women, as observed in other African studies (Djigma et al., 2011). These differences in the pattern of HPV type distribution in countries and regions may be related to different sexual habits and migrations of people (Piras et al., 2011).
The low sensitivity for HPV positivity suggests that VIA will have little impact on cervical cancer incidence and mortality in the country. Therefore, the actors involved in the screening of precancerous lesions would like to undergo continuous training or retraining on this technique which seems simple and better adapted in a poor country like Cote d’Ivoire. It would be interesting to couple each screening by the IVA / L to a HPV DNA screening by PCR.
The main limitation of this study was the small number of women included thus limits the generalization of our data to the whole Ivoirians populations. Then, all positive samples were not confirmed cytologicaly. The DNA extraction was done by the method of phenol chloroform and this kind of methods deemed too long have been abandoned in favor of extraction kit. Despite these limitations, this study shows the necessity to implement co-testing (VIA/L) associated to HPV assay in low-income countries.
The authors have not declared any conflict of interests.
The authors are extremely grateful to Dr. Tsakiogiannis and Prof Cantarelli for their helpful technical advices as well as to the Pepfar for the material support.
REFERENCES
|
Adami HO, Ponten J, Sparen P, Bergstrom R, Gustafsson L, Friberg LG (1994). Survival trend after invasive cervical cancer diagnosis in Sweden before and after cytologic screening. Cancer 73(1):140-147.
Crossref
|
|
|
|
Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, Nouhou H, Sakande B, Wesley R, Somanathan T, Sharma A (2008). Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int. J. Cancer 123(1):153-160.
Crossref
|
|
|
|
|
Ausubel FM, Brent R, Kingston RE, Moore DD (1999). Short Protocols in Molecular Biology. New York: Gre Pub. Asso and Wil. Inter. Available at:
View
|
|
|
|
|
Djigma FW, Ouedraogo C, Karou DS, Sagna T, Bisseye C, Zeba M, Ouermi D, Gnoula C, Pietra V, Ghilat-Avoid-Belem NW, Sanogo K (2011). Prevalence and genotype characterization of human papillomaviruses among HIV-seropositive in Ouagadougou, Burkina Faso. Act. Trop. 117(3):202-206
Crossref
|
|
|
|
|
GLOBOCAN (2008). Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer. Available at:
View
|
|
|
|
|
Globocan Cancer Fact sheets (2012). Cervical Cancer. Available online:
View. Accessed 11 Aug 2015.
|
|
|
|
|
Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, Schiffman MH, Scott DR, Apple RJ (2000). Improved amplification of genital human papillomaviruses. J. Clin. Microbiol. 38(1):357-361
|
|
|
|
|
Horo A, Jaquet A, Ekouevi DK, Toure B, Coffie PA, Effi B, Messou E, Jacquet A, Horo A, Charbonneau V, Ekouevi DK, Roncin L, Toure B, Coffie P, Minga A, Sasco AJ, Garrigue I, Fleury H, Dabis F (2010), for the IeDEA West Africa collaboration. Cervical human papillomavirus and HIV infection in women of child-bearing age in Abidjan, Cote d'Ivoire 2010. Br. J. Cancer 2012:1-8.
|
|
|
|
|
Lazcano-Ponce EC, de Ruíz PA, López-Carrillo L, Nájera-Aguilar P, Avila-Ceniceros R, Escandón-Romero C, Cisneros MT, Hernández-Avila M (1997). Validity and reproducibility of cytologic diagnosis in a sample of cervical cancer screening centers in Mexico. Act. Cytol. 41(2):277-284.
Crossref
|
|
|
|
|
Miller AB, Chamberlain J, Day NE, Hakama M, Prorok PC (1990). Report on a Workshop of the UICC Project on Evaluation of Screening for Cancer. Int. J. Cancer. 46(5):761-769.
Crossref
|
|
|
|
|
Miller AB, Sankaranarayanan R, Bosch FX, Sepulveda C (2003). Can screening for cervical cancer be improved, especially in developing countries? Int. J. Cancer 107(3):337-340
Crossref
|
|
|
|
|
Minga A, Moh R, Kone M, Dabis F, Sasco AJ (2012) Cervical cancer screening by visual inspection in Cote d'Ivoire, operational and clinical aspects according to HIV status. BMC Public Health 12(1):237.
Crossref
|
|
|
|
|
Moussavou PB, Koumakpayi IH, Andriniaina, Nkili-Meyong A, Labouba I, Bisvigou U, Chansi JK, Engohan-Aloghe C, Dissanami F, Ambounda N, Delannoy-Vieillard AS, Diancourt L, Nkoghe D, Leroy EM, Belembaogo E and Berthet N (2016). Molecular analysis of human Papillomavirus detected among women positive for cervical lesions by visual inspection with acetic acid/Lugol's iodine (VIA/VILI) in Libreville, Gabon. Infect. Agent Cancer 11:50
Crossref
|
|
|
|
|
Ngoma T, Muwonge R, Mwaiselage J, Kawegere J, Bukori P, Sankaranarayanan R (2010). Evaluation of cervical visual inspection screening in Dar es Salaam, Tanzania. Int. J. Gynaecol. Obstet. 109(2):100-104.
Crossref
|
|
|
|
|
Okolo C, Franceschi S, Adewole I, Thomas JO, Follen M, Snijders PJ, Meijer CJ, Clifford GM (2010). Human papillomavirus infection in women with and without cervical cancer in Ibadan, Nigeria. Infect Agent Cancer 5(1):24
Crossref
|
|
|
|
|
Piras F, Piga M, De Montis A, Zannou AR, Minerba L, Perra MT, Murtas D, Atzori M, Pittau M, Maxia C, Sirigu P (2011). Prevalence of Human Papillomavirus infection in women in Benin, West Africa. Virol. J. 8:514.
Crossref
|
|
|
|
|
Sankaranarayanan R, Budukh AM, Rajkumar R (2001). Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull. World Health Organization 79(10):954-962.
|
|
|
|
|
Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, Chithrathara K, Parkin DM. Nair MK (2003). Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol's iodine (VILI) in cervical cancer screening in Kerala. Ind. Int. J. Cancer 106(3):404-408.
Crossref
|
|
|
|
|
Sankaranarayanan R, Wesley RS (2003). A Practical Manual on Visual Screening for Cervical Neoplasia. IARC Tech. Pub. 41. IARC Press: Lyon.
|
|
|
|
|
Teguete I, Muwonge R, Traore C, Dolo A, Bayo S, Sankaranarayanan R (2012) . Can visual cervical screening be sustained in routine health services? Experience from Mali, Africa. BJOG 119:220-226.
Crossref
|
|
|
|
|
Thomas JO, Herrero R, Omigbodun AA, Ojemakinde K, Ajayi IO, Fawole A, Oladepo O, Smith JS, Arslan A, Munoz N, Snijders PJ, Meijer CJ, Franceschi S (2004) Prevalence of papillomavirus infection in women in Ibadan, Nigeria: A population-based study. Br. J. Cancer 90(3):638-645
Crossref
|
|
|
|
|
Tsakogiannis D, Diamantidou V, Toska E, Kyriakopoulou Z, Dimitriou TG, Ruether IGA, Gortsilas P, Markoulatos P (2015). Multiplex PCR assay for the rapid identification of human papillomavirus genotypes 16, 18, 45, 35, 66, 33, 51, 58, and 31 in clinical samples. Arch. Virol.160(1):207-214.
Crossref
|
|
|
|
|
University of Zimbabwe/JHPIEGO Cervical Cancer Project (1999). Visual inspection with acetic acid for cervical-cancer screening: test qualities in a primary-care setting. Lancet 353(9156):869-873.
Crossref
|
|
|
|
|
zur Hausen H (2009). Papillomaviruses in the causation of human cancers¬¬¬-a brief historical account. Virology 384(2):260-265.
Crossref
|
|