ABSTRACT
Carbohydrates which comprise of monosaccharides, disaccharides and polysaccharides are heterogeneous complex structure in living systems and are found to bind to other organic molecules such as proteins and lipids. Advanced glycation end products (AGEs) are also heterogeneous group of molecules that accumulate in plasma and other body fluids and in cell and tissues. This review focuses on the impact of polysaccharides and AGEs on immune function. A number of in vitro and in vivo studies have demonstrated that polysaccharides and AGEs modulate both innate and adaptive immune responses. Polysaccharides such as lectins are reported to activate innate immune cells and T-helper cells leading to the up-regulation of T-lymphocytes, B-lymphocytes and macrophages and release of different cytokine profiles such as tumour necrosis factor (TNF-α), interleukin (IL)-1, IL-6, IL-8, IL-10, IL-12, interferon gamma and beta (IFN-γ and IFN-β2). Both lectins which are non-enzymatic proteins present in plants and animals that preferentially bind to specific carbohydrate structures and AGE which triggers inflammatory response by binding to RAGE do so there by mediating inflammation known as a key underlying cause in the development of vascular complications leading to an increased expression of cytokines, growth factors and adhesion molecules with mediate and immune response.
Key words: Immunomodulation, carbohydrates, advanced glycation end products (AGEs), receptors, lectins, in vitro and in vivo studies.
Immunomodulation through natural substances could be considered an alternative for the prevention and cure of various diseases. There is growing evidence suggesting that polysaccharides from natural plants can significantly enhance immune system responses. Plant polysaccha-rides are known to possess various biological and phar-macological activities hence are regarded as promising immunomodulatory agents which are relatively nontoxic and so have no significant side effects (Kim et al., 2011; Jin-Kyung et al., 2011; Tang et al., 2012; Xia et al., 2012). It has been demonstrated that most of the poly-saccharides are activators of immune cells through their capacity to enhance production of immune mediators, such as reactive oxygen species (ROS), nitric oxide (NO), TNF-α and IL-10 and also their immunomodulatory characteristics are mainly dependent on their unique molecular structures which activate varied surface receptors (Jeurink et al., 2008; Weng et al.,2011).
Macrophages and lymphocytes are important immune cells which play a pivotal role in immune responses by releasing cytokines that plays a critical role in mediating signal transduction and stimulating the immune defence system (Chen et al., 2008; Schepetkin and Quinn, 2006; Zha et al., 2007). Cytokines which are soluble glycol-proteins ought to critically involve in the immune response. The functions of these proteins are diverse and include roles in normal humoral and T- lymphocyte mediated immune response (Schepetkin and Quinn, 2006; Zha et al., 2007).
The killer cell immunoglobulin-like receptors (KIR) and C-type lectin-like receptors are the two major families of receptors expressed by human NK cells which recognize class I MHC molecules. The receptors within each family have similar features with highly homologous extracel-lular domains attached to various transmembrane regions and cytoplasmic tails. However, the inhibitory receptors belonging to both KIR and C-type lectin-like receptor families carry a pair of ITAMs in their cytoplasmic tails (Baba et al., 2000). Moreover, due to their early production of cytokines and chemokines and their ability to lyse target cells without prior sensitisation NK cells are crucial components of the innate immune system, providing a first line of defence against infectious agents. Hence, they can be activated non-specifically by several cytokines, including IL-2, IL-15 and IL-18 (Middleton et al., 2002)
Numbers of pathologic conditions are accompanied by changes in cytokine levels and by disturbances in the cytokine-mediated interplay between innate and acquired immune responses (Stanilova et al., 2005). IL-2 is found to be an essential cytokine for T lymphocyte growth. IFN-γ promotes the differentiation of Th1 cells and functions principally on macrophages to enhance antimicrobial properties. IL-4 is crucial for the differentiation of naive Th cells into the Th2 cells that promote humoral immunity and also has a central role in the pathogenesis of allergic inflammation (Chen et al., 2008). A study by Zha et al. (2007) using Dendrobium huoshanense in an in vitro study showed that the polysaccharide of HPS-1B23 was able to stimulate IFN-γ and TNF-α production in the culture medium of splenocytes and macrophages, respectively with no observed toxicity effect of the samples (Zha et al., 2007).In the human body, the immune system is divided into the innate and adaptive immunities. Innate immunity refers to a non-specific immune response in encountering a particular antigen, while the adaptive immunity shows a high specificity to antigens invaded and is responsible for a memory response after the first exposure of the same antigen. These two systems do not work separately, they act closely together to help fight against the external threats by triggering a series of immune responses (Shao et al., 2004; Wong et al., 2011).
Carbohydrates are storage components in living system and comprise monosaccharides, disaccharides and polysaccharides (Khodse et al., 2007). Their role in biological processes makes them to be considered as vital biomolecules (Ghazarian et al., 2011; Hu and Xu, 2011). Carbohydrates decorate the surfaces of all cells (glycocalyx) in the form of polysaccharides, glycopro-teins, glycolipids and other glycoconjugates. The saccha-ride binding proteins mediate cell-cell interactions in numerous biological processes, such as blood coagula-tion, immune response, viral infection, pathological processes, inflammation, embryogenesis and cellular signal transfer (Han et al., 2011; Hu and Xu, 2011), thus they are recognized as ligands for carbohydrate recognition proteins (for example, lectins) (Hu and Xu, 2011; Nakamura et al., 2013; Wu et al., 2006).
AGEs are heterogeneous group of molecules that accumulate in plasma and other body fluids and in cell and tissues (Kasper and Funk, 2001). They are main pathogenic agent of advanced age, diabetes, atherosclerosis, renal failure and other chronic diseases. Researches has shown that AGEs are risk factors of disease as are known to be generated by the non-enzymatic reaction of amino groups with different biomolecules such as proteins and lipids(Hirasawa et al., 2011; Kasper and Funk, 2001; Wu et al., 2011). AGEs accumulate in the living system due to an increased level of sugars and reactive dicarbonyl compounds such as glucose, fructose, deoxyglucose, glyoxal, methylglyoxal and triosephosphates and their formation is irreversible, causing protease-resistant cross-linking of peptides and proteins, which can lead to protein deposition and amyloidosis (Krautwald and Münch, 2010).
CARBOHYDRATE STRUCTURE AND FUNCTION
Organic molecules in living systems are classified into four major groups: proteins, lipids, nucleic acids and carbohydrates. However carbohydrates are by far the most abundant organic molecules found in nature, and nearly all organisms synthesize and metabolize carbohydrates. It has the empirical formula CnH2nOn, where n is ≥3 and glucose is the common monosaccharide that may be linked to form a variety of other macromolecules. Carbohydrates appear to form a heterogeneous complex structure in living systems by binding to other organic molecules such as proteins and lipids by the action of glycosyltransferases and glycosi-dases. The combined activity levels of these enzymes in the endoplasmic reticulum (ER) and the Golgi apparatus, and perhaps on the cell surface, determine the glycosylation patterns (carbohydrate domains) of glycolipids and glycoproteins (Ghazarian et al., 2011).
The structural features of a polysaccharide are defined by molecular weight, monosaccharide composition, sequence of monosaccharide, configuration and position of glycosidic linkages, type and polymerization degree ofbranch, spatial configuration, etc (Wu et al., 2011). The extent to which polysaccharides exerts their bioactivities is closely related to their chemical composition, molecular weight, branching, chain conformation and water solubility. The basic understanding of both the primary and secondary structures for the polysaccharides is essential for the successful interpretation of their bioactivities, and therefore, the monosaccharide compo-sition analysis of polysaccharides is the most important step for the further discovery of its physicochemical properties, structure and structure-bioactivity relationship (Huang et al., 2007; Xie et al., 2013a).

Possible structures are O- or N-linked glycans and composition varies, giving a wide variety of structures able to bind to many different ligands (Figure 1). However, it is the secondary structure that determines the biological activity.Most of the polysaccharides, especially those found in the cell wall of the plant are mainly composed of monosaccharides such as xylose (xyl), arabinose (ara), mannose (man), sorbinose (sor), ribose (rib), Fuc, Glc, Gal, GlcNAc and GalNAc, along with small proportions of rhamnose (rha), fructose (fur), and 4-O-methyl galactosamine. The glycosyl linkages of these monosac-charide residues are those determined to give an insight into the structure of polysaccharides (Hsieh et al., 2008; Wang et al., 2012).
CELLULAR RECEPTORS FOR CARBOHYDRATES
Certain carbohydrates found on circulating cells, proteins and pathogens are recognized by specific lectin receptors involved in clearance or immunity (Nangia-Makker et al., 2002). Cell-surface carbohydrates, composed of several different kinds of monosaccharides, interact with many types of lectins as part of a communication signalling network between cells. Since the biogenesis of the cell-surface carbohydrates is tightly regulated, alterations in glycosylation patterns may play key roles in governing the invasive and metastasis potentials of many types of cancers. Viral virulence as well as bacterial and parasite infections are also mediated by host-cell surface carbohydrates, especially their terminal saccharides, which are recognized by invading pathogens (Hyun et al., 2007).
Complex carbohydrates (polysaccharides) are attached to the majority of secreted proteins and cover the surface of cells as a dense layer. However their importance, availability and structural variations reflect a key role in many biological functions. Lectins which are carbohydrate-binding proteins that are able to detect subtle differences between complex carbohydrate structures, decipher glycocodes recognizing certain sugars to carry out various functions like cell attachment, migration or invasion and that is why among all of the variety ofadhesion molecules, cell-surface carbohydrate structures have been a focus of many investigations (Nangia-Makker et al., 2002). Lectins are found throughout nature in plants, bacteria and animals. The major types of animal lectins include galectins, C-type, I-type, P-type and R-type lectins. Most lectins contain a carbohydrate recognition domain (CRD) that binds with high specificity to the outermost sugars of N-linked and O-linked glycans as well as glycospingolipids (Sørensen et al., 2012).

Among other members, C-type lectin receptors (CLRs) are considered to be suitable target in innate immunity and cell specific drug delivery due to their endocytotic or cell activation properties. They represent a large receptor family including collectins, selectins, lymphocyte lectins and proteoglycan and share a structurally homologous carbohydrate-recognition domain (CRD) as well as often binding to carbohydrates in a Ca2+-dependent pattern. Myeloid CLRs such as DC-SIGN, mannose receptor (MR), DEC-205 and others are crucial to initiate immune responses against various pathogens including bacteria, viruses, parasites and fungi (Figure 2a). However, some CLRs serve solely as phagocytic receptors whereas others activate signalling pathway by inducing the activation of tyrosine kinases such as spleen tyrosine kinase (SYK) through classical immunoreceptor tyrosine-based activation motifs (ITAMs) or hemITAMs (Figure 2b) (Lepenies et al., in press, accessed 2013).The signaling pathway elicited upon CLR engagement depends on their cytoplasmic signaling motifs. Some CLRs contain an intracellular hemITAM or are associated with ITAM-bearing adaptor proteins leading to the activation of the tyrosine kinase Syk. In contrast, other CLRs contain an intracellular ITIM that induces the recruitment of phosphatases such as SHP-1 and SHP-2. Another group of myeloid CLRs including DC-SIGN or MR does not possess intracellular signaling motifs, thus signaling is independent of Syk or phosphatases (Lepenies et al., in press, accessed 2013).
CARBOHYDRATES IN CELLULAR FLUIDS
Cellular fluids, especially extracellular have been isolated from various bodily fluids and used as biological markers for various diseases among which are cancer and diabetes mellitus (Tzimagiorgis et al., 2011). Carbohy-drates appeared to be basic to virtually every aspect of extracellular circulation, fulfilling roles from purely structural to mediating the highly specific recognition events that underlie cell-cell communication. Sugars are naturally chosen by nature to suitably mediate such an extraordinary variety of interactions. They are linked and similar to nucleotides and amino acids, also are able to form branched structures with stereospecific linkages, features that confer exponential capacity for structural diversity. Extracellular sugars are often elaborated with covalent modifications, such as sulfation, acetylation andand phosphorylation that impart further structural variety (Bowman and Bertozzi, 1999).Several conjugates including glycolipids and glycol-proteins can adhere to each other by trans carbohydrate-carbohydrate interactions between apposed membranes. For example, myelin, the membranous sheath which is spirally wound around nerve axons is rich in two glycosphingolipids, GalC and its sulphated form, galactosylceramide I3-sulfate. These interactions are often mediated by divalent cations. Water disrupts these interactions between single sugar molecules, but the interactions between polymeric multivalent glycoconju-gates or membrane lipid domains, that present a multivalent array of carbohydrate head groups, are stronger and persist in water. These can be strong enough to cause specific adhesion of sponge cells into a multicellular organism. In the case of mammalian cells, they may cause transient adhesion of cells allowing up-regulation of proteins such as integrins to maintain adhesion and the interaction can be homotypic, similar to that for the lexganglioside (Boggs et al., 2008).
Immunomodulatory activity of polysaccharides
Polysaccharides isolated from various traditional medicinal plants, especially higher plants (Xu et al., 2009) have been shown to greatly affect the immune system both in vivo and in vitro by stimulating cytokine and chemokine production, ROS production and cell proliferation. They are reported to be non-toxic, with no significant side effects, which is a major problem associated with many other immunomodulatory agents, such as bacterial polysaccharides and synthetic compounds. These polysaccharides therefore have the potential as immunomodulators (Belska et al., 2010; Schepetkin and Quinn, 2006) as they have been shown to increase macrophage cytotoxic activity against tumour cells and microorganisms, activate phagocytic activity and enhance secretion of cytokines and chemokines (Chen et al., 2010; Schepetkin and Quinn, 2006).
Several studies have shown the immunomodulatory effects of certain polysaccharides and how they activate innate immune cells and T-helper cells leading to the up-regulation of T-lymphocytes, B-lymphocytes, and macro-phages and release of different cytokine profiles (Chen et al., 2010; Cheng et al., 2008; Fan et al., 2012; Kouakou et al., 2013; Makino et al., 2006; Wang et al., 2013; Wong et al., 2011; Xia et al., 2012; Xu et al., 2009; Zhang et al., 2013). For example, an in vivo immunomodulatory study by Wong et al. (2011) using sclerotial polysaccharides isolated by hot water extraction from Pleurotus tuber-regium and Pheidole rhinocerus (PTW and PRW, respectively) on both healthy BALB/c mice and healthy athymic nude mice had shown that innate immune cells such as monocyte/macrophages and T-helper cells dendritic cells are activated by the mushroom are activatedby the mushroom sclerotial polysaccharides upon detection of release cytokines like IL-1β, IFN-γ, TNF-α, IL-2 and IL-6 (Wong et al., 2011).Also, in a study by Zhang et al. (2013) on hot water extracted polysaccharides from stems of Taxillus chinensis and Uncaria rhyncophylla in a dose-dependent manner (10 and 100 µg/ml) have shown an increase production of TNF and NO, especially on 100 µg/ml concentration in treated macrophages when compared with untreated ones. Hence it can be seen that these polysaccharide fractions display significant immunomodu-latory activities as indicated by increased production of TNF-α as well as NO (Zhang et al., 2013).Another example is on the recent study by Wang et al. (2013) on Kadsura marmorata. This plant has been used in traditional Chinese medicine as one of the major ingredients for the treatment of bronchial asthma, eczema and acute and chronic infection due to either viruses or bacteria. The study which was investigated in vitro on chicken lymphocytes and macrophages has shown that K. rmorata had up-regulate the production of chicken T-lymphocytes, B-lymphocytes and peritoneal macro-phages which in turn could stimulate the cell proliferation. It also significantly enhances cytokine secretion. This was ascertained when different concentrations (dose dependent manner) of K. marmorata were used. Hence was suggested as having potential effects on regulating the immune system (Wang et al., 2013).
Protein glycation
During the lifetime of the cells, proteins are exposed to a multitude of modification process or non-enzymatic glycosylation reaction between reducing sugars and amine residues in proteins which usually occurred as a result of elevation of glucose concentrations (Shuvaev et al., 2001; Vlassopoulos et al., 2013). Oxidation and glycation are among the major non enzymatic mecha-nisms. Such kind of alterations can affect tissues and are accumulated in the cells with an indisputable effect on metabolism. In the reaction known as Maillard, that involves three main stages: early, intermediate and late, glucose or other reducing sugars such as fructose, pentoses, galactose, mannose, ascorbate, xylulose, at the early stage, react with a free amino group of several molecules, including proteins, nucleic acids and lipids, to form an unstable aldimine compound called the Schiff base. However, through rearrangement, this base gives rise to a stable ketoamine, known as Amadori product (Figure 3) (Lapolla et al., 2005). These molecular and cellular alterations, though not dangerous at the initial stage, can become damaging and pathogenic when sufficiently abundant. Among the various targets that are sensitive to alteration, blood plasma is sustainably and continually exposed to numerousmetabolites, which in turn induce an oxidative stress (Rondeau and Bourdon, 2011). Products of AGE are believed to be risk markers or risk factors of disease (Kasper and Funk, 2001; Vlassopoulos et al., 2013). Soluble AGE compounds in the circulation and extracellular fluids and another glycation product known as glycated hemoglobin (HbA1c) have been demonstrated to be the biomarkers for the diagnosis of diabetes and monitoring of glucose control in diabetic individuals as well as macrovascular diseases (Indurthi et al., 2012; Vlassopoulos et al., 2013).

Cellular receptors for advanced glycation end products
The AGEs that are in the circulation or extracellular space can interact with two types of cell surface receptors. Clearance and scavenger receptors are predo-minantly involved in AGE capture, removal and degradation. This group of receptors includes p60 (OST-48), p90 (80 K-H), galectin-3, type I and type II macro-phage scavenger receptors, CD-36, FEEL-1 and -2, scavenger receptor proteins SR-BI and SR-BII and the lectin-like oxidized low-density lipoprotein receptor 1 (Lox-1). The other types of AGE-receptors initiate specific cellular signalling events in response to AGE exposure. Among these various AGE-receptors, a receptor for advanced glycation end-products (RAGE) is the receptor for AGEs which accumulate in various diseases and inflammations and is suggested to be involved in cellular migration and spreading over binding of amphoterin, a developmental-regulated heparin-binding protein (Indurthi et al., 2012; Kasper and Funk, 2001).RAGE is a 35 kDa member of the immunoglobulin superfamily of cell surface proteins that interacts with a wide range of ligands, including AGE products, modified low density lipoproteins, amyloid fibrils, amphoterin (HMGB1) and various S100 proteins. It shares structural homology with other immunoglobulin like receptors (Leclerc et al., 2009) and is a specific binding protein for AGE moieties which have been detected on monocytes/macrophages, smooth muscle cells, hepato-cytes, neurons, pericytes, T-lymphocytes, mesangial cells, type I pneumocytes, osteoblast-like cells and endothelial cells and its expression can be abnormally up or down-regulated in human disease (Kasper and Funk, 2001; Xie et al., 2013b).
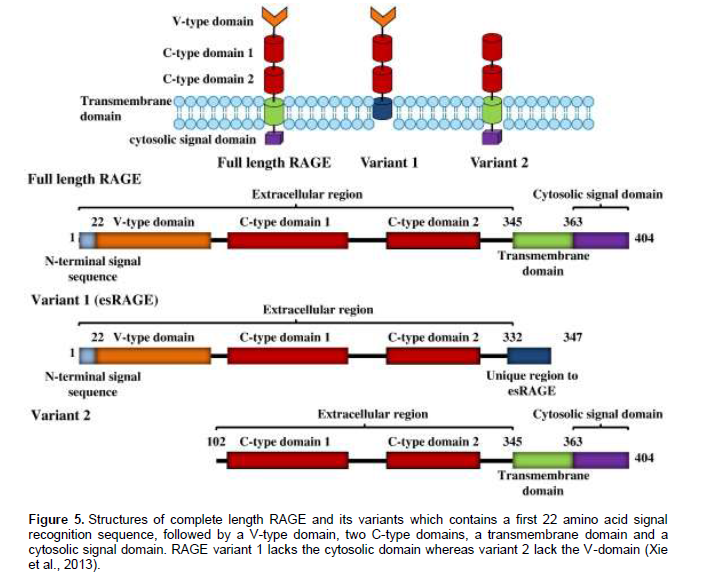
Activation of the RAGE pathway has been shown to be important in wound healing, tumour growth andmetastasis, as well as in systemic amyloidosis (Germanová et al., 2010; Mahajan et al., 2010). The mature 382 amino-acid long RAGE is composed of an extracellular part (314 aa), a single transmembrane spanning helix (27 aa) and a short cytosolic domain (41 aa) (Figure 4) (Leclerc et al., 2009). Complete length RAGE contains a V-type domain, which functions for ligand binding, two C-type domains, a transmembrane spinning helix, and a C-terminal cytosolic domain (ctRAGE) which is required for signal transduction. However RAGEs with the deletion of V-type domain (variant 2) are unable to interact with its ligands and therefore irresponsive to any stimuli, whereas RAGEs without ctRAGE (variant 1) have negative actions by competing with endogenous full-length RAGE for ligand binding, thereby blunting the RAGE-dependent signal transduction (Figure 5) (Xie et al., 2013).RAGE has been documented in promoting inflam-mation and endothelial activation, which in turn also accelerates coronary atherosclerotic development. Its activation is enhanced by accumulation of its ligands in inflammatory compartments, initiating a vicious cycle causing further up-regulation of the receptor and sustained cell activation. These molecules has also been shown to be highly expressed in human atherosclerotic lesions where it co-localizes with pro-inflammatory and pro-oxidative mediators (Mahajan et al., 2010).

Advanced glycation end products in cellular fluids
Normal tissue remodelling is thought to be contributed by AGE modified proteins. However, under pathological conditions such as diabetes, rheumatoid arthritis (RA) and osteoarthritis, renal insufficiency and in smokers, AGEs can be found elevated in body fluids such as serum, synovial fluid and urine. Among them, serum and synovial fluid pentosidine is the superior indicator for so many diseases status (Kasper and Funk, 2001). Soluble receptor for advanced glycation end products (sRAGE) which is the alternative splicing of RAGE mRNA and the proteolytic cleavage of membrane-bound RAGE has the ability to bind with the same ligands, sRAGE acts as a competitive inhibitor of RAGE, and precludes the cell-bound RAGE signaling. RAGE adversely involves in several pathological processes of human diseases, such as diabetes, Alzheimer's disease and chronic inflamma-tory diseases such as RA (Chayanupatkul and Honsawek, 2010). It has been reported recently that another extracellular newly identified RAGE-binding protein, known as EN-RAGE, has been found. It is a member of the S100 protein family, also called S100A12or CAAF1 or calgranulin C, which is comprised of closely related low molecular weight (10-14 kDa) acidic calcium-binding proteins with EF hands. These proteins were purifiedfrom bovine lung extract and expressed abundantly in the esophageal epithelium, neutrophils and monocytes/macrophages in humans. In the other hand to sRAGE, EN-RAGE is a natural pro-inflammatory ligand for RAGE. EN-RAGE binding to RAGE activates intracellular signal cascades including MAP-kinase and NKκB which subsequently induces secretion of cytokines such as IL-1β and TNF-α, expression of adhesion molecules there by activating inflammatory responses and enhancing the activation and migration of monocytes/macrophages to damaged vessels leading to the pathogenesis of inflam-matory diseases (Hasegawa et al., 2003; Kalousová et al., 2012; Kim et al., 2012). Additionally, S100A12 is able to interact with scavenger receptors; it is also chemotactic for neutrophils and monocytes and by binding copper. It is able to generate ROS and so acts as a pro-oxidant agent. Increased level of EN-RAGE are found in various inflammatory com-partments like synovial fluid and its serum concentrations are increased in inflammatory diseases and correlated with the disease activity. High concentrations of S100A12 are described in patients with neurodegenerative diseases and atherosclerosis. EN-RAGE interacting with RAGE could also be implicated in cancerogenesis particularly at sites with chronic and persistent inflammation (Kalousová et al., 2012).
Immunomodulatory effects of advanced glycation end-products
AGE triggers inflammatory response by binding to RAGE there by mediating inflammation known as a key under-lying cause in the development of vascular complications leading to an increased expression of cytokines, growth factors and adhesion molecules with mediate an immune response (Choi et al., 2013; Hirose et al., 2011). Intracellularly, AGEs that are formed inside and outside of cells induces a more unspecific chemical reaction, the production of ROS increases which causes cross-linking with other cellular proteins and generation of lipid peroxidation product (Kasper and Funk, 2001). RAGE, found in most tissues, have potent immunomodulatory actions, promoting ROS production and inflammation (Vlassopoulos et al., 2013). AGEs are known to be high in patients with poorly controlled disease, and its accumulation is a fundamental mechanism that causes the complications in diseases like diabetes. T lymphocytes contain a surface receptor for AGE-modified proteins that is inducible by the stimulation of the T-lymphocyte mitogen phytohemagglutinin. AGEs have also been found to act on macrophages through the AGE receptor on the plasma membrane, though it is not known whether the proliferation of activated T lymphocytes would be affected by AGEs (Liu et al., 2005).Several targets of advanced glycation include structural proteins, such as collagen and aggrecan, plasma proteins, including immunoglobulins and albumin, and intracellular proteins, such as haemoglobin and lens crystallin. Protein glycation in particular has the potential to alter many cellular functions, and as a consequence AGE has been suggested to contribute towards the pathogenesis of many diseases. Moreover, AGE-modified proteins have been shown to generate free radicals such as superoxide and NO, and to up-regulate pro-inflammatory cytokines by monocyte and/or macrophages. Other responses elicited by AGE include induction of IL-1 and expression of tissue factor by monocytes and release of TNFα by THP-1 monocytic cells incubated with AGE-human serum albumin (HSA) (Perty?ska-Marczewska et al., 2004). Other consequences observed in AGE-RAGE interaction are an increase in the procoagulant response to TNF-α, a reduction in thrombomodulin expression, and an increase in endothelin-1 levels (Figure 6) (Lapolla et al., 2005).

Carbohydrates are favourable candidates for disease control, because they are present on the cell surface and act as an identification tag, through which they can interact with their surroundings, whether the extracellular matrix or homotypic or heterotypic cells. Interfering with the normal cell recognition phenomenon using a large or a small sugar molecule has been reported to block the progression of tumours by interfering with angiogenesis, cell-cell, cell-matrix interactions, tumour invasion and metastasis. Modification of a natural plant carbohydrate to facilitate its absorption by the body is a more cost-effective and easier way to prevent disease progression than synthesizing a competitive sugar molecule. The generation of AGEs is an inevitable process in vivo and their accumulation in tissues has been implicated in the process of aging and also in the pathogenesis of several pathological conditions. A number of in vitro and in vivo studies have demonstrated that carbohydrates and AGEs modulate various indices of immune function. Further analysis of monosaccharide composition of polysac-charides could be an essential parameter for the evaluation of quality of standard of polysaccharide materials and basic information on it before being applied for any investigation. Methods of isolating a particular polysaccharide is also paramount: their extraction by different methods might yield different chemical com-ponents, as such reliable and effective methods should be applied. Although, so far investigations of the receptor-mediated pathways for the immunomodulatoryactivity of certain polysaccharides have been reported, a more detailed mechanistic study is required to explain how the innate immune cells such as macrophages are activated by polysaccharides. Future studies could be directed not only to its ability to control cancer which is widely and commonly known it for, but also in other diseases. Further studies should be applied on the most effective compounds that could suppress AGE-mediated inflammatory signalling cascades, which involved the expression of pro-inflammatory intermediates and NF-κB transcriptional activity. Suppression of intracellular ROS production by scavenging ROS and reducing NADPH oxidase activity was a key regulatory mechanism for inhibiting AGE-mediated inflammatory response. Given the key role of AGE-RAGE signalling in the progression of diabetic complications, the suppressive effects of those compounds on AGE-mediated inflammation could be applied to prevent the development of diabetic complications.
The author(s) have not declared any conflict of interests.
REFERENCES
Baba E, Erskine R, Boyson JE, Cohen GB, Davis DM, Malik P, Strominger JL (2000). N-linked carbohydrate on human leukocyte antigen-C and recognition by natural killer cell inhibitory receptors. Hum. Immunol. 61(12):1202-1218.
Crossref |
|
|
Belska NV, Guriev AM, Danilets MG, Trophimova ES, Uchasova EG, Ligatcheva AA, Belsky YP (2010). Water-soluble polysaccharide obtained from Acorus calamus L. classically activates macrophages and stimulates Th1 response. Int. Immunopharmacol. 10(8):933-942.
Crossref |
|
|
Boggs JM, Gao W, Hirahara Y (2008). Myelin glycosphingolipids, galactosylceramide and sulfatide, participate in carbohydrate-carbohydrate interactions between apposed membranes and may form glycosynapses between oligodendrocyte and/or myelin membranes. Biochim. Biophys. Acta 1780(3):445-455.
Crossref |
|
|
Bowman KG, Bertozzi CR (1999). Carbohydrate sulfotransferases: mediators of extracellular communication. Chem. Biol. 6(1):R9-R22.
Crossref |
|
|
Chayanupatkul M, Honsawek S (2010). Soluble receptor for advanced glycation end products (sRAGE) in plasma and synovial fluid is inversely associated with disease severity of knee osteoarthritis. Clin. Biochem. 43(13-14):1133-1137.
Crossref |
|
|
Chen J-R, Yang Z-Q, Hu T-J, Yan Z-T, Niu T-X, Wang L, Wang M (2010). Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia 81(8):1117-1124.
Crossref |
|
|
Chen Z, Kwong Huat Tan B, Chan SH (2008). Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int. Immunopharmacol. 8(12):1663-1671.
Crossref |
|
|
Cheng A, Wan F, Wang J, Jin Z, Xu X (2008). Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis fish. Int. Immunopharmacol. 8(1):43-50.
Crossref |
|
|
Choi H-J, Jang H-J, Chung T-W, Jeong S-I, Cha J, Choi J-Y, Ha K-T (2013). Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia 86:19-28.
Crossref |
|
|
Fan L, Ding S, Ai L, Deng K (2012). Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohydr. Polym. 90(2):870-874.
Crossref |
|
|
Germanová A, Koucký M, Hájek Z, PaÅ™ízek A, Zima T, Kalousová M (2010). Soluble receptor for advanced glycation end products in physiological and pathological pregnancy. Clin. Biochem. 43(4-5):442-446.
Crossref |
|
|
Ghazarian H, Idoni B, Oppenheimer SB (2011). A glycobiology review: Carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 113(3):236-247.
Crossref |
|
|
Han E, Ding L, Jin S, Ju H (2011). Electrochemiluminescent biosensing of carbohydrate-functionalized CdS nanocomposites for in situ label-free analysis of cell surface carbohydrate. Biosensors Bioelectron. 26(5):2500-2505.
Crossref |
|
|
Hasegawa T, Kosaki A, Kimura T, Matsubara H, Mori Y, Okigaki M, Iwasaka T (2003). The regulation of EN-RAGE (S100A12) gene expression in human THP-1 macrophages. Atherosclerosis 171(2):211-218.
Crossref |
|
|
Hirasawa Y, Sakai T, Ito M, Yoshimura H, Feng Y, Nagamatsu T (2011). Advanced-glycation-end-product-cholesterol-aggregated-protein accelerates the proliferation of mesangial cells mediated by transforming-growth-factor-beta 1 receptors and the ERK-MAPK pathway. Eur. J. Pharmacol. 672(1-3):159-168.
Crossref |
|
|
Hirose J, Yamabe S, Takada K, Okamoto N, Nagai R, Mizuta H (2011). Immunohistochemical distribution of advanced glycation end products (AGEs) in human osteoarthritic cartilage. Acta Histochem. 113(6):613-618.
Crossref |
|
|
Hsieh YSY, Chien C, Liao SKS, Liao S-F, Hung W-T, Yang W-B, Wong C-H (2008). Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg. Med. Chem. 16(11):6054-6068.
Crossref |
|
|
Hu M-X, Xu Z-K (2011). Carbohydrate decoration of microporous polypropylene membranes for lectin affinity adsorption: comparison of mono- and disaccharides. Colloids Surf. B Biointerfaces 85(1):19-25.
Crossref |
|
|
Huang Q, Jin Y, Zhang L, Cheung PCK, Kennedy JF (2007). Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohydr. Polym. 70(3):324-333.
Crossref |
|
|
Hyun S, Kim J, Kwon M, Yu J (2007). Selection and syntheses of tentacle type peptides as 'artificial' lectins against various cell-surface carbohydrates. Bioorg. Med. Chem. 15(1):511-517.
Crossref |
|
|
Indurthi VSK, Leclerc E, Vetter SW (2012). Interaction between glycated serum albumin and AGE-receptors depends on structural changes and the glycation reagent. Arch. Biochem. Biophys. 528(2):185-196.
Crossref |
|
|
Jeurink PV, Noguera CL, Savelkoul HFJ, Wichers HJ (2008). Immunomodulatory capacity of fungal proteins on the cytokine production of human peripheral blood mononuclear cells. Int. Immunopharmacol. 8(8):1124-1133.
Crossref |
|
|
Kalousová M, KubÄ›na AA, Benáková H, Dusilová-Sulková S, TesaÅ™ V, Zima T (2012). EN-RAGE (extracellular newly identified receptor for advanced glycation end-products binding protein) and mortality of long-term hemodialysis patients: Aprospective observational cohort study. Clin. Biochem. 45(7-8):556-560.
Crossref |
|
|
Kasper M, Funk RHW (2001). Age-related changes in cells and tissues due to advanced glycation end products (AGEs). Arch. Gerontol. Geriatr. 32(3):233-243.
Crossref |
|
|
Khodse VB, Fernandes L, Gopalkrishna VV, Bhosle NB, Fernandes V, Matondkar SGP, Bhushan R (2007). Distribution and seasonal variation of concentrations of particulate carbohydrates and uronic acids in the northern Indian Ocean. Mar. Chem. 103(3-4):327-346.
Crossref |
|
|
Kim J-K, Cho ML, Karnjanapratum S, Shin I-S, You SG (2011). In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 49(5):1051-1058.
Crossref |
|
|
Kim J-K, Park S, Lee MJ, Song YR, Han SH, Kim SG, Yoo T-H (2012). Plasma levels of soluble receptor for advanced glycation end products (sRAGE) and proinflammatory ligand for RAGE (EN-RAGE) are associated with carotid atherosclerosis in patients with peritoneal dialysis. Atherosclerosis 220(1), 208-214.
Crossref |
|
|
Kouakou K, Schepetkin IA, Yapi A, Kirpotina LN, Jutila MA, Quinn MT (2013). Immunomodulatory activity of polysaccharides isolated from Alchornea cordifolia. J. Ethnopharmacol. 146(1):232-242.
Crossref |
|
|
Krautwald M, Münch G (2010). Advanced glycation end products as biomarkers and gerontotoxins - A basis to explore methylglyoxal-lowering agents for Alzheimer's disease? Exp. Gerontol. 45(10):744-751.
Crossref |
|
|
Lapolla A, Traldi P, Fedele, D. (2005). Importance of measuring products of non-enzymatic glycation of proteins. Clin. Biochem. 38(2):103-115.
Crossref |
|
|
|
Leclerc E, Fritz G, Vetter SW, Heizmann CW (2009). Binding of S100 proteins to RAGE: An update. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1793(6):993-1007. |
|
|
|
Lepenies B, Lee J, Sonkaria S (in press, accessed 2013). Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv. Drug Deliv. Rev. 65(9):1271-1281. |
|
|
Liu C-T, Chen K-M, Lee S-H, Tsai L-J (2005). Effect of supplemental l-arginine on the function of T lymphocytes and the formation of advanced glycosylated end products in rats with streptozotocin-induced diabetes. Nutrition 21(5):615-623.
Crossref |
|
|
Mahajan N, Bahl A, Dhawan V (2010). C-reactive protein (CRP) up-regulates expression of receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in THP-1 cells: Inhibitory effects of atorvastatin. Int. J. Cardiol. 142(3):273-278.
Crossref |
|
|
Makino S, Ikegami S, Kano H, Sashihara T, Sugano H, Horiuchi H, Oda M (2006). Immunomodulatory Effects of Polysaccharides Produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 89(8):2873-2881.
Crossref |
|
|
Middleton D, Curran M, Maxwell L (2002). Natural killer cells and their receptors. Transplant Immunol. 10(2-3):147-164.
Crossref |
|
|
Nakamura M, Tsutsumi M, Ishikawa Y, Umemiya H, Hasegawa T, Izawa K, Hasegawa T (2013). Glycosylated tris-bipyridine ferrous complexes to provide dynamic combinatorial libraries for probing carbohydrate-carbohydrate interactions. Tetrahedron 69(14):3019-3026.
Crossref |
|
|
Nangia-Makker P, Conklin J, Hogan V, Raz A (2002). Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends Mol. Med. 8(4):187-192.
Crossref |
|
|
Pertyńska-Marczewska M, Kiriakidis S, Wait R, Beech J, Feldmann M, Paleolog EM (2004). Advanced glycation end products upregulate angiogenic and pro-inflammatory cytokine production in human monocyte/macrophages. Cytokine 28(1):35-47.
Crossref |
|
|
Pesesse X, Backers K, Moreau C, Zhang J, Blero D, Paternotte N, Erneux C (2006). SHIP1/2 interaction with tyrosine phosphorylated peptides mimicking an immunoreceptor signalling motif. Adv. Enzyme Regul. 46(1):142-153.
Crossref |
|
|
Ponnusamy S, Zhang H, Kadam P, Lin Q, Lim TK, Sandhu JS, Choolani M (2012). Membrane proteins of human fetal primitive nucleated red blood cells. J. Proteom. 75(18):5762-5773.
Crossref |
|
|
Rondeau P, Bourdon E (2011). The glycation of albumin: Structural and functional impacts. Biochimie 93(4):645-658.
Crossref |
|
|
Schepetkin IA, Quinn MT (2006). Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 6(3):317-333.
Crossref |
|
|
Shao B-M, Xu W, Dai H, Tu P, Li Z, Gao X-M (2004). A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 320(4):1103-1111.
Crossref |
|
|
Shuvaev VV, Laffont I, Serot J-M, Fujii J, Taniguchi N, Siest G (2001). Increased protein glycation in cerebrospinal fluid of Alzheimer's disease. Neurobiol. Aging 22(3):397-402.
Crossref |
|
|
Sørensen ALT, Clausen H, Wandall HH (2012). Carbohydrate clearance receptors in transfusion medicine. Biochim. Biophys. Acta 1820(11):1797-1808.
Crossref |
|
|
Stanilova SA, Dobreva ZG, Slavov ES, Miteva LD (2005). C3 binding glycoprotein from Cuscuta europea induced different cytokine profiles from human PBMC compared to other plant and bacterial immunomodulators. Int. Immunopharmacol. 5(4):723-734.
Crossref |
|
|
Tang X-H, Yan L-F, Gao J, Yang X-L, Xu Y-X, Ge H-Y, Yang H-D (2012). Antitumor and immunomodulatory activity of polysaccharides from the root of Limonium sinense Kuntze. Int. J. Biol. Macromol. 51(5):1134-1139.
Crossref |
|
|
Tzimagiorgis G, Michailidou EZ, Kritis A, Markopoulos AK, Kouidou S (2011). Recovering circulating extracellular or cell-free RNA from bodily fluids. Cancer Epidemiol. 35(6):580-589.
Crossref |
|
|
Vlassopoulos A, Lean MEJ, Combet E (2013). Role of oxidative stress in physiological albumin glycation: A neglected interaction. Free Radic. Biol. Med. 60:318-324.
Crossref |
|
|
Wang H, Deng X, Zhou T, Wang C, Hou Y, Jiang H, Liu G (2013). The in vitro immunomodulatory activity of a polysaccharide isolated from Kadsura marmorata. Carbohydr. Polym. 97(2):710-715.
Crossref |
|
|
Wang X-M, Sun R-G, Zhang J, Chen Y-Y, Liu N-N (2012). Structure and antioxidant activity of polysaccharide POJ-U1a extracted by ultrasound from Ophiopogon japonicus. Fitoterapia 83(8):1576-1584.
Crossref |
|
|
Weng BB-C, Lin Y-C, Hu C-W, Kao M-Y, Wang S-H, Lo D-Y, Chiou R Y-Y (2011). Toxicological and immunomodulatory assessments of botryosphaeran (β-glucan) produced by Botryosphaeria rhodina RCYU 30101. Food Chem. Toxicol. 49(4):910-916.
Crossref |
|
|
Wong K-H, Lai CKM, Cheung PCK (2011). Immunomodulatory activities of mushroom sclerotial polysaccharides. Food Hydrocoll. 25(2):150-158.
Crossref |
|
|
Wu JH, Singh T, Herp A, Wu AM (2006). Carbohydrate recognition factors of the lectin domains present in the Ricinus communis toxic protein (ricin). Biochimie 88(2):201-217.
Crossref |
|
|
Wu L, Ma L, Nicholson LFB, Black PN (2011). Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir. Med. 105(3):329-336.
Crossref |
|
|
Xia L, Liu X, Guo H, Zhang H, Zhu J, Ren F(2012). Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J. Funct. Foods 4(1):294-301.
Crossref |
|
|
Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM (2013a). Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 25(11):2185-2197.
Crossref |
|
|
Xie J-H, Shen M-Y, Nie S-P, Liu X, Zhang H, Xie M-Y (2013b). Analysis of monosaccharide composition of Cyclocarya paliurus polysaccharide with anion exchange chromatography. Carbohydr. Polym. 98(1):976-981.
Crossref |
|
|
Xu H, Yao L, Sun H, Wu Y (2009). Chemical composition and antitumor activity of different polysaccharides from the roots of Actinidia eriantha. Carbohydr. Polym. 78(2):316-322.
Crossref |
|
|
Zha X-Q, Luo J-P, Luo S-Z, Jiang S-T (2007). Structure identification of a new immunostimulating polysaccharide from the stems of Dendrobium huoshanense. Carbohydr. Polym. 69(1):86-93.
Crossref |
|
|
Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bailey T, Longvah T (2013). Immunomodulatory activities of polysaccharides isolated from Taxillus chinensis and Uncaria rhyncophylla. Carbohydr. Polym. 98(2):1458-1465.
Crossref |