Full Length Research Paper
ABSTRACT
Onoclea sensibilis L. is a temperate fern species of horticultural importance, and widely distributed in the natural environment of North America, Eastern Europe, and parts of Asia. With increasing climate change, including excessive heat and unpredictable, sometimes severe precipitation events, ferns such as O. sensibilis may come under increasing loss of habitat and possibly survival threat. This is a study of the photophysiology and dark respiration in O. sensibilis growing on the cliffs overlooking the Hudson River in Palisades New York, United States of America (USA), with documentation of changes it incurred after severe heat and drought-like conditions ensued following a moderate spring season. After the extreme summer events, photosynthesis and respiration rates declined, but leaf fluorescence analyses indicated no major change in quantum yield of photosystem II or electron transport per reaction center, suggesting that O. sensibilis may have survival strategies to succeed if climate change is not too severe. Data are also presented on the photosynthesis rate in relation to variations in light intensity expressed as photosynthetic active radiation (PAR) from 20 to 100 µmol photons m-2 s-1, and the results are discussed in relation to prior published findings.
Key words: Habitat loss, leaf chlorophyll fluorescence analysis, physiological ecology, plant conservation, plant ecology.
INTRODUCTION
This is a study of the photophysiology and respiration of the temperate fern Onoclea sensibilis L. (Figure 1), commonly known as the ‘sensitive fern’ due to its intolerance of early winter frost. The geographic range of O. sensibilis encompasses temperate locales in the northern hemisphere, including much of mid to eastern regions of North America, Eastern Europe including Russia, China and temperate Eastern Asia; although it also has been reported from New Zealand.
During the summer of 2022, the extreme climate in the northeastern United States of America (USA), that is, drought-like conditions and elevated peak temperatures exceeding 35°C (including the Hudson River palisade cliffs where this study was done) produced severe stress for many species of shade plants, including ferns and forbs dwelling in the forested areas and open locations on the cliffs. The effects were most pronounced for understory plants on higher ground where insufficient ground water and soil moisture led to wilting, decreased new growth and die back. However, other understory plants growing at the base of steep slopes, or in ravines where down-slope ground water seepage contributed to intermittent flowing springs, often were more resilient; especially in locations where gaps in the tree canopy provided sun flecks and dappled shade. Multiple factors (e.g., topography, elevation, microclimate, and quality and quantity of root substrate) contribute to variations in understory plant survival. Fern species that are particularly adapted to shaded understory environments, with typically consistent sources of moisture, are most susceptible to extreme heat and drought conditions in summer. In addition to the aesthetic qualities of ferns (Singh and Johari, 2018), they provide important ecological services (Moran, 2004; Sharpe et al., 2010); and their conservation in the face of increasing extreme climate events is a growing concern, including other environmental challenges that pose serious threats to a stable global habitat (Arcand and Ranker, 2013; Nowicki and Kowalska, 2018; Turkmen, 2022).
With increasing climate change, especially elevated summer temperatures and less predictable moderate rainfall, temperate fern species globally may be particularly susceptible to habitat stress with consequent decline in species richness and density (Sharpe, 2019; Testo and Watkins, 2013). Additional research evidence is needed to evaluate the effects of extreme climatic conditions on temperate species of ferns that are likely to incur deleterious growth effects or die back, especially if conservation efforts are to be successful in ensuring the survival of possibly sensitive species across broad geographic ranges (Amberber et al., 2014; Anderson, 2021; Ibars and Estrelles, 2012; Mehltreter, 2010; Ramírez-Barahona et al., 2011).
Although O. sensibilis is widely grown as an ornamental fern and is distributed broadly geographically in the natural environment; it appears there are relatively few research studies on its response to adverse environmental events. Khrapko and Tsarenko (2015) studied the adaptive strategies of O. sensibilis and Matteucia struthiopteris (both members of the Onocleaceae family) located in the south of the Russian Far East, and reported that the adaptive strategies of the two species vary depending on the ecological and coenotic conditions, but these variations were more significant in M. strutiopteris than in O. sensibilis. They also noted that under favorable conditions, O. sensibilis is a codominant and constitutes the herbaceous layer with other herb species. However, in locations with unfavorable soil moisture and light conditions, the density of the fern is much lower, and the plants are largely non-fertile. The competitive capacity of O. sensibilis was also noted by Cousens et al. (1985) who studied ferns growing on marsh hummocks in Florida (USA) and reported that biomass of O. sensibilis growing alone was much greater than when it was growing competitively with a co-inhabiting fern, Lorinseria areolata.
A comparative study of the physiological and morphological properties of deciduous and wintergreen ferns growing in southeastern Pennsylvania (USA), including O. sensibilis, Dryopteris intermedia, Polystichum acrostichoides and Polypodium virginianum, was published by Reudnik et al. (2005). Among other relevant ecophysiological variables, they reported that O. sensibilis had the highest total chlorophyll (2000 µg ml-1) and largest chlorophyll a:b ratio (ca. 3.0). They also assessed leaf chlorophyll fluorescence for the ratio of variable fluorescence to maximum fluorescence (Fv/Fm). The value for O. sensibilis (0.76) was intermediate to that of the other three species that varied between ca. 0.71 and 0.78. They noted that O. sensibilis is most typically found in the sun, but was also occasionally found under forest canopy. The capacity for O. sensibilis to occur in sun-laden environments as well as forest floor habitats suggests it is potentially adaptable to widely different ecological and coenotic conditions.
The sampling site for this study was a fern patch growing at the top of a north-facing moderate slope, surrounded by broad leaf trees on the east and west margins, located on Torrey Cliff, Palisades, New York (part of the Lamont-Doherty Earth Observatory campus of Columbia University). As explained more fully previously, this patch of mixed fern species included scattered individuals of O. sensibilis situated among other fern species at the top of this north facing slope with minimal surrounding elevated topography that could serve as a watershed source. Thus, this locale was particularly affected by the decreased precipitation that accrued during summer 2022. Consequently, it was a suitable location to study the possible effects of extreme summer heat and drought on the physiological status of O. sensibilis, a species that may be a good indicator species for climate change effects on survival of fern species with adaptive capacity to varied environmental locales.
The objectives of this study were as follows:
(1) To assess the photophysiology (photosynthesis rate, chlorophyll fluorescence indicators of photosystem II effectiveness) and dark respiration of O. sensibilis leaves in late spring (June, 2022) as a potential baseline for comparisons to mid-summer climate effects.
(2) To reassess these variables in mid to late July 2022 to determine what effects on O. sensibilis leaves, if any, could be attributed to the extreme climatic conditions.
(3) To document the rate of photosynthesis of O. sensibilis leaves under varying light intensities, and to determine the photosynthetic compensation point during mid to late July, as evidence of primary productive potential while under climatic stress.
MATERIALS AND METHODS
Study site
The study site was located at the northern edge of the Lamont-Doherty Earth Observatory campus on Torrey Cliff, Palisades New York (41° 00’ 17.19” N, 73° 54’ 24.71” W; elevation 113 m). The fern patch containing O. sensibilis was located at the top of a north-facing slope surrounded by broad-leaf trees on the east and west sides, and shaded by a two-story building façade on the south side. Consequently, it received largely diffuse sunlight most of the day, with occasional dappled sunlight filtering through the tree canopy to the east during early morning hours. At mid-day under cloudless, open sky, the photosynthetic active solar radiation (PAR) was ca. 100 µmol m-2 s-1 (LiCor solar monitor Li-1776; LiCor Biosciences, Lincoln, Nebraska). In addition to scattered stands of O. sensibilis (Figure 1), other ferns at the site included Athyrium species, Dryopteris marginalis (wood fern), Cystopteris fragilis and also sparsely occurring grass.

Sample collection of O. sensibilis and laboratory analysis
Sample collection
Representative leaf samples of O. sensibilis were collected in the morning on each sampling date, enclosed in zip-lock bags with a small quantity of deionized water, and immediately taken to the nearby laboratory for analysis. Three leaf samples were collected in late spring 2022 (June 18th) to obtain some baseline data before the onset of mid-summer. Six leaf samples were taken in mid-summer 2022 (July 24 - 28th ) to obtain a more representative sample of the status of O. sensibilis after exposure to a week-long heat wave that enveloped much of the north eastern, U.S.A., including Rockland County, New York where the Lamont-campus is located on Torrey Cliff. Temperatures varied from 32.2 to 35.6°C during the extreme temperatures, and there was minimal precipitation in the preceding months, incurring a deficit of precipitation that was 50 to 75% of the norm.
Photosynthesis and respiration measurements
The photosynthesis rate of the O. sensibilis leaves was assessed using an infra-red gas analyzer (IRGA) system, with an optically clear, 163 cm3 cuvette (Vernier, Beaverton Oregon), and illuminated with a Light Emitting Diode (LED) light source at 100 µmol m-2 s-1 PAR (LiCor Biosciences, Lincoln, Nebraska), equivalent to the ambient PAR during mid-day at the sampling site, and at a temperature of 25°C. In addition, photosynthesis rates were determined at four light intensities (20, 40, 80 and 100 µmol m-2 s-1 PAR) for the six samples collected in July to obtain evidence of potential photosynthesis rates under varying PAR for O. sensibilis plants during the summer climate stress. The data for each PAR was expressed as the mean assimilation rate ± Standard Error of the Mean (SEM). The mean photosynthesis rate as a function of the four PAR levels is reported in the text and plotted as a graph to display the gain in photosynthesis rate with increasing PAR. Based on these data, linear regression analysis (GraphPad Software, San Diego, California) was used to derive an equation representing the predicted rate of CO2 assimilation during net photosynthesis as a function of varying light intensity (PAR). A Kolmogorov-Smirnov test confirmed that the data were sufficiently normally distributed to apply the parametric linear regression analysis. Furthermore, the compensation point (where CO2 gain by photosynthesis is balanced by respiratory loss) was determined by assessing the PAR intensity where the net photosynthesis rate approached zero.
Dark respiration was measured by enclosing the IRGA cuvette containing the leaf sample in an opaque enclosure to determine the rate of CO2 production in complete darkness at 25°C. The leaf sample was maintained in the dark condition until the reaction centers of the photosystems of the leaves came to equilibrium with the darkened state, and measurements were begun when there was a steady state respiration rate.
Each leaf was imaged digitally and the total area was computed based on pixel count analysis with reference to a standard, 4 cm2 opaque square. The mean photosynthesis and respiration rates ± SEM were expressed per leaf area as µmol CO2 m-2 s-1 based on the digital estimated area of each leaf preparation.
Leaf stomatal density estimates
Mean stomatal density, on the abaxial surface of the O. sensibilis leaves sampled in July, was made by using a leaf peel method (acetate varnish was applied to the abaxial surface, and a replica of the leaf epidermis was obtained by gently peeling away the thin layer of hardened varnish). A wet mount slide preparation using deionized water was made, and the acetate peel was examined at a magnification of 400X using a Nikon phase contrast compound light microscope (Nikon Instruments, Melville, NY). Twenty optical fields were viewed, and the number of stomata per field was tabulated, then the counts were converted to equivalent number per cm2 based on the diameter of the objective visual field. Mean number of stomata per cm2 was determined based on the composite counts in the twenty observations.
Assessment of Fv/Fm and ET0/RC
An OS-30p+ Chlorophyll Fluorometer (Opti-Sciences, Inc., Hudson, New Hampshire) was used to obtain the quantum yield efficiency for variable fluorescence/maximum fluorescence (Fv/Fm) and electron transport (ET0) per reaction center (RC) (ET0/RC) based on the JIP test application in the OS-30p+ instrument. Leaf samples were dark adapted for 20 to 30 min before the measurements were made to ensure that the reaction centers (RC) had come to equilibrium with the darkened state. Measurements were made in triplicate for each leaf sample taken in June and in July. Thus, for the nine samples of leaves (three in June and six in July), 27 leaf fluorescence measurements were made. The results were expressed as the mean ± SEM for the Fv/Fm and ET0/RC measurements.
RESULTS
Results for photosynthesis rate, dark respiration, and leaf fluorescence data (Fv/Fm and ET0/RC) are presented in Table 1.

The mean rates of photosynthesis and respiration in June were higher than for leaves collected in July after the month-long deficits in precipitation and several days of excessive heat. However, the leaf fluorescence results show that the photosystem two (PSII) quantum efficiency estimates (Fv/Fm) are nearly equivalent for the June and July samples, at ca. 0.75. This is a reasonably strong Fv/Fm value, indicating that the quantum efficiency of the leaves is not so severely affected as was the net photosynthesis rate in the leaf samples collected subsequent to the excessive heat. The electron transfer estimates from PSII to the quinone intermediate (QA) and beyond in the electron transfer chain (TR0/RC), are substantial for both the June and July leaf samples. Given the appreciable values of the leaf fluorescence results, these data suggest that O. sensibilis is down regulating net photosynthesis through response mechanisms further along the carbon fixation pathway beyond the light photon reaction centers, perhaps morphologically through stomatal down regulation of gas and water vapor exchange with the atmosphere, as discussed more fully earlier. To further augment the leaf physiology data, the mean stomatal density for the abaxial surface of the leaves of O. sensibilis collected in July was determined to be 2164 stomata per cm2.
The results of the analysis of mean photosynthesis rate related to varying intensities of PAR (20 to 100 µmol m-2 s-1) are as shown in Figure 2.
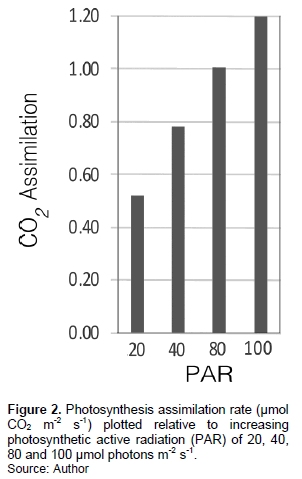
The mean net photosynthesis rate ± SEM expressed as µmol CO2 m-2 s-1 per variation in light intensity (20 to 100 µmol photons m-2 s-1) is as follows: 20 (0.52 ± 0.07), 40 (0.78 ± 0.09), 80 (1.00 ± 0.10) and 100 (1.20 ± 0.08). A linear regression estimate (p << 0.01) of the net photosynthesis CO2 assimilation rate (A) relative to the varying levels of PAR light intensity (I) yielded Equation 1:
A = 0.00788* I +0.4025 (1)
Where, A is expressed as µmol CO2 assimilated m-2 s-1 and I is in units of µmol photons m-2 s-1. This is applicable only within the limits of the variation in PAR used in this analysis (20 to 100 µmol m-2 s-1), which spans the range in PAR found at the sampling site as explained earlier. The photosynthetic compensation point was estimated to be at a PAR of ca. 1 to 2 µmol m-2 s-1.
DISCUSSION
As a largely temperate fern, widely distributed in the northern hemisphere and of considerable horticultural interest, O. Sensibilis is worthy of much more research attention; especially its role in the understory of forests and its growth in open locations including wetlands, marsh hummocks, and sunny margins of wooded areas (Cousins et al., 1985; Khrapko and Tsarenko, 2015; Reudnik et al., 2005). It has a rich biogeographic and geological history, with a long and abundant fossil record that includes most of the major forested regions of the northern hemisphere. This includes substantial evidence throughout the Paleocene in North America (Rothwell and Stacey, 1991). Its history spans late Cretaceous and Tertiary in some regions of North America, and the Eocene in northwest Europe and Japan. Its modern, less widely distributed location (largely absent in western and mid European regions), is probably due to extinction of populations in these intervening geographic locales due to climate change, resulting in its present disjunctive global distribution across north America, eastern Europe and parts of Asia (Barrington, 1993).
With increasing major changes in climate in the northern hemisphere, and globally, additional research is needed to clarify how increasing annual temperatures, and less predictable (but likely more severe) precipitation events may affect plant communities, including those of ferns and other herbaceous plants that have become adapted to more amenable annual climate regimes. Although some ferns have diversified and become adapted to harsh environments including desert and drylands (e.g., cheilanthoid ferns, and others), many fern species have evolutionary, long-established niches in the understory of forests or relatively moist, moderate temperate or tropical environments (Anderson, 2021; Sessa, 2018; Watkins and Cardelús, 2012).
Given evidence that O. sensibilis may be sufficiently adaptable to survive in varying habitat regimes (Reudnik et al., 2005), more research on its susceptibility to, or resilience against, major changing climate patterns may be worthwhile. The results of the current study spanning late spring and into mid-summer, when the northeastern USA incurred major heat waves and limited precipitation, indicate that the sample of leaves from O. sensibilis had a lower net photosynthetic rate following a major heat wave in mid-July than in late spring (June, 2022) of that year. The mean net photosynthesis rate in July was ca. 75% of the rate in June, and there was sufficient difference between the two means that there was no overlap of the ± SEM values.
However, the July mean rate of 1.20 µmol CO2 m-2 s-1 at 100 µmol m-2 s-1 PAR is still appreciable, although the June rate (ca. 1.6 µmol CO2 m-2 s-1) is closer to the value reported for other ferns, such as the widely distributed fern Asplenium platyneuron. Based on graphical data of Anderson and Griffin (2021), the estimated net photosynthesis rate for A. platyneuron growing in a partially shaded location was ca. 1.6 µmol CO2 m-2 s-1 at PAR of 100 µmol m-2 s-1. It was higher for A. platyneuron plants exposed to brighter sunlight (2.2 µmol CO2 m-2 s-1 at PAR of 100 µmol m-2 s-1). In addition to the immediate or direct effects of climate stress on plant physiology, the lack of appreciable new leaf growth may have skewed the existing foliage of O. sensibilis toward a more mature or somewhat senescent state. This situation could also contribute to differences in the photophysiology and respiration of the samples collected in July compared to June. However, overall, the leaf samples were in good condition morphologically and rather typically chlorophyllous as shown in Figure 1.
With a compensation point for O. sensibilis of 1 to 2 µmol m-2 s-1 PAR, and evidence of appreciable net photosynthesis rates with variations in PAR from 20 to 100 µmol m-2 s-1 (Figure 2), there is further evidence that O. sensibilis appears to be adaptable to habitats that are widely different in light intensities. Moreover, the substantial values of PSII quantum efficiency (Fv/Fm = 0.75) during the late spring season, and also after the summer climate extremes, suggest that O. sensibilis has strong potential to rebound from some adverse climate effects. The value of Fv/Fm = 0.75 reported here is substantially similar to the value of 0.76 published by Reudnik et al. (2005) for O. sensibilis growing in Pennsylvania (USA).
It is not possible, presently, to determine the precise physiological response that accounts for the net photosynthesis decline in July. However, given the decrease in respiration as well as net photosynthesis rates during July compared to June, there may be increased stomatal closure during the climatic stressful events; thus, decreasing transpiration loss, and also lowering gas exchange with the atmosphere, thereby limiting the amount of photosynthetic CO2 assimilation as well as decreasing CO2 loss during respiration.
Further research on the physiological status of the leaf gas exchange and more generally on hydraulic status of water use by O. sensibilis during extreme climate events is warranted. It is worth noting that the stomatal densities on the abaxial leaf surface of O. sensibilis (ca. 2000 cm-2) is somewhat less than reported for other naturally occurring ferns where values are closer to 3000 to 7000 cm-2 (Anderson and Griffin, 2021; Ludlow and Wolf, 1975).
Stomatal density in combination with dynamic control of stomatal gas conductance (gs), through regulation of stomatal opening, may contribute to variations in gas exchange rates with the atmosphere; and hence, affect net photosynthesis rates. There is a strong limiting role of gs on photosynthesis, as confirmed in numerous prior publications–a topic reviewed more generally by Xiong and Flexas (2020). Hence, stomatal density and regulation of stomatal conductance (gs) are likely factors in the varied rates of photosynthesis in O. sensibilis and warrant more intensive investigation. Further research on the physiological status of the leaf gas exchange, and more generally on the hydraulic status of water use, including water use efficiency (WUE), during extreme climate events for this fern species is likely to be productive in better understanding the resilience and potential adaptive capacity of O. sensibilis during increasing challenges of climate change.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Amberber M, Argaw M, Asfaw Z (2014). The role of homegardens for insitu conservation of plant biodiversity in Holeta Town, Oromia National Regional State, Ethiopia. International Journal of Biodiversity and Conservation 6(1):8-16. |
|
|
Anderson OR (2021). Physiological ecology of ferns: Biodiversity and conservation perspectives. International Journal of Biodiversity and Conservation 13(2):49-63. Available at: |
|
|
Anderson OR, Griffin KG (2021). Preliminary ecophysiological observations of the fern Asplenium platyneuron growing in two microenvironments varying in light intensity at an urban location in New York City. Journal of the Torrey Botanical Society 148(4):308-317. |
|
|
Arcand NN, Ranker TA (2013). Conservation Biology. In. Ranker TA, Haufler CH (eds.), Biology and Evolution of Ferns and Lycophytes. Cambridge: Cambridge University Press. pp. 257-283. |
|
|
Barrington DS (1993). Ecological and historical factors in fern biogeography. Journal of Biogeography 20(3):275-279. |
|
|
Cousens MI, Lacey DG, Kelly EM (1985). Life-history studies of ferns: a consideration of perspective. Proceedings of the Royal Society of Edinburgh 86B:371-380. |
|
|
Ibars AM, Estrelles E (2012). Recent developments in ex situ and in situ conservation of ferns. Fern Gazette 19(3):67-86. Available at: |
|
|
Khrapko OV, Tsarenko NA (2015). Adaptive strategies of two species from the Family Onocleaceae. Contemporary Problems of Ecology 8(2):148-154. |
|
|
Ludlow CJ, Wolf FT (1975). Photosynthesis and respiration rates of ferns. American Fern Journal 65:43-48. |
|
|
Mehltreter K (2010). Fern conservation. In Mehltreter K, Walker LR, Sharpe JM (eds.), Fern Ecology. Cambridge: Cambridge University Press. pp. 323-359. |
|
|
Moran RC (2004). A Natural History of Ferns (302 p). Portland, OR, USA: Timber Press. |
|
|
Nowicki L, Kowalska A (2018). Ferns: Ecology, Importance to Humans and Threats. New York: Nova Scientific Publishers. 178p. Available at: |
|
|
Ramírez-Barahona S, Luna-Vega I, Tejero-Díez D (2011). Species richness, endemism, and conservation of American tree ferns (Cyatheales). Biodiversity and Conservation 20:59-72. |
|
|
Reudnik MW, Snyder JP, Xu B, Cunkelman A, Balsamo RA (2005). A comparison of physiological and morphological properties of deciduous and wintergreen ferns in southeastern Pennsylvania. American Fern Journal 95(2):45-56. |
|
|
Rothwell GW, Stockey RA (1991). Onoclea sensibilis in the Paleocene of North America, a dramatic example of structural and ecological stasis. Review of Palaeobotany and Palynology 70(1-2):113-124. |
|
|
Sessa EB (2018). Evolution and classification of ferns and lycophytes. In. Ferna?ndez H (ed.), Current Advances in Fern Research. New York, USA: Springer International Publishing AG. pp. 179-200. |
|
|
Sharpe JM (2019). Fern Ecology and Climate Change. Indian Fern Journal 36:179-199. Available at: |
|
|
Sharpe JM, Mehltreter K, Walker LR (2010). Ecological Importance of Ferns. In. Mehltreter K, Walker LR, Sharpe JM (eds.), Fern Ecology. Cambridge: Cambridge University Press. pp. 1-21. |
|
|
Singh AP, Johari D (2018). Scope of ferns in horticulture and economic development. In. Ferna?ndez H (ed.), Current Advances in Fern Research (pp. 153-175). New York, USA: Springer International Publishing AG. |
|
|
Testo WL, Watkins JE Jr. (2013). Understanding mechanisms of rarity in pteridophytes: Competition and climate change threaten the rare fern Asplenium scolopendrium var. americanum (Aspleniaceae). American Journal of Botany 100(11):2261-2270. |
|
|
Turkmen M (2022). Emerging Challenges in Environment and Earth Science Vol. 3 (67 pp.). West Bengal, India: Book Publisher International. |
|
|
Watkins JE Jr., Cardelu?s CL (2012). Ferns in an angiosperm world: Cretaceous radiation into the epiphytic niche and diversification on the forest floor. International Journal of Plant Science 173(6):695-710. |
|
|
Xiong D, Flexas J (2020). From one side to two sides: the effects of stomatal distribution on photosynthesis. New Phytologist 228:1754-1766. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0