Full Length Research Paper
ABSTRACT
Habitat type and their vegetation composition play important role in determining the abundance and diversity of animals including small mammals, hence any change in habitat type will influence their abundance and spatial distribution. This study aimed at investigating the influence of habitat types (that is, Wetland grassland, Miombo and Vachellia woodlands) on small mammal abundance, diversity, and richness in the Usangu area, in southern part of Ruaha National Park, in Tanzania. Sherman and pitfall plastic bucket traps were employed both for trapping small mammals. A total of 92 small mammals were captured in 2124 trap nights giving 13% trap success representing two families, namely, Muridae (6 species) and Soricidae (1 species). Furthermore, trap success differed among habitat types with the highest being in wetland grassland. Species diversity was higher in Vachellia woodlands compared to other habitat types. It would therefore seem that wetland grassland and Vachellia woodland habitats are very crucial in maintaining small mammal abundance and diversity in Usangu Area. Therefore, future management plan should incorporate these refuge habitats for continued existence of small mammals in Usangu. Further study is warranted in wet season in order to have comparison information that will assist in management of small mammal in Usangu.
Key words: Conservation, habitat preferences, habitat restoration, Miombo woodlands, rodents, Ruaha National Park, Tanzania, Vachellia woodlands, wetland grassland.
INTRODUCTION
Small mammals form an important component in all most every terrestrial ecosystems despite their low status among wildlife enthusiast, especially when compared with charismatic mega-fauna and the abundant avifauna found in the tropical regions (Gbogbo et al., 2017). It is well documented that they are fundamental component of the food chain in almost every ecosystem as they feed on various foodstuff including plants, lichens, fungi, invertebrates and in turn they are also preyed by large array of mammals, avian predators and snakes (Byrom et al., 2014; Ecke et al., 2002; Kiwia, 2009). Based on this important facts, small mammals presence as well as changes in their diversity and abundance is used to influence to a great extent the dynamics of these organisms as well as their future existence (Angerbjörn et al., 1999; Ecke et al., 2001). Previous studies have shown that small mammals can also act as keystone species (Delibes-Mateos et al., 2011; Kelt, 2011)as they facilitate carbon cycle and energy flow and influence soil fertility (Mbugua, 2004; Michael et al., 2016)as well as affect the structure and composition of habitats through the consumption and dissemination of plants (including seeds and fruits), lichen, and fungal spores (Carey and Johnson, 1995; Angelici and Luiselli, 2005).
The abundance and population dynamics of small mammals in heterogeneous landscape are most likely influenced by various factors (Batzli, 1992; Stenseth et al., 2002). Such factors are the distribution and abundance of habitat resources which may influence their distribution pattern (Hieronimo et al., 2014). In addition, the most critical factors such as food availability and shelter have been found to influence small mammals population dynamics and abundance (Hansson, 1997), and they are of great importance for their reproduction and survival (Batzli, 1983). On the other hand, land use/land cover types (Fraschina et al., 2014)and inter-specific competition for crucial resources have also been shown to be of great importance in explaining the distribution of small mammals species in different habitats (Morris, 1995; Johannesen and Mauritzen, 1999).
The principal factor for small mammals composition and abundance within their geographic range is habitat conditions (Geier and Best, 1980). The connection of these species to a particular habitat is very steady and their conservatism in the selection of habitats is believed to be amongst their ecological adaptations (Peterson et al., 1999; Yakimova and Gaidysh, 2021). Small mammals are good indicator of habitat condition and environmental health and they are among the first to respond to any habitat alterations (Bock et al., 1984; Magige, 2013). Despite the nature and extent of disturbance, if vegetation is changed and habitat is altered the composition and abundance of some species may benefit while others may be affected negatively.
Little is known about the small mammal’s community composition, abundance and diversity of Usangu area in Ruaha National Park. The Usangu area was previously a game reserve that was fragmented and degraded by uncontrolled human activities (WWF and WCS, 2003). In 2008, the area was gazetted to be part of Ruaha National Park resulting into prohibited anthropogenic activities. Following the exclusion of human activities in the Usangu area, natural vegetation has been recovered since then and wetlands grassland has also been re-established (Kihwele et al., 2012). However, the information on the distribution and abundance of small mammals of this area with contrasts in landforms and habitats types is not well understood. The most recent publicity available of the area was conducted over seven years ago documenting their presence and absence in four study sites, however the results of the study so far do not allow the derivation of small mammals-habitat type relationship (Stanley et al., 2015). This study therefore attempts to investigate the spatial variation of small mammal abundance, composition, diversity and species richness in the three main habitat types (wetland grassland, Miombo and Vachellia woodlands), that are found within the Usangu area in Ruaha National park and providing a baseline for future work. The hypothesis to be tested is that the small mammals are randomly distributed in Usangu area, irrespective of type of the habitat.
MATERIALS AND METHODS
Study area
The study was conducted in Usangu area located on 08° 30′ South and 34° 15′ East in southern part of Ruaha National Park (Figure 1). It orientates slightly SW to NE from 33° 05′ East to 34° 50′ East. In west, the park is bordered with volcanic hills of Mbeya, to the SW the volcanic heights of the Poroto Mountains and to the south the ancient crystalline mountains of the Kipengere and Poroto ranges. Formerly Usangu area was a game reserve; in 2008, the Usangu Game Reserve and other adjacent important and remarkable wetlands were annexed into Ruaha National Park, making it the largest National Park in Tanzania and East Africa with an area of about 20,226 km2 (7,809 m2) (Sirima, 2010; Tanzaniatourism, 2021). All sampling sites were selected in the Usangu area within Ruaha National Park. The climate of the park is characterized by tropical semi-arid with a pronounced dry season from May to November every year. The average annual rainfall is about 650 mm increasing towards the West with increasing altitude. Mean annual temperature is 24°C with three hottest months from October to December (39-40°C), while June through August is the three coolest months (21.7°C).
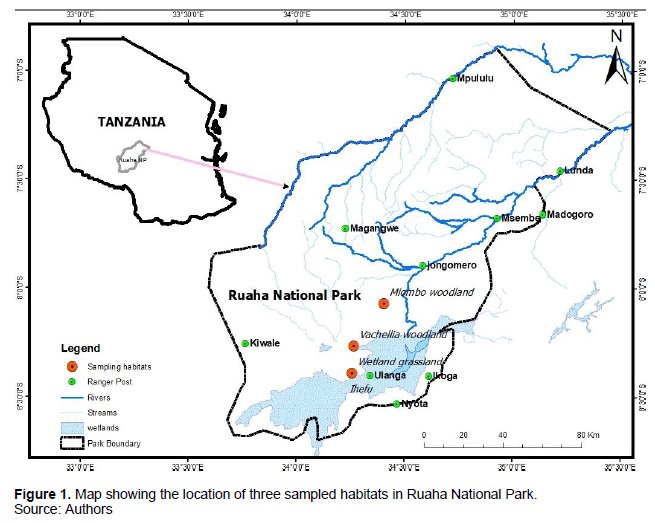
Habitat type selection
The study consisted of three main habitat types in Usangu area: Miombo woodland, Vachellia woodland and wetland grassland. Each habitat type was selected based on their percentage coverage, whereby each habitat selected consisted of more than 80% cover of one of the selected species in each habitat (Figure 1). The Miombo woodland harbored a variety of trees species including Brachystegia spiciformis, Julbernadia globiflora, Pterocarpus angolensis, Combretum psidioides, Cassipourea mollis, Gardenia ternifolia, Catunaregam taylorii and Phyllanthus inflata. Also, the ground floor was devoid of grass cover including Themeda triandra, Hyparrhenia species and Andropogon species. Vachellia woodland was dominated by trees such as the Vachellia tortilis, but other selected areas were mixed with Vachellia dreponolobium, Senegalia mellifera, Vachellia kirkii and Vachellia nigrescens and with Commiphora species. Common forbs include Solanum, Leonotis, Vernonia, Hygrophyla, and Sesamum. The grass layer was dominated by annuals including Aristida, Dactyloctenium, Urochloa and Dichanthium species. Finally, the wetland grassland habitat was dominated mostly by Vachellia seyal and Vachellia dreponolobium. Herbs include Ormocarpum trichocarpum, ygrophilla auriculata and Blepharis affinis. Dominant grasses were Andropogon mannii, Andropogon gayanus, and Exotheca abyssinica, Setaria incrassata and Themeda triandra. Hygrophilla auriculata was also present and was an indicator of constant wetness.
Small mammals trapping
Small mammals were trapped in the three habitats from 15 to 20th October 2018, during the dry season. A total of nine separate trapping points each covering a total area of approximately 4900 m2 were established in the Usangu area in the three habitats. In each selected habitat, a total of three trapping points were established and spaced at a distance of 500 to 600 m from each point. 49 commercially available Sherman’s live traps made of Aluminium, (230 mm × 95 mm × 80 mm, H. B. Sherman Traps, Inc., Tallahassee, Florida, USA) were placed per trapping point in 7 lines, each with 7 traps spaced at 10 m apart. The Sherman’s traps were baited following every check with a mixture of lightly fried fresh coconut, peanut butter and mixed with sardines and millet. Pitfall plastic buckets with a volume of 10 L in lines were buried in the soil such that the rim was at the level with the ground (Timbuka and Kabigumila, 2009). Each pitfall line contained ten buckets spaced at intervals of 10 m apart. All buckets had tiny drainage holes at the bottom to allow rain water to drain away (Sangiwa and Magige, 2019). A polythene drift fence was placed to intercept and redirect animals moving on the ground into pitfall traps in each trapping points (Bury and Corn, 1987). Global Positioning System (GPS) was used to record the location and altitudes of all sampling sites. Traps and pitfall lines were checked twice daily for four consecutive days, immediately after sunrise and in the evening. The live captured animals were identified, weighed and marked using permanent marker pen on their first capture before being released into the field.
Data analysis
Small mammal abundance
Small mammals in this study refer to rodents and shrew. The percent occurrence (R) of small mammals was calculated using Equation 1:

Small mammal relative abundance (R.A) was expressed as percentage trapping success which is the proportion of captures relative to the number of traps set over a given period (Odhiambo et al., 2005). Trap success usually expressed as the number of individual of particular species per 100 trap-nights or bucket nights (that is, the proportion of catches relative to the number of traps set over a given period), was used to determine the relative abundance of the caught species( Stanley et al., 1996). Trap success (TS) was calculated using the Equation 2:
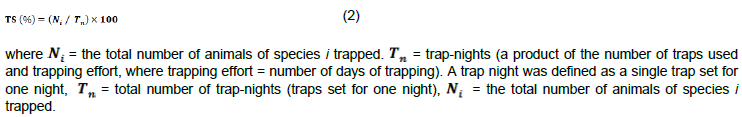
Community similarities
Similarities in pairs of small mammals’ communities were determined with Jaccard Index given in Equation 3:

where SJ is the similarity index, c is the number of shared species between the two sites and a and b are the number of species unique to each site.
Small mammals’ diversity
Diversity indices for the rodents were calculated using Shannon-Weiner diversity indices (Shannon, 1948)by using Equation 4:

where H? is the diversity index and pi represent the proportion of species i in the total number of animals captured.
All data were tested for normality using tests for kurtosis and skewness. Because all datasets were not normally distributed, the relative abundance and diversity index values of the small mammal species across the three different habitats were compared using Kruskal-Wallis a non-parametric test. All statistical analyses were carried out using R software version 3.6.3.
RESULTS
Small mammal’s species composition
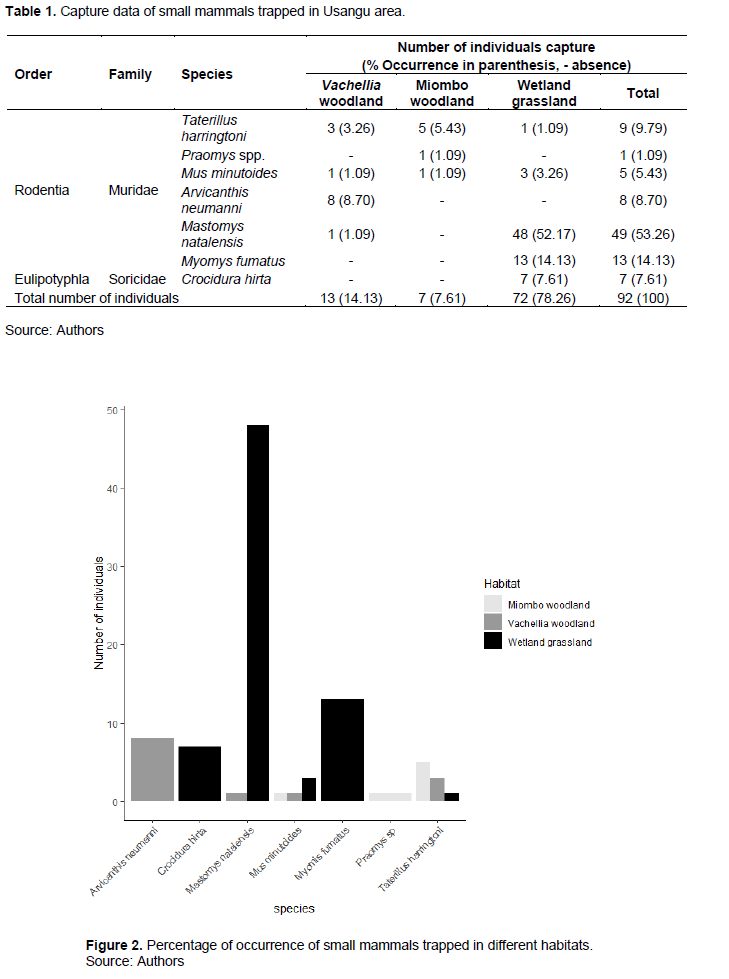
Ninety-two (92) individuals of small mammals belonging to 7 species, 2 families and two orders (Rodentia and Eulipotyphla) were captured from 2,124 sample-nights (1,764 Sherman trap-nights and 360 bucket-nights). The Rodentia which constituted 92.39% of the total number of captures, included Arvicanthis neumanni (Neumann's grass rat) (8.70%), Mastomys natalensis (multimammate rats) (53.26%), Mus minutoides (The African pygmy mouse) (5.43%), Myomysfumatus (14.13%), Praomys species (1.09%) and Taterillus harringtoni (Harrington's tateril) (9.79%). The Eulipotyphla consisted of 7.61% of the total number of captures with all individuals being Crocidura hirta (Lesser red musk shrew) shown in Table 1 and Figure 2. The wetland grassland habitat accommodated a total number of 5 species, whereby four species (that is, Taterillus harringtoni, Mus minutoides, Mastomys natalensis and Myomys fumatus) belonged to Muridae family while only one species, that is, C. hirta belonged to Soricidae family. On the other hand, Vachellia woodland habitat comprised of 4 species all from Muridae family while Miombo woodland habitat contained only 3 species all belonging to Muridae family (Figure 1 and Table 1). The number of species did not differ significantly between the three habitats (H (2) = 2.4363, P=0.2958).
Abundance and distribution of species of small mammals in the three habitats of Usangu
The present study found that, wetland grassland habitat had the highest trap success (10.169%) followed by Vachellia woodland habitat (1.836%), while Miombo woodland habitat had the lowest trap success rate (0.989%) (Table 2). M. natalensis was the most abundant species accounting about 6.921% of the total captures. Other abundant species was M. fumatus whereas Praomys species was the least common species, accounting for only 0.141% of the total captures (Table 2). A. neumanni had the highest trap success in Vachellia woodland (1.130%) while in Miombo woodland habitat T. harringtoni was the most abundant species (0.706%) and in Wetland habitat M. natalensis was the most dominant species (6.780%) (Table 2). Two species M. fumatus (1.836%) and C. hirta (0.989%) were trapped only in wetland grassland habitat while A. neumanni (1.130%) and Praomys spp. (0.141%) were trapped only in Vachellia and Miombo woodlands habitats, respectively. M. natalensis was trapped in two habitats, that is, Vachellia woodland (0.141%) and wetland (6.780%) while M. minutoides and T. harringtoni were the most widely dispersed species, trapped in all three habitats (Table 2).
Species diversity
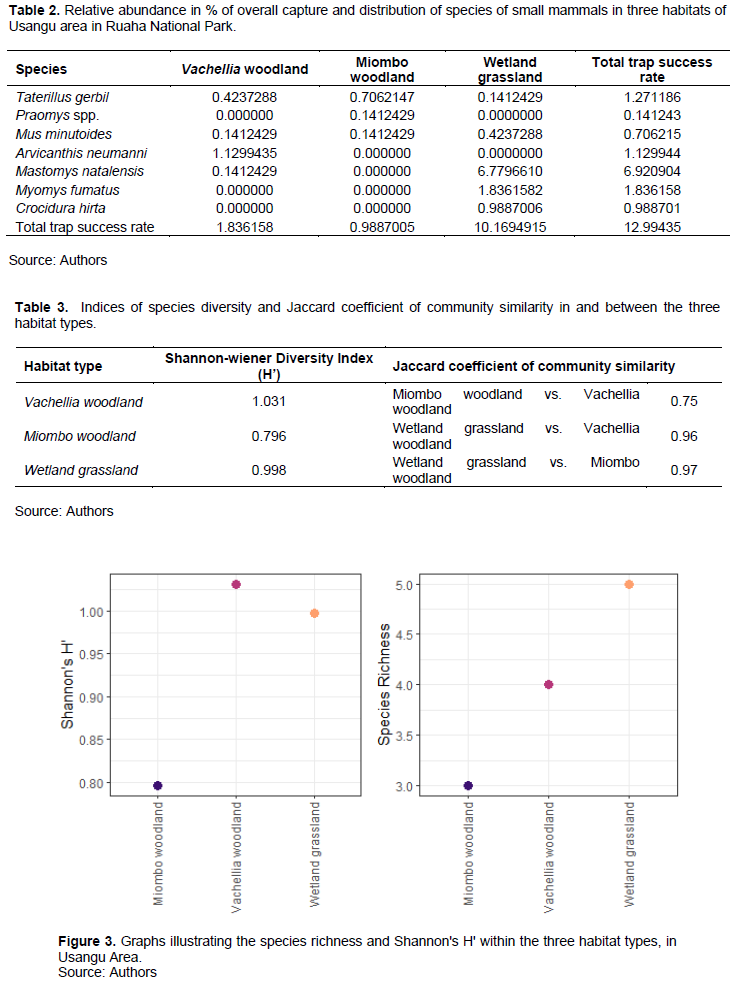
The highest Shannon-Weaver Index (H′) of 1.03 was recorded in Vachellia woodland habitat while Miombo woodland had the lowest H′ (0.796) (Table 3 and Figure 3). The three diversities were not significant different from each other (H (2) = 2, P = 0.3679).
Coefficient of community similarities
The 4 species encountered in the Vachellia woodland against 3 species in the Miombo woodland and 5 species in Wetland resulted into highest Jaccard coefficient index value between Wetland and Miombo woodland habitats (0.97) and lowest similarity index between Miombo and Vachellia woodlands (0.76) (Table 3).
DISCUSSION
Small mammal’s species composition, trap success and richness
This study reveals spatial patterns of small mammal assemblages across the three main habitats in the Usangu Area, in Ruaha National Park (Figure 1). This study was considered to contribute to a database for small mammal communities in wetlands grassland and woodlands habitats of southern Tanzania. Although our research results reveal the common species of the small mammals across the three main habitat types in the Usangu area, in Ruaha National Park, the comparative studies that can be used to determine whether our findings typify these habitats are still lacking. The only comparable study is the one carried out by Stanley et al. (2015), who recorded a total of 20 small mammals (three species of shrew and 17 species of Rodentia) in Isunkaviola Plateau and Makindi Springs of the Ruaha National Park. In the study, fewer species were found at the Usangu area compared to what was found across the Ruaha National Park in Stanley et al. (2015). Interestingly, different small mammals’ community composition was not found compared to Stanley et al. (2015) findings at Ruaha National Park study sites. In addition, these disparities in species richness between the two studies might be related to sample design, survey length, timing, or study coverage.
The type of traps used in this study might have also influenced the lower abundance and richness of small mammals captured. Numerous studies have shown that, the use of combination of variety of trap types is the best means for examining general composition and structure of small mammal community (Voss and Emmons, 1996; Woodman et al., 1996; Astúa et al., 2006; Santos-Filho et al., 2006). Stanley et al. (2015)employed three types of traps (Museum Specials, Victor Rat Traps and Sherman Traps), and sampled a large number of small mammal species than the present study which used only two types of traps (Sherman Traps and Pitfall Plastic buckets). Similarly, Stanley et al. (1998)in Eastern Arc Mountains, Tanzania documented the small mammal individuals using Museum Specials, Victor Rat Traps and Sherman Traps and obtained a higher number of 28 species than that of present study. In addition, Magige (2016)in Serengeti ecosystem, Tanzania employed four types of traps (employed Sherman’s live traps, tomahawks, wire mesh trap and pitfall traps) to assess small mammal population across different habitat types and captured ten small mammal individual species which is higher than the present study. However, a number of studies have shown that a complimentary use of both live traps and pitfall is effective at documenting wide range of taxa of the small mammal species in many sites or habitats (Stanley et al., 1998; Lyra-Jorge and Pivello, 2001; Dizney et al., 2008; Caceres et al., 2011). Therefore, Sherman Traps and Pitfall Plastic buckets were used in this study in order to maximize the capture, as none of the two methods is enough to be used alone, and thus, both methods positively complemented each other as it has been indicated in other small mammal studies (Stanley et al., 1998; Nicolas and Colyn, 2006; Shilereyo et al., 2021). In addition, the effectiveness of Sherman Traps and Pitfall traps have also been widely tested, and the use of these methods in capturing a wide variety of small mammal with different taxa, sizes and weight has become somewhat acceptable (Pizzimenti, 1979; Dickman, 1995; Voss and Emmons, 1996; Goodman et al., 2001; Astúa et al., 2006; Santos-Filho et al., 2006; Umetsu et al., 2006). Furthermore, the complimentary use of Sherman Traps and Pitfall Plastic buckets together with different types of baits in this study is thought to be efficient in evaluating the small-mammal community in Usangu area. Numerous studies have emphasized the use of different types of baits as they increases the number of species and individuals that are captured (HiCe and VeLazCo, 1961; Woodman et al., 1996). In this study, a mixture of four different types of baits was placed in the Sherman’s traps. The use of combination of baits in this study was particularly important and is highly encouraged for small mammals study because different species get attracted to different types of baits (Timbuka and Kabigumila, 2009).
Generally, the present study findings found higher species richness in the wetland grassland habitat than in the Vachellia and Miombo woodlands habitats. Higher number of species might be due to dense vegetation ground cover which was available in wetland grassland habitat compared to other habitats, that could be responsible for providing good shelter for small mammals and accounts more species richness. Results from the present study were consistent with the multiple studies that have found higher species richness in wetland and grassland areas compared to other habitats. Bowland and Perrin (1993)found higher species richness and abundance in wetland habitats in Kamberg Nature Reserve, South Africa. In addition, Scott et al. (2008) recorded higher species richness in habitat with tall grasses compared to developing woodland habitats. Furthermore, Aubry et al. (1991)found both wetland and grassland habitats contained higher species richness in KwaZulu-Natal, South Africa. Small mammals prefer habitats with tall grasses because they provide them with enough food, vegetation cover and protection from predators and most of them tend to avoid open patches such as those found within woodland habitats as they provide less food and protection (Eccard et al., 2000; Tattersall et al., 2001; Scott et al., 2008). Conversely, the results did not agree with those of Magige (2013)and Mulungu et al. (2008)who found that woodlands habitat contained more species of rodents and shrew than grassland habitats.
The study findings indicated that the abundance of small mammals varied among the habitat type, with wetland grassland habitat was found to contain a great number of individuals compared to other habitats. According to Bowland and Perrin (1993), the higher abundance of small mammals in wetland grassland habitat might be due to the fact that wetland usually act as their reservoir during drought. Furthermore, wetland grassland habitat was clearly identifiable by higher plant cover than woodland habitats. Manson et al. (1999)and Hamilton et al. (2015)have noted that habitats with high plant cover are often selected by small mammals as a mechanism to reduce predation. Additionally, the Vachellia woodland contained higher diversity of small mammals compared to other habitats which is similar to previous findings (Magige, 2013; Byrom et al., 2014). The higher species diversity in Vachellia woodland habitat might be due to the availability of vegetation cover from predation and nesting sites (Nyirenda et al., 2020). From this study variation of species diversity was contributed to variations in vegetation physiognomy. Slightly diversity differences were found possibly due to differences of habitats in term of supporting ability for the survival of small mammal’s species. Despite of the normal situation that past anthropogenic disturbances lessen the diversity and abundance in the ecosystems; this study findings show that diversity was higher in the former disturbed areas as a result of cultivation and livestock keeping in Vachellia woodland than in Miombo woodland which was not used for agricultural activities and settlement. On the other hand, the Miombo woodland harbored the least number of small mammal diversity and abundance in the study area due to poor suitable habitats for small mammals (Caro, 2001, 2002; Nkwabi et al., 2018). This is also reflected by other investigators. For instance, Bayo (2019)reported lowest diversity and abundance of small mammals in the miombo woodland compared to other habitats in Handeni Hill Forest Reserve in Tanzania. The lower abundance and diversity in Miombo woodlands could possibly be due to frequent disturbances from fires (Bayo, 2019)as well as grazing from large herbivores as Miombo woodlands are known to provide the suitable habitat for large wild herbivores but in turn these species can cause significant impacts on vegetation which can indirectly affect small?mammal populations (Dewees et al., 2010; Ellis and Cushman, 2018). Furthermore, Miombo habitats are considered as a vegetation formation growing on soils that have low nutrient content, hence not productive and are mostly marked with low faunal biodiversity (Dewees et al., 2010). Various studies (Ecke et al., 2001; Lambert et al., 2006; Mengistu et al., 2015; Magige, 2016; Shilereyo et al., 2019)have also reported variation in small mammals abundance in response to variation in habitat types and composition since vegetation diversity and composition can influence the availability of food and shelter which remain key factors for small mammals’ survival and reproduction.
Small mammals chiefly Muridae were highly caught in Vachellia woodland and wetland than in Miombo woodland which seems not a favorable habitat type as previously reported (Magige, 2016). However, there was a higher similarity in the species between Wetland and Vachellia woodland, Wetland and Miombo woodland. The presence of similarities in species between these habitat types was probably contributed by the presence of good availability feeding resources, soil types and cover. Similarly, the wetland grassland had a slight heterogamous habitat including variety of grazing vegetation, fruits, seeds, arthropods, some shrubs to escape from predators and vast open land which facilitates easy detection of crawling predators in particular. On the other side, the Miombo habitat had good cover except for category of homogenous vegetation which could provide fewer grains and grazing varieties. The habitat heterogeneity hypothesis developed initially by Macarthur and Mac-Arthur (1961), proposes that an increase in number of different habitats can lead to an increase in species diversity and abundance which corroborates to the findings of this study. Furthermore, a study done in Serengeti kopjes revealed a high diversity of small mammals associated with the availability of food and cover in different habitats (Timbuka and Kabigumila, 2009). Another possible explanation of comparatively higher small mammals’ similarities in wetland and Vachellia woodlands could also be related to secondary succession of the former Usangu as recovery from anthropogenic disturbances. Secondary succession could have supplied a great variety of food materials which favor population growth of different species. The results on similarities of small mammals in between different habitats indicate the health and state of wetlands and Vachellia/Miombo woodlands had rapid turnover rate, high biotic potential, ability to invade reclaimed areas and sensitivity to environmental disturbance (Griffin et al., 2011). Therefore, the three sampled habitats support small mammal’s communities and represent area of considerable conservation importance.
Out of seven species recorded during this study, the M. natalensis was the only species that was significantly more abundant species as indicated by the multiple captures. Multiple captures are known to be used as an index of high density (Leirs et al., 1995; Timbuka and Kabigumila, 2009). M. natalensis was also the most dominant species in the wetland grassland habitat. High abundance of M. natalensis in Usangu area, particularly in wetland grassland may be due to their being an omnivorous species and generalists and the availability of other environmental resources, such as vegetation cover for protection from predators (Mulungu et al., 2011; Mamba et al., 2019; Nyirenda et al., 2020). Furthermore, M. natalensis has been recorded as the most adaptable in a wide range of habitats and environmental condition and most prevalent small mammals species in East Africa (Andresen, 1972; Byrom et al., 2015; Fichet-Calvet et al., 2008; Mulungu et al., 2011; Shilereyo et al., 2019; Timbuka and Kabigumila, 2009)as well as throughout sub-Saharan Africa (Granjon et al., 1997; Leirs et al., 1995).
M. fumatus was the second most abundant species in the study area. This species was captured in wetland grassland habitat but not in Vachellia and Miombo woodlands. This result agrees with the findings of Gezahegn et al. (2016)from Yetere Forest and Venance (2009)from Mikumi National Park. Several factors could be responsible for the higher abundance of M. fumatus in wetland grassland habitat. Food availability and cover may be higher and predator abundance might be lower in wetland grassland habitat compared to woodlands habitat (Bantihun and Bekele, 2015; Shilereyo et al., 2019; Nyirenda et al., 2020). However, this species has been recorded in different habitats, ranging from forests at 1000 m up to the Afro Alpine moorlands above 4000 masl, and is most widely distributed across African countries (Gezahegn et al., 2016).
Praomys spp. was the least abundant species of rodents recorded during the present study. This species was trapped only from the Miombo woodland habitat. This result goes in line with Bayo (2019), who found that Praomys spp. was confined only to the Miombo woodland habitat and avoided dense vegetation and moist areas. In addition, the presence of this species in Miombo woodland in Usangu area can be attributed to numerous factors, such as the availability of vegetation cover and array of food items across seasons (Nyirenda et al., 2020). Although Miombo woodland has been documented to contain relatively low fauna species, the presence of rodents could have contributed by the resource-rich termite mounds found in these habitats (Fleming and Loveridge, 2003). However,Meliyo et al. (2014)reported that this species was among the most abundant and dominant species of rodents in most of the study habitats, with more abundance in plateau and plain habitats. In addition, Isabirye-Basuta and Kasenene (1987)have found this species in both tropical evergreen forest and undisturbed mature forest habitats. Praomys spp. is also one of the most widely distributed and abundant rodents in Africa in the intertropical zone (Nicolas et al., 2005).
CONCLUSIONS
This study has demonstrated that biodiversity management should aim at incorporate refuge habitats such as wetland grassland and Vachellia woodlands within Ruaha National Park as they have been found to be not only of major importance for maintaining terrestrial small mammal abundance and diversity, but also very crucial for their population recovery. Miombo woodland habitat seems to be poor in small mammal diversity and abundance compared to wetland grassland and Vachellia woodlands habitats. Differences and similarities on small mammal species richness, diversity, and abundance in three habitats appear to be influenced by general effect on habitat conditions for the small mammals, such as the amount of vegetation cover and food as well as the recovery rate of the formerly Usangu area inhabited by human. The presence of few species recorded justifies the reasons for repeated sampling in different habitats and seasons is of paramount importance for reliable information and better comparison of spatial and temporal species abundance, richness and diversity in Usangu area.
ACKNOWLEDGEMENTS
The authors thank ASILIA LODGES AND CAMPS LTD for their financial support through Usangu Biodiversity Survey Project. They thank Tanzania National Parks (TANAPA), Tanzania Wildlife Research Institute (TAWIRI) and Tanzania Commission for Science and Technology (COSTECH) for permission to conduct the study.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Andresen HC (1972). Observations on the Life Histories and Behaviour of some Small Rodents from Tanzania. African Zoology 7(2):419-449. |
|
|
Angelici FM, Luiselli L (2005). Patterns of specific diversity and population size in small mammals from arboreal and ground-dwelling guilds of a forest area in southern Nigeria. Journal of Zoology 265(1):9-16. |
|
|
Angerbjörn A, Tannerfeldt M, Erlinge S (1999). Predator-prey relationships: Arctic foxes and lemmings. Journal of Animal Ecology 68(1):34-49. |
|
|
Astúa D, Moura RT, Grelle CEV, Fonseca MT (2006). Influence of baits, trap type and position for small mammal capture in a Brazilian lowland Atlantic Forest. Boletim do Museu Biológico Mello Leitão 19(1):31-44. |
|
|
Aubry KB, Crites MJ, West SD (1991). Regional patterns of small mammal abundance and community composition in Oregon and Washington. U S Forest Service General Technical Report Pnw (2857344):285-294. |
|
|
Bantihun G, Bekele A (2015). Population structure of small mammals with different seasons and habitats in Arditsy Forest , Awi Zone , Ethiopia. International Journal of Biodiversity and Conservation 7(8):378-387. |
|
|
Batzli GO (1983). Responses of Arctic Rodent Populations to Nutritional Factors. Oikos pp. 396-406. |
|
|
Batzli GO (1992). Dynamics of Small Mammal Populations: A Review. In: Wildlife 2001: Populations pp. 831-850. |
|
|
Bayo MJ (2019). Rodent diversity and habitat association in Handeni Hill Forest Reserve, North Eastern Tanzania (Doctoral dissertation, Sokoine University of Agriculture). |
|
|
Bock CE, Bock JH, Kenney WR, Hawthorne VM (1984). Responses of Birds, Rodents, and Vegetation to Livestock Exclosure in a Semidesert Grassland Site. Rangeland Ecology and Management/Journal of Range Management Archives 37(3):239-242. |
|
|
Bowland JM, Perrin MR (1993). Wetlands as reservoirs of small-mammal populations in the Natal Drakensberg. South African Journal of Wildlife Research-24-Month Delayed Open Access 23(2):39-43. |
|
|
Bury RB, Corn PS (1987). Evaluation of Pitfall Trapping in Northwestern Forests: Trap Arrays with Drift Fences. The Journal of Wildlife Management pp. 112-119. |
|
|
Byrom AE, Craft ME, Durant SM, Nkwabi AJK, Metzger K, Hampson K, Mduma SAR, Forrester GJ, Ruscoe WA, Reed DN, Bukombe J, Mchetto J, Sinclair ARE (2014). Episodic outbreaks of small mammals influence predator community dynamics in an east African savanna ecosystem. Oikos 123(8):1014-1024. |
|
|
Byrom AE, Ruscoe W, Nkwabi AJK, Metzger K, Forrester GJ, Durant S, Makacha S, Bukombe J, Mchetto J, Mduma SAR, Reed DN, Hampson K, Sinclair ARE (2015). Small mammal diversity and population dynamics in the greater Serengeti ecosystem. In: Serengeti IV: Sustaining Biodiversity in a Coupled Human-Natural System. (Eds ARE Sinclair, KL Metzger, JM Fryxell and SAR Mduma.) pp. 323-357. |
|
|
Caceres NC, Nápoli RP, Hannibal W (2011). Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia. |
|
|
Carey AB, Johnson ML (1995). Small mammals in managed, naturally young, and old-growth forests. Ecological Applications 5(2):336-352. |
|
|
Caro TM (2001). Species richness and abundance of small mammals inside and outside an African national park. Biological Conservation 98(3):251-257. |
|
|
Caro TM (2002). Factors affecting the small mammal community inside and outside Katavi National Park, Tanzania. Biotropica 34(2):310-318. |
|
|
Delibes-Mateos M, Smith AT, Slobodchikoff CN, Swenson JE (2011). The paradox of keystone species persecuted as pests: A call for the conservation of abundant small mammals in their native range. Biological Conservation 144(5):1335-1346. |
|
|
Dewees PA, Campbell BM, Katerere Y, Sitoe A, Cunningham AB, Angelsen A, Wunder S (2010). Managing the miombo woodlands of Southern Africa: Policies, incentives and options for the rural poor. Journal of Natural Resources Policy Research 2(1):57-73. |
|
|
Dickman CR (1995). Diets and habitat preferences of three species of crocidurine shrews in arid southern Africa. Journal of Zoology 237(3):499-514. |
|
|
Dizney L, Jones PD, Ruedas LA (2008). Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwestern Naturalist 89(3):171-180. |
|
|
Eccard JA, Walther RB, Milton SJ (2000). How livestock grazing affects vegetation structures and small mammal distribution in the semi-arid Karoo. Journal of Arid Environments 46(2):103-106. |
|
|
Ecke F, Löfgren O, Hörnfeldt B, Eklund U, Ericsson P, Sörlin D (2001). Abundance and diversity of small mammals in relation to structural habitat factors. Ecological Bulletins pp.165-171. |
|
|
Ecke F, Löfgren O, Sörlin D (2002). Population dynamics of small mammals in relation to forest age and structural habitat factors in northern Sweden. Journal of Applied Ecology 39(5):781-792. |
|
|
Ellis TD, Cushman JH (2018). Indirect effects of a large mammalian herbivore on small mammal populations: Context-dependent variation across habitat types, mammal species, and seasons. Ecology and Evolution 8(23):12115-12125. |
|
|
Fichet-Calvet E, Lecompte E, Koivogui L, Daffis S, Ter Meulen J (2008). Reproductive characteristics of Mastomys natalensis and Lassa virus prevalence in Guinea, West Africa. Vector-Borne and Zoonotic Diseases 8(1):41-48. |
|
|
Fleming PA, Loveridge JP (2003). Miombo woodland termite mounds: Resource islands for small vertebrates? Journal of Zoology 259(2):161-168. |
|
|
Fraschina J, León VA, Busch M (2014). Role of Landscape Scale in the Distribution of Rodents in an Agroecosystem of Argentina. |
|
|
Gbogbo F, Tabiri K, Yahaya M (2017). Diversity and abundance of small mammals along a disturbance gradient on a university campus in Ghana. International Journal of Ecology and Development 32(1):54-65. |
|
|
Geier AR, Best LB (1980). Habitat Selection by Small Mammals of Riparian Communities: Evaluating Effects of Habitat Alterations. The Journal of Wildlife Management pp. 16-24. |
|
|
Gezahegn G, Mundanthra B, Afework B (2016). Species Composition and Habitat Association of Rodents in Yetere Forest, Central Ethiopia. (June 2018). |
|
|
Goodman SM, Hutterer R, Ngnegueu PR (2001). A report on the community of shrews (Mammalia: Soricidae) occurring in the Minkébé Forest, northeastern Gabon. Zeitschrift fur Saugetierkunde. |
|
|
Granjon L, Duplantier JM, Catalan J, Britton-Davidian J (1997). Systematics of the genus Mastomys (Thomas, 1915) (Rodentia: Muridae): a review. Belgian Journal of Zoology (Belgium). |
|
|
Griffin MJ, Trewick SA, Wehi PM, Morgan-Richards M (2011). Exploring the concept of niche convergence in a land without rodents: The case of weta as small mammals. New Zealand Journal of Ecology pp. 302-307. |
|
|
Hamilton BT, Roeder BL, Hatch KA, Eggett DL, Tingey D (2015). Why is small mammal diversity higher in riparian areas than in uplands? Journal of Arid Environments 119:41-50. |
|
|
Hansson L (1997). Population growth and habitat distribution in cyclic small rodents: To expand or to change? Oecologia 112(3):345-350. |
|
|
HiCe CL, VeLazCo PM (1961). Relative effectiveness of seveRal Bait and tRap types foR assessing teRRestRial small mammal communities in neotRopical RainfoRest. South African Journal of Economics 29(3):225-225. |
|
|
Hieronimo P, Kimaro DN, Kihupi NI, Gulinck H, Mulungu LS, Msanya BM, Leirs H, Deckers JA (2014). Land use determinants of small mammals abundance and distribution in a plague endemic area of Lushoto district, Tanzania. Tanzania Journal of Health Research 16(3). |
|
|
Isabirye-Basuta G, Kasenene JM (1987). Small Rodent Populations in Selectively Felled and Mature Tracts of Kibale Forest, Uganda. Biotropica pp. 260-266. |
|
|
Johannesen E, Mauritzen M (1999). Habitat selection of grey-sided voles and bank voles in two subalpine populations in southern Norway. Annales Zoologici Fennici (pp. 215-222). Finnish Zoological and Botanical Publishing Board. |
|
|
Kelt DA (2011). Comparative ecology of desert small mammals: A selective review of the past 30 years. Journal of Mammalogy 92(6):1158-1178. |
|
|
Kihwele E, Mnaya B, Meng'ataki G, Birkett C, Wolanski E (2012). The role of vegetation in the water budget of the Usangu wetlands, Tanzania. Wetlands Ecology and Management 20(5):389-398. |
|
|
Kiwia H (2009). Species richness and abundance estimates of small mammals in Zaraninge coastal forest in Tanzania. Tanzania Journal of Science. |
|
|
Lambert TD, Malcolm JR, Zimmerman BL (2006). Amazonian small mammal abundances in relation to habitat structure and resource abundance. Journal of Mammalogy 87(4):766-776. |
|
|
Leirs H, Verhagen R, Verheyen W, Perrin MR (1995). The biology of Elephantulus brachyrhynchus in natural miombo woodland in Tanzania. Mammal Review 25(1?2):45-49. |
|
|
Lyra-Jorge MC, Pivello VR (2001). Combining live trap and pitfall to survey terrestrial small mammals in savanna and forest habitats, in Brazil. Mammalia (Paris) 65(4):524-530. |
|
|
Magige FJ (2013). Rodent species diversity in relation to altitudinal gradient in Northern Serengeti, Tanzania. African Journal of Ecology 51(4):618-624. |
|
|
Magige FJ (2016). Variation of small mammal populations across different habitat types in the Serengeti ecosystem. Tanzania Journal of Science 42(1):15-22. |
|
|
Mamba M, Fasel NJ, Mahlaba TAM, Austin JD, McCleery RA, Monadjem A (2019). Influence of sugarcane plantations on the population dynamics and community structure of small mammals in a savanna-agricultural landscape. Global Ecology and Conservation 20:e00752. |
|
|
Manson RH, Ostfeld RS, Canham CD (1999). Responses of a small mammal community to heterogeneity along forest-old-field edges. In: Landscape Ecology 14(4):355-367. |
|
|
Mbugua S (2004). The influence of land use patterns on diversity and abundace of rodents in Gachoka division of meerere district, Kenya 91. |
|
|
Meliyo JL, Kimaro DN, Msanya BM, Mulungu LS, Hieronimo P, Kihupi NI, Gulinck H, Deckers JA (2014). Predicting small mammal and flea abundance using landform and soil properties in a plague endemic area in Lushoto district, Tanzania. Tanzania Journal of Health Research 16(3). |
|
|
Mengistu A, Kiros W, Yonas M (2015). Abundance and community composition of small mammals in different habitats in Hugumburda forest, northern Ethiopia. International Journal of Biodiversity and Conservation 7(2):119-125. |
|
|
Michael N, Ringo J, Ratnayeke S (2016). Diversity, Composition and Richness of Small Mammals in Natural and Agricultural Areas in Mbeya Region, Tanzania. International Journal of Modern Plant and Animal Sciences 4(1):35-46. |
|
|
Morris DW (1995). Habitat selection in mosaic landscapes. Mosaic landscapes and ecological processes (pp. 110-135). Springer, Dordrecht. |
|
|
Mulungu LS, Mahlaba TA, Massawe AW, Kennis J, Crauwels D, Eiseb S, Monadjem A, Makundi RH, Katakweba AAS, Leirs H, Belmain SR (2011). Dietary differences of the multimammate mouse, Mastomys natalensis (Smith, 1834), across different habitats and seasons in Tanzania and Swaziland. Wildlife Research 38(7):640-646. |
|
|
Mulungu LS, Makundi RH, Massawe AW, Machang'u RS, Mbije NE (2008). Diversity and distribution of rodent and shrew species associated with variations in altitude on Mount Kilimanjaro, Tanzania. Mammalia 72(3):178-185. |
|
|
Nicolas V, Colyn M (2006). Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136(1):107. |
|
|
Nicolas V, Verheyen E, Verheyen W, Hulselmans J, Dillen M, Akpatou B, Dudu A, Wendelen W, Colyn M (2005). Systematics of African lowland rainforest Praomys (Rodentia, Muridae) based on molecular and craniometrical data. Zoological Journal of the Linnean Society 145(4):539-553. |
|
|
Nkwabi AK, Kija H, John B, Otsyina RM, Monjare JF, Kajuni AR (2018). Abundance and distribution of small mammals relative to human activities in the wildlife management areas of Ruvuma landscape, southern Tanzania. In: International Journal of Fauna and Biological Studies 5(2):156-162. |
|
|
Nyirenda VR, Namukonde N, Simwanda M, Phiri D, Murayama Y, Ranagalage M, Milimo K (2020). Rodent assemblages in the mosaic of habitat types in the zambezian bioregion. Diversity 12(10):365. |
|
|
Odhiambo R, Makundi R, Leirs H, Verhagen R (2005). Community structure and seasonal abundance of rodents of maize farms in southwestern Tanzania. |
|
|
Peterson AT, Soberón J, Sánchez-Cordero V (1999). Conservatism of ecological niches in evolutionary time. Science 285(5431):1265-1267. |
|
|
Pizzimenti JJ (1979). The relative effectiveness of three types of traps for small mammals in some Peruvian rodent communities. Acta Theriologica 24:351-361. |
|
|
Sangiwa MW, Magige FJ (2019). Effects of roads on small mammal diversity and abundance in the northern Serengeti, Tanzania. African Journal of Ecology 57(4):565-574. |
|
|
Santos-Filho M, Da Silva D, Sanaiotti T (2006). Efficiency of four trap types in sampling small mammals in forest fragments, Mato Grosso, Brazil. Mastozoología Neotropical 13(2):217-225. |
|
|
Scott DM, Joyce CB, Burnside NG (2008). The influence of habitat and landscape on small mammals in Estonian coastal wetlands. Estonian Journal of Ecology 57(4). |
|
|
Shannon CE (1948). A Mathematical Theory of Communication. Bell System Technical Journal 27(3):379-423. |
|
|
Shilereyo MT, Magige FJ, Ogutu JO, Røskaft E (2019). Spatial and temporal variation in small mammal abundance and diversity under protection, pastoralism and agriculture in the Serengeti Ecosystem, Tanzania. bioRxiv, 727206. |
|
|
Shilereyo MT, Magige FJ, Ogutu JO, Røskaft E (2021). Land use and habitat selection by small mammals in the Tanzanian Greater Serengeti Ecosystem. Global Ecology and Conservation 27:e01606. |
|
|
Sirima A (2010). Protected Areas, Tourism and Human Displacement: Interests anc Challenges behind Ruaha National Park Expansion. Environmental Science Department MSc. Leisu. |
|
|
Stanley WT, Goodman SM, Hutterer R (1996). Notes on the insectivores and elephant shrews of the Chome Forest, South Pare Mountains, Tanzania (Mammalia: Insectivora et Macroscelidea). Zoologische Abhandlungen (Dresden). |
|
|
Stanley WT, Kihaule PM, Howell KM, Hutterer R (1998). Small Mammals of the Eastern Arc Mountains, Tanzania. Journal of East African Natural History 87(1):91-100. |
|
|
Stanley WT, Rogers MA, Kihaule PM (2015). Preliminary Results of a Survey of Small Mammals in Ruaha National Park, Tanzania. Journal of East African Natural History 104(1-2):195-211. |
|
|
Stenseth NC, Viljugrein H, J?drzejewski W, Mysterud A, Pucek Z (2002). Population dynamics of Clethrionomys glareolus and Apodemus flavicollis: Seasonal components of density dependence and density independence. Acta Theriologica 47(1):39-67. |
|
|
Tanzaniatourism (2021). Ruaha National Park - Tanzania Tourism. |
|
|
Tattersall FH, Macdonald DW, Hart BJ, Manley WJ, Feber RE (2001). Habitat use by wood mice (Apodemus sylvaticus) in a changeable arable landscape. Journal of Zoology 255(4):487-494. |
|
|
Timbuka C, Kabigumila J (2009). Diversity and abundance of small mammals in the Serengeti kopjes, Tanzania. Tanzania Journal of Science 32(1):1-12. |
|
|
Umetsu F, Naxara L, Pardini R (2006). Evaluating the efficiency of pitfall traps for sampling small mammals in the neotropics. Journal of Mammalogy 87(4):757-765. |
|
|
Venance J (2009). Small mammal communities in the mikumi national park, Tanzania. Hystrix-the Italian Journal of Mammalogy 20(2). |
|
|
Voss RS, Emmons LH (1996). Mammalian diversity in neotropical lowland rainforests: A preliminary assessment. Bulletin of the American Museum of Natural History; no. 230. |
|
|
Woodman N, Timm RM, Slade NA, Doonan TJ (1996). Comparison of Traps and Baits for Censusing. Jounal of Mammalogy 77(1):274-281. |
|
|
WWF, WCS (2003). Technical Report on Water Availability in the Ruaha River and The State of Usangu Game Reserve, November 2003. (November):1-12. |
|
|
Yakimova AE, Gaidysh IS (2021). The species composition and abundance of terrestrial small mammals in the Finnish-Russian Friendship Nature Reserve. Nature Conservation Research 6(S1):127-136. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0