ABSTRACT
Cellulases (enzymes of great potential in biotechnology) are currently of interest due to their applicability in the hydrolysis of cellulose from lignocellulosic materials, producing fermentable sugars for alcohol production. The great demand for efficient enzymes is the driving force to prospect new cellulases-producing microorganisms, as well as to optimize the enzyme production step. Many variables can be optimized in microorganism cultivation, such as pH, type of microorganism, induction, temperature, type and concentration of substrate, among others. This work aimed to evaluate the production of cellulases by submerged fermentation from three strains of filamentous fungi (Trichoderma koningii, Penicillium species, Rhizomucor species) and two strains of bacteria (Bacillus megaterium and Bacillus subtilis), using sugarcane bagasse as substrate. Variations in substrate concentrations (0.5, 1.6 and 2.7%, w/v) and temperature (28, 33 and 38°C) were evaluated on volumetric activity. The best fungus was T. koningii (3130.4 IU/L) using 2.7% natural sugarcane bagasse at 28°C. Among the bacteria, B. megaterium stood out with an enzyme production in range of 130 to 156.7 IU/L (at 28-33°C using natural and acid-alkaline pretreated bagasses), although up to around 20 times lower than the production by the T. Koningii.
Key words: Microorganisms, lignocellulosic biomass, cellulolytic enzymes, pretreated biomass.
Cellulases are among the most important hydrolytic enzymes, being the third largest industrial enzyme in the world market. This is due to the wide application of these enzymes, such as starch processing, animal food production, extraction of fruit and vegetable juices, pulp and paper industry, and textile industry (Singhania et al., 2010; Akinyele et al., 2014; Jasani et al., 2016). Currently, there is a great interest in cellulases for use in ethanol production from lignocellulosic biomass, and some enzyme companies have launched cellulases specific to the lignocellulosic biomass processing industries (Singhania et al., 2010). Cellulases hydrolyze the β-1,4 glucosidic linkages of cellulose resulting in glucose, which can be fermented to produce second-generation ethanol (2G ethanol).
Cellulase is industrially produced mainly by submerged fermentation (SmF) (Lin et al., 2017), although in the last two decades solid-state fermentation (SSF) has awakening interest due to the high enzyme productivity, reduced energy requirements, etc. (Acharya et al., 2010; Singhania et al., 2010). The scientific literature has reported studies concerning both solid-state and submerged fermentation (Hernández-Domínguez et al., 2014; Zanirun et al., 2014; Jasani et al., 2016; Bentil et al., 2018; Wang et al., 2018). Bentil et al. (2018) reported that submerged fermentation is more efficient than solid state fermentation to obtain higher cellulase production rates when using white-rot basidiomycetous fungi. Submerged fermentation provides a homogeneous environment, continuous oxygen supply and better control over several parameters, such as pH, temperature, dissolved oxygen (DO), and froth formation. Moreover, there is no problem of mass transfer and heat removal (Singhania et al., 2015; Wang et al., 2018).
Regarding microorganisms, there is a wide range of cellulase-producing species, which may be fungi, yeast, aerobic bacteria and actinomycetes (Mmango-kaseke et al., 2016; Behera et al., 2017). Among them, the filamentous fungi are the best producers of cellulases, mainly of the genus Trichoderma and Aspergillus (Baraldo-Junior et al., 2014; Borges et al., 2014; Juturu and Wu, 2014). The screening and isolation of microbes from nature is one of the important ways to get cellulases with diverse properties and high activity (Juturu and Wu, 2014); thus, researchers are continually searching for new microorganisms. Cellulase is produced as a primary metabolite associated with microorganism growth (Zanirun et al., 2014).
The production of cellulase also depends on the growth parameters, which include pH, temperature, carbon source, nitrogen source, agitation rate, and others (Nagar and Kumar, 2010; Jasani et al., 2016). Agro-industrial residues are generally used as carbon sources, as they are a low-cost raw material and also act as inducers of cellulase production (Zanirun et al., 2014; Catelan and Pinotti, 2019). Different types of lignocellulosic substrate may contribute to different cellulase production (Pandey et al., 2016). Some of the substrates significantly stimulate enzyme production without supplementation of the culture medium with specific inducers (Singhania et al., 2010). Moreover, the use of pretreated biomass can promote the growth of microorganisms since the pretreatment reduces the recalcitrance of the material and facilitates the access to the cellulose (Rodríguez-Zúñiga et al., 2011). Therefore, the selection of suitable substrate capable to induce high yield of cellulase is a very important subject.
In this work, we investigated the production of cellulases by submerged fermentation using different filamentous fungi (Trichoderma koningii, Penicillium species, Rhizomucor species) and two bacterial species (Bacillus megaterium and Bacillus subtilis). For each microorganism, three types of sugarcane bagasses were evaluated (natural and pretreated with acid-alkaline and with hydrogen peroxide solutions) and two cultivation variables, temperature (28, 33 and 38°C) and concentration of sugarcane bagasse (0. 5, 1.6 and 2.7% w/v). The experiments were performed according to a 32 factorial design, in which the temperature and concentration of bagasse were varied for each microorganism and for each type of sugarcane bagasse.
Microorganisms and substrate
T. koningii INCQS 4031 (CFAM 422) and B. subtilis (ATCC 6633) were obtained from Oswaldo Cruz Foundation (Rio de Janeiro, RJ, Brazil). Penicillium and Rhizomucor spp. were isolated and supplied by the Department of Environmental Engineering of the Federal University of Espírito Santo (Vitória, ES, Brazil). B. megaterium ATCC 14945 was donated by the Department of Chemical Engineering of the Federal University of São Carlos (São Carlos, SP, Brazil). The fungi and bacteria were maintained on potato dextrose agar (42 g/L) and nutrient agar slant (23 g/L), respectively, and kept at 4°C prior to use.
Sugarcane bagasse was supplied by Destilaria Itaúnas S/A (DISA, Espírito Santo, Brazil) and the procedure performed to obtain the natural bagasse and pretreated with acid-alkaline and hydrogen peroxide solution was as described in previous work (Salomão et al., 2019).
Inoculum preparations
Bacteria
The inoculum medium was composed of 1% (w/v) peptone, 0.5% (w/v) yeast extract and 1% (w/v) sodium chloride and incubated for 12 h at 30°C and 200 rpm (adapted from Fernandes, 2007).
Fungi
To obtain the precultures, the fungi strains were grown on an agar plate containing 3.9% (w/v) potato dextrose agar at 28°C for 5 days. After this period, spores were harvested by adding 10 mL of 0.1% (v/v) Tween-80 and counted in a Neubauer chamber (Menoncin et al., 2009).
Enzyme production by SmF
For enzyme production, sugarcane bagasse was used with particle diameter sizes between 0.85 and 2.36 mm (Fernandes, 2007). The bagasse was added to 250 mL Erlenmeyer flasks containing 100 mL mineral salt solution of Mandels and Weber (1969) at pH 5.3. The flasks were autoclaved at 121°C for 20 min and inoculated with concentration of 106 spores/mL. The flasks were incubated (28, 33 and 38°C) on a shaker (100 rpm) during 48 h for bacteria and 72 h for fungi. At the end of fermentation, the medium was filtered and the filtrate was used for determination of enzyme activity.
For this study the following conditions were evaluated: cultivation temperature (28, 33 and 38°C) and sugarcane bagasse concentration (0.5, 1.6 and 2.7% w/v) for each microorganism (B. megaterium, B. subtilis, T. koningii, Penicillium spp. and Rhizomucor spp.) and for each type of sugarcane bagasse (natural and pre-treated with acid-alkaline and hydrogen peroxide solutions, as described in Salomão et al. (2019)). The values of concentration and temperatures selected here were based on works found in the literature, which used agro-industrial residues to produce cellulases (Akinyele et al., 2014; Mesa et al., 2016; Jasani et al., 2016; Irfan et al., 2017; Fernandes et al., 2018). For these experiments, a 32 factorial design with two central points was used. Statistical analysis of the data was performed using Statistic v. 13.0, and the values were considered significant for p-values < 0.05. The model obtained had the assumption of normal distribution of errors (difference between the values estimated by the model and the “correct value”) admitted as valid and the model equations that correlate the activity of cellulases with the temperature and the concentration of sugarcane bagasse of sugar were obtained using the uncoded variables.
Enzyme activity
The endoglucanase (EG) activity was determined according to Ghose (1987) using 2% (w/v) carboxymethyl cellulose (CMC) in 0.05 M citrate buffer, pH 4.8 as substrate. The mixture containing 0.5 mL of CMC and 0.5 mL of enzyme sample was incubated at 50°C for 30 min. The reaction was stopped by adding 2.0 mL of dinitrosalicylic acid (DNS) reagent and boiled for 5 min. The released reducing sugar was measured according to the DNS curve with glucose as standard (Miller, 1959). One International Unit (IU) of endoglucanase activity was defined as the amount of enzyme that produced 1 μmol of glucose equivalent per milliliter per minute under the assay conditions.
Production of cellulases by fungi
The SmF results with the different fungi (T. koningii, Penicillium spp. and Rhizomucor spp.) and with the different substrates (natural sugarcane bagasse and pretreated with acid-alkaline and with hydrogen peroxide solutions) are shown in Table 1. The best enzyme producers followed the order: T. Koningii (3130.4 IU/L at 28°C using 2.7% w/v natural bagasse), Penicillium spp. (111.8 IU/L at 33°C using 1.6% w/v acid-alkaline pretreated bagasse) and Rhizomucor spp. (63.5 IU/L at 28°C using 1.6% w/v natural bagasse). Salomão et al. (2019) reported similar findings for solid-state fermentations of the same fungi. Under the conditions evaluated in our study reported here, T. Koningii was capable of producing 28 and 49 times more cellulose activity than Penicillium and Rhizomucor spp., respectively. The fact that we observe the best production with the genus Trichoderma is important for perspectives from an industrial point of view, since the genus Trichoderma is the most culturable and industrially exploited for the production of several multipurpose enzymes. This excellent characteristic of synthesizing a multitude of enzymes, for numerous applications, makes this genus a magnificent industrial cell factory of enzymes (Gautam and Naraian, 2020).
There are few studies in the scientific literature on the production of cellulases with the species T. koningii by SmF. Besides, enzymatic activities are measured with different substrates, making a deeper comparison difficult to achieve. In terms of CMCase (endoglucanase activity), Wang et al. (2012) reported a production of 40,300 IU/L by T. koningii D-64 isolated from soil samples from Singapore. This production was obtained after the optimization of the production medium, with the addition of 1% cellulose and 2% wheat bran; however, initially the volumetric activities were between 200 and 7000 IU/L using different carbon sources.
Liu et al. (2012) compared the endoglucanase activity of 4 species of fungi (T. koningii, Trichoderma reesei, Trichoderma viride and Aspergillus niger) and found that T. koningii was the best cellulose producer (31,300 IU/L), followed by T. viride (22,000 IU/L), in a medium containing 20 g/L of microcrystalline cellulose. This work corroborates our results, that is, T. koningii is the best producer of cellulases by SmF, at least among the evaluated fungi. Also, Wang et al. (2013) found enzyme activities of 28,300 IU/L by a mutant T. koningii hyperproducer of cellulase in cultures induced by bran and corncob powder.
With regard to Penicillium, it is possible to find very different values of cellulase production in the scientific literature, which depends on the species, culture medium and physical conditions used. Mesa et al. (2016), in a study of culture medium optimization, obtained a production of 13 IU/L (CMCase) by a wild strain of Penicillium spp., with the addition of 1.5 g/L of sugarcane bagasse pretreated with acid-alkaline solution. Santa-Rosa et al. (2018) found an activity of 600 IU/L by a strain of Penicillium spp. isolated from the Amazon region in a medium containing 7.5 g/L of carboxymethylcellulose as a carbon source. Fernandes et al. (2018) used natural Soybean hulls as well as fractions obtained from pretreatment as carbon sources on the production of cellulases by Penicillium spp. Results showed production of 130 IU/L (CMCase) using 1% of the in natura residue and 200 UI/L (Avicelase) with pretreated residue. Vázquez-Montoya et al. (2020) found an activity of 1683 UI/L using Moringa straw as carbon source in a submerged fermentation of Penicillium funiculosum.
In a search of the scientific literature, no reports were found for studies of production of cellulases by Rhizomucor spp. As we found in our work, this genus is not a good cellulase producer.
Regarding the solid substrate used for fermentation, we found that the microorganisms can produce enzymes using different sugarcane bagasses (natural and pretreated with acid-alkaline and hydrogen peroxide solutions), but the enzyme production by T. koningii is more expressive using natural sugarcane bagasse. For both pretreated sugarcane bagasses, the higher the substrate concentration (1.6 and 2.7%, w/v) and the temperature (33 and 38°C), the lower the enzyme production by this fungus. Probably, the generation of toxic substances in the pretreatment (Vasconcellos et al., 2015) negatively affected the production of cellulases by T. koningii when a high pretreated bagasse concentration was used. On the contrary, Penicillium spp. seems to be more tolerant to temperature and toxic compounds, because the highest CMCase activities were obtained at 33°C and using 1.6% (w/v) acid-alkaline pretreated bagasse.
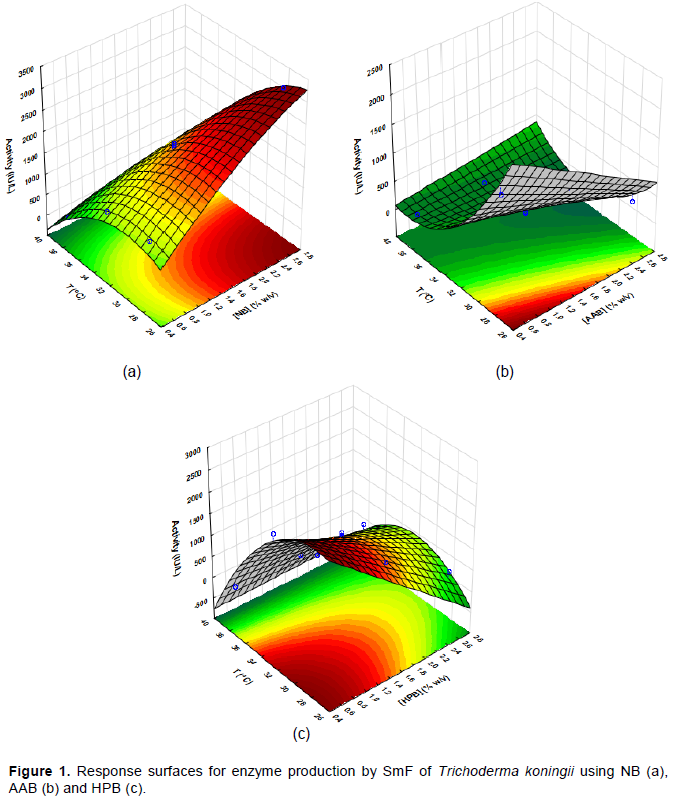
The effects of temperature and concentration of sugarcane bagasse on the enzyme production by SmF of T. koningii (the best enzyme producer in our work) were statistically analyzed and the results (ANOVA) are shown in Table 2. The variables that were not statistically significantly (p <0.05) were removed, and thus the new values of the effects of the variables on the enzymatic production were obtained (Table 3), as well as the three equations (Equations 1 to 3) that describe the behavior of the variables in the enzyme activity and the response surfaces (Figure 1a, b and c) for natural sugarcane bagasse, pretreated with base-acid and hydrogen peroxide solution, respectively. Both concentration of sugarcane bagasse ([B]) and temperature (T) influenced the production of cellulases (A.E.). When natural sugarcane bagasse was used, higher bagasse concentrations and lower temperatures led to better results in the enzyme production, with the temperature having an effect 1.5 times higher. However, for pretreated bagasse, both the increase in bagasse concentration and temperature interferes negatively in the enzyme production. As pointed out earlier, chemically pretreated bagasse can contain toxic substances (Vasconcellos et al., 2015) that inhibit the growth of the microorganisms; therefore, higher concentrations of bagasse lower the enzyme production. The effect of the concentration of bagasse pretreated with hydrogen peroxide solution was greater when compared with the bagasse pretreated with acid-alkaline solution.
A.E (IU/L) = -12515.9 + 3409.8 × [B] - 181.5 × [B]2 + 847.1 × T - 13.7 × T2 - 69.9 × T × [B] (1)
A.E (IU/L) = 22522.2 - 2078.4 × [B] - 1149.8 × T + 14.7 × T2 + 55.0 × [B] × T (2)
A.E (IU/L) = -11059.1 - 3416.9 × [B] + 1054.2 × T -19. 9 × T2 + 86.4 × T × [B] (3)
Production of cellulases by bacteria
The results of enzymatic production with the bacteria B. megaterium and B. subtilis are shown in Table 4. Among the bacteria, B. megaterium showed better results (CMCase activity of 156.7 IU/L at 33°C using acid-alkaline pretreated sugarcane bagasse at 0.5%, w/v), even better than the yields found by SmF of the fungi Penicillium and Rhizomucor.
Shahid et al. (2016) reported a production of 710 IU/L (CMCase activity) by B. megaterium BM 05 using wheat straw as substrate. Al-Gheethi (2015) investigated the potential of different sewage sludge as medium of production of cellulases by B. megaterium. The highest and lowest activities were 14,300 and 1400 IU/L, respectively, which did depend on the studied sewage. In addition, the authors studied the use of bagasse as a carbon source and the achieved enzyme activity was 9800 IU/L. The enzyme activities reported by the authors were much higher than those found in this work, which corroborates with the statement that the species of microorganism and the working conditions result in very different enzyme productions.
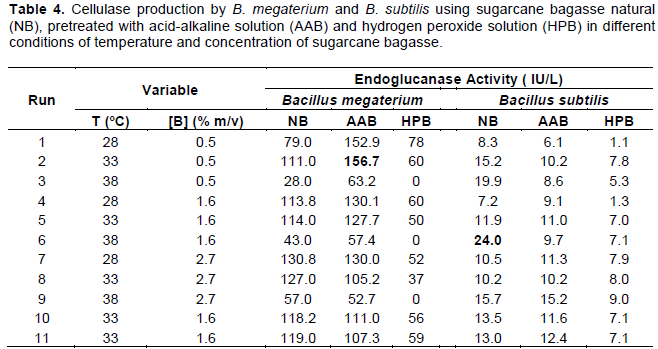
literature, depending on the species used and the working conditions. Jiménez-Leyva et al. (2017) evaluated three different strains of B. subtilis in the production of cellulases using carboxymethylcellulose and microcrystalline cellulose as carbon sources. The authors found CMCase activities between 150 and 450 IU/L. Vaid and Bajaj (2017) studied different sources of carbon and nitrogen in the production of cellulases by the strain B. subtilis G2 and the highest activity was 2179 IU/L using banana peel, which was very close to the activity using sugarcane bagasse (2000 IU/L).
Regarding to the substrates used in this work, we can see that the sugarcane bagasse treated with hydrogen peroxide led to the lowest values of enzyme activity, both for B. megaterium and B. subtilis. For B. megaterium, a slightly higher enzyme production was observed when bagasse pretreated with acid-alkaline solution was used. For B. subtilis, the enzyme productions were close to the natural bagasse and pretreated with acid-alkaline solution.
The effects of the temperature and the concentration of sugarcane bagasse on the enzyme production were statistically analyzed and the results of ANOVA are shown in Table 5. Statistical analysis was performed only for B. megaterium, which showed the best enzyme production. After removing the variables that did not significantly affect the enzyme activities (p> 0.05), the values of the effects of the variables on the enzyme production were obtained (Table 6), as well as the equations (Equations 4 to 6) that describe the behavior of the variables in the enzyme activity, and the surface response (Figure 2a, b, and c), when using natural bagasse and bagasses pretreated with acid-base and peroxide solutions, respectively. It can be seen that both variables influenced the enzyme production, similarly to the results obtained with T. koningii. When natural sugarcane bagasse was used, higher concentrations produced higher enzymatic activities; however, an increase in the temperature resulted in a decrease in the activity. The effect of the temperature was twice greater than that of the bagasse concentration. For the other two types of pretreated bagasse, there is a decrease in the enzyme production by increasing both substrate concentration and temperature, where temperature is the variable that most influenced the enzyme production.
A.E. (IU/L) = -1545.0 + 14.7 × [B] + 105.9 × T - 1.7 × T2 (4)
A.E (IU/L) = - 633.6 - 12.9 × [B] + 55.0 × T - 0.9 × T2 (5)
A.E (IU/L) = - 567.5 - 46.4 × [B] + 46.5 × T - 0.8 × T2 + 1.2 × [B ] × T (6)
This study showed that T. koningii is a good choice to produce cellulases by SmF using natural sugarcane bagasse as carbon source and relatively low temperature of cultivation. These findings could be attractive from an economic point of view, because pretreatment of the bagasse increases its final cost, as well as higher temperature is energy consuming and therefore not environmentally friendly.
The authors have not declared any conflict of interests.
The authors appreciate the financial support from Foundation for Research Support of Espírito Santo (FAPES), Federal University of Espírito Santo (UFES) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
REFERENCES
|
Acharya BK, Mohana S, Jog R, Divecha J, Madamwar D (2010). Utilization of anaerobically treated distillery spent wash for production of cellulases under solid-state fermentation. Journal of Environmental Management 91:2019-2027.
Crossref
|
|
|
|
Akinyele JB, Falade OE, Olaniyi OO (2014). Screening and optimization of culture conditions for cellulase production by Aspergillus niger nspr012 in submerged fermentation. Journal of Microbiology, Biotechnology and Food Sciences 4(3):189-193.
Crossref
|
|
|
|
|
Al-Gheethi AAS (2015). Recycling of sewage sludge as production medium for cellulase by a Bacillus megaterium strain. International Journal of Recycling of Organic Waste in Agriculture 4:105-119.
Crossref
|
|
|
|
|
Baraldo Junior A, Borges DG, Tardioli PW, Farinas CS (2014). Characterization of β-glucosidase produced by Aspergillus niger under solid-state fermentation and partially purified using MANAE-Agarose. Biotechnology Research International.
Crossref
|
|
|
|
|
Behera BC, Sethi BK, Mishra RR, Dutta SK, Thatoi HN (2017). Microbial cellulases-Diversity & biotechnology with reference to mangrove environment: A review. Journal of Genetic Engineering Biotechnology 15:197-210.
Crossref
|
|
|
|
|
Bentil JA, Thygesen A, Mensah M, Lange L, Meyer AS (2018). Cellulase production by white-rot basidiomycetous fungi: solid-state versus submerged cultivation. Applied Microbiology and Biotechnology 102:5827-5839.
Crossref
|
|
|
|
|
Borges DG, Junior AB, Farinas CS, Giordano RDLC, Tardioli PW (2014). Enhanced saccharification of sugarcane bagasse using soluble cellulase supplemented with immobilized β-glucosidase. Bioresource Technology 167:206-213.
Crossref
|
|
|
|
|
Catelan TC, Pinotti LM (2019). Avanço das pesquisas envolvendo Aspergillus niger e bagaço da cana-de-açúcar como fonte de carbono visando à produção de celulases: Uma análise bibliométrica. Matéria (Rio J.) 24:2.
Crossref
|
|
|
|
|
Fernandes MLM (2007). Produção de lipases por fermentação no estado sólido e sua utilização em biocatálise. Ph.D. thesis. Brazil: Universidade Federal do Paraná.
|
|
|
|
|
Fernandes TG, López JA, Silva LA, Polizeli MTM, Silva DP, Ruzene DS, Carvalho MLS, Carvalho IF (2018). Prospecting of soybean hulls as an inducer carbon source for the cellulase production. Preparative Biochemistry and Biotechnology 48(8):743-749.
Crossref
|
|
|
|
|
Gautam RL, Naraian N (2020). Trichoderma, a Factory of Multipurpose Enzymes: Cloning of Enzymatic Genes. In: Hesham AL, Upadhyay R, Sharma G, Manoharachary C, Gupta V (eds) Fungal Biotechnology and Bioengineering 137-162. Fungal Biology. Springer, Cham.
Crossref
|
|
|
|
|
Ghose TK (1987). Measurement of cellulase activities. Pure and Applied Chemistry 59(2):257-268.
Crossref
|
|
|
|
|
Hernández-Domínguez EM, Rios-Latorre RA, Álvarez-Cervantes J, Loera-Corral O, Román-Gutiérrez AD, Díaz-Godínez G, Mercado-Flores Y (2014). Xylanases, cellulases, and acid protease produced by Stenocarpella maydis grown in solid-state and submerged fermentation. Bioresources 9(2):2341-2358.
Crossref
|
|
|
|
|
Irfan M, Mushtaq Q, Tabssum F, Shakir HA, Qazi JI (2017). Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K‑18 for saccharification using response surface methodology AMB Express 7:29.
Crossref
|
|
|
|
|
Jasani H, Umretiya N, Dharajiya D (2016). Isolation, Optimization and Production of Cellulase by Aspergillus niger from agricultural waste. Journal of Pure and Applied Microbiology 10(2):1159-1166.
|
|
|
|
|
Jiménez-Leyva MF, Beltrán-Arredondo LI, Cervantes-Gámez R, Cervantes-Chávez J, López-Meyer M, Castro-Ochoa D (2017). Effect of CMC and MCC as Sole Carbon Sources on Cellulase Activity and eglS Gene Expression in Three Bacillus subtilis strains Isolated from corn stover. BioResources 12(1):1179-1189.
Crossref
|
|
|
|
|
Juturu V, Wu JC (2014). Microbial cellulases: engineering, production and applications. Renewable and Sustainable Energy Reviews 33:188-203.
Crossref
|
|
|
|
|
Menoncin S, Domingues NM, Freire DMG, Oliveira JV, Di Luccio M, Treichel H, de Oliveira D (2009). Immobilization of lipases produced by solid state fermentation from Penicillium verrucosum on hydrophobic supports. Ciência e Tecnologia de Alimentos 29(2):440-443.
Crossref
|
|
|
|
|
Lin J, Zhang X, Song B, Xue W, Su X, Chen X, Dong Z (2017). Improving cellulase production in submerged fermentation by the expression of a Vitreoscilla hemoglobin in Trichoderma reesei. AMB Express 7(203).
Crossref
|
|
|
|
|
Liu HQ, FengY, Zhao DQ, Jiang JX (2012). Evaluation of cellulases produced from four fungi cultured on furfural residues and microcrystalline cellulose. Biodegradation 23:465-472.
Crossref
|
|
|
|
|
Mandels M, Weber J (1969). The production of cellulases. Advances in Chemistry 95:391-414.
Crossref
|
|
|
|
|
Mesa L, Salvador CA, Herrera M, Carrazana DI, González E (2016). Cellulases by Penicillium sp. in different culture conditions. Bioethanol 2:84-93.
Crossref
|
|
|
|
|
Miller GL (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31:426-428.
Crossref
|
|
|
|
|
Mmango-Kaseke Z, Okaiyeto K, Nwodo UU (2016). Optimization of Cellulase and Xylanase Production by Micrococcus Species under Submerged Fermentation. Sustainability 8:1168.
Crossref
|
|
|
|
|
Nagar S, Kumar V (2010). Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. Journal of Industrial Microbiology and Biotechnology 37:71-83.
Crossref
|
|
|
|
|
Pandey AK, Edgard G, Negi S (2016). Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renewable Energy 98:51-56.
Crossref
|
|
|
|
|
Rodríguez-Zúñiga UF, Farinas CS, Bertucci Neto V, Couri S, Crestana S (2011). Produção de celulases por Aspergillus niger por fermentação em estado sólido. Pesquisa Agropecuária Brasileira. 46(8):912-919.
Crossref
|
|
|
|
|
Salomão GSB, Agnezi JC, Paulino LB, Hencker LB, de Lira TS, Tardioli PW, Pinotti LM (2019). Production of cellulases by solid state fermentation using natural and pretreated sugarcane bagasse with different fungi. Biocatalysis and Agricultural Biotechnology 17:1-6.
Crossref
|
|
|
|
|
Santa-Rosa PS, Souza AL, Roque RA, Andrade EV, Astol S, Mota AJ, Nunes-Silva CG (2018). Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology 31:84-92.
Crossref
|
|
|
|
|
Shahid ZH, Irfan M, Nadeem M, Syed Q (2016). Production, Purification, and Characterization of Carboxymethyl Cellulase from Novel Strain Bacillus megaterium. Environmental Progress Sustainable Energy 35(6):1741-1749.
Crossref
|
|
|
|
|
Singhania RR, Sukumaran RK, Patel AK, Larroche C, Pandey A (2010). Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme and Microbial Technology 46:541-549.
Crossref
|
|
|
|
|
Singhania RR, Sustainability E, Patel AK, Thomas L, Giri BS (2015). Industrial Enzymes. In: Pandey A., Höfer R, Taherzadeh M, Nampoothiri K, Larroche C (eds.) Industrial biorefineries and White Biotechnology, pp. 473-497. Elsevier
Crossref
|
|
|
|
|
Vaid S, Bajaj BB (2017). Production of Ionic Liquid Tolerant Cellulase from Bacillus subtilis G2 Using Agroindustrial Residues with Application Potential for Saccharification of Biomass Under One Pot Consolidated Bioprocess. Waste and Biomass Valorization 8:949-964.
Crossref
|
|
|
|
|
Vasconcellos VM, Tardioli PW, Giordano RLC, Farinas CS (2015). Production efficiency versus thermostability of (hemi) cellulolytic enzymatic cocktails from different cultivation systems. Process Biochemistry 50:1701-1709.
Crossref
|
|
|
|
|
Vázquez-Montoya EL, Castro-Ochoa LD, Maldonado-Mendoza IE, Luna-Suárez S, Claudia Castro-Martínez C (2020). Moringa straw as cellulase production inducer and cellulolytic fungi source. Revista Argentina de Microbiología 52(1):4-12.
Crossref
|
|
|
|
|
Zanirun Z, Bahrin E K, Lai-Yee P, Hassan MA, Abd-Aziz S (2014). Effect of Physical and Chemical Properties of Oil Palm Empty Fruit Bunch, Decanter Cake and Sago Pith Residue on Cellulases Production by Trichoderma asperellum UPM1 and Aspergillus fumigatus UPM2. Applied Biochemistry and Biotechnology 172:423-435.
Crossref
|
|
|
|
|
Wang Z, Ong HX, Geng A (2012). Cellulase production and oil palm empty fruit bunch saccharification by a new isolate of Trichoderma koningii D-64. Process Biochemistry https://doi.org/10.1016/j.procbio.2012.07.001
Crossref
|
|
|
|
|
Wang S, Liu G, Yu J, Tian S, Huang B, Xing M (2013). RNA interference with carbon catabolite repression in Trichoderma koningii for enhancing cellulase production. Enzyme and Microbial Technology 53:104-109.
Crossref
|
|
|
|
|
Wang H, Kaur G, Pensupa N, Uisan K, Du C, Yang X, Lin CSK (2018). Textile waste valorization using submerged filamentous fungal fermentation. Process Safety and Environmental Protection 118:143-151.
Crossref
|
|