Full Length Research Paper
ABSTRACT
Surface and ground water resources in urban areas are at risk of contamination from many human activities such as oil spills due to improper location of fuel filling stations and auto mobile repair workshops in fast developing cities in third world countries like Cameroon. The study was aimed at determining the physicochemical properties of surface water resources around fuel filling stations and auto-mobile repair workshops in the Bamenda city, North West Region of Cameroon. Ten water samples were collected from surface water sources around two fuel filling stations and three auto-mobile repair workshops. The pH, temperature and electrical conductivity were tested using a Bluelab pH meter, Mercury in glass thermometer, and a WTW LF 91 meter, respectively. The total hardness of water was measured using EDTA titrimetric method; alkalinity was determined using titrimetric method with 0.01 M concentration of HCl as titrant while the concentrations of calcium, magnesium and chlorine were measured using complexometric titration. Results showed that the pH values ranged from 5.62 to 6.24 for auto-mobile repair workshops and 6.15 to 6.70 for fuel filling stations which were below the World Health Organization (WHO) range (6.5 to 8.5) recommended for potable water. Other physicochemical parameters like electrical conductivity, water hardness, temperature and alkalinity were all within WHO recommended range. Ca+ and Mg2+ concentration was within limits while Cl was above WHO recommended limits. The study concludes that the amount of waste oil generated at fuel filling stations and auto mobile repair workshops is significant to cause negative effects on the environment in the Bamenda city, North West Region of Cameroon.
Key words: Fuel filling stations, auto-mobile repair workshops, physicochemical properties, WHO limits.
INTRODUCTION
One of the adverse environmental impacts of urbanization globally is the changes in the physical, chemical and the biological properties of basic natural resources such as soils, air and water. In the case of water for example, the pollution and contamination of this resource does not only affect its quality or physicochemical properties but equally serves as a threat to public health since humans depend on it for various purposes such as domestic use and agriculture (Nganje et al., 2007). Surface and ground water resources in urban areas are at risk of contamination from many human activities such as oil spills due to haphazard location of fuel filling stations and auto mobile repair workshops along surface water resources especially in developing countries. Spilled oil contains carcinogenic or toxic components such as heavy metals, total petroleum hydrocarbons (TPH) which affects human health both directly and indirectly (Nganje et al., 2007). With the increasing rate of urbanization in developing countries like Cameroon, there has been an increasing number of fuel filling stations and author-mobile repair workshops with high potentials risk of contamination of water resources which is yet to be investigated.
At gas stations, fuel is stored and transferred between tanker trucks, storage tanks, and vehicle tanks. During both storage and transfer, a small fraction of unburned fuel is typically released. If a spill occurs while runoff occurs, the hydrocarbon can be expected to float on top of the water sheet, because gasoline, diesel oil, and lubricants are typically less dense than water. While the fraction may be small, the cumulative release can be substantial because of the large quantities of fuel sold (Hilpert et al., 2015). At auto-mobile repair shops, spilled oil is often used oil. The US Environmental Protection Agency (EPA) (2001), defined used motor oil as “any petroleum-based or synthetic oil that has been used for vehicle lubrication and as a result of normal use, motor oil becomes contaminated with various impurities such as dirt, water, chemicals or metals from vehicle engine”. Once degraded motor oil escapes the engine, it has the potential to pollute waterways in the form of runoff and soil. The US EPA reported that 1 gallon of used motor oil can contaminate 1 million gallons of fresh water. Used oils are therefore considered as one of the most hazardous mainstream categories of environmental pollutants, posing a major threat to the environment and public health.
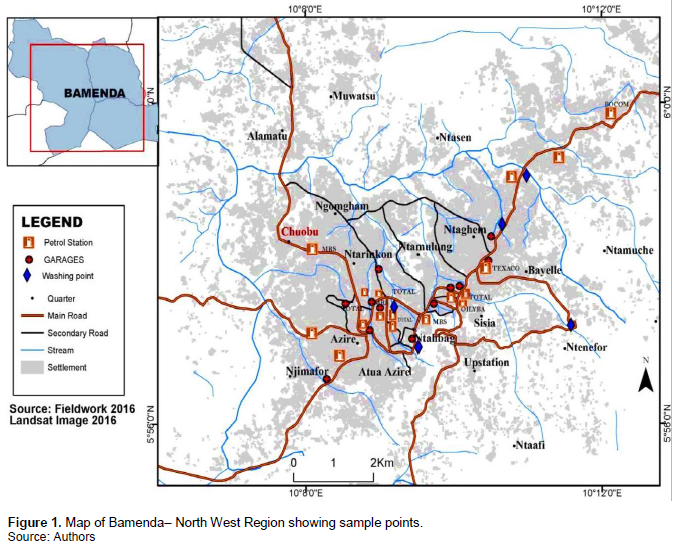
A common practice in Cameroonian cities is to allocate large tracts of land, sometimes reaching 5 ha or more to groups of small-scale auto-mechanic businesses and designate these as villages where they locate their workshops and repair yards to offer their services to the public (Adelekan and Abegunde, 2011). In most cities, fuel feeling stations are also located near sensitive ecosystems without any proper environmental impact assessment (Adelekan and Abegunde, 2011). Most of these fuel filling stations and auto-mobile repair workshops are located near streams and rivers which the population depend on for domestic and agricultural purposes. The city of Bamenda has witnessed an increase in the number of fuel filling stations and auto-mobile repair impacts of these activities on the surface water sources are yet to be investigated. It is against the backdrop of this knowledge gap that this study is conceived. In this research, the impacts of oil spills from fuel filling stations and auto-mobile repair workshops on the physico-chemical properties of surface water systems in some selected sites in the Bemanda city were investigated. The findings can then be used as a basis for improving the management of fuel filling stations and autho-mobile repair shops as well as guide environmental lanners on the suitable location of these activities in urban areas in Cameroon and other developing countries.
Study area
This study was carried out in Mezam division in the Bamenda highlands (17,300 km2) in the North West Region of Cameroon. The area is located within latitude 5°40’ and 7° North of the equator and within longitudes 9°45 and 11°10’ East of the Meridian (Law, 2010).
MATERIALS AND METHODS
Sampling criteria and sample collection
Study sites selected for this study were based on their proximity to surface water resources to auto mobile repair workshops and fuel filling stations (Figure 1). Using this criterion, ten surface water sources which were located within a 10 to 20 m distance from the auto-mobile repair workshops and fuel filling stations were selected. Ten water samples were collected from five surface water resources (two fuel filling stations and three auto-mobile repair workshops) in the month of August 2017. Two samples were collected from each surface water system within a 100 m spacing. These samples were collected in 250 ml polythene bottles pre-washed with non-ionic detergents, rinsed with distilled water three times. Before sampling of water, the bottles were rinsed three times with the sampled water.
Sample analysis
Parameters like pH, temperature and electrical conductivity were tested in the field using, a Bluelab pH meter, Mercury in glass thermometer, and a WTW LF 91 meter, respectively. Samples were put in an ice cooler and transported to the chemistry laboratory of the University of Buea, Cameroon for further analysis of other physicochemical parameters.
The total hardness of water was measured using Ethylene Diamine Tetra Acid (EDTA) as titrant with Ammonium Chloride and Ammonium Hydroxide buffer solution (pH-10) and erichrome black T as the indicator. Alkalinity was determined using titrimetric method using standard solution of 0.01 M concentration of HCl and methyl orange used as indicator. Concentrations of Calcium, Magnesium and Chlorine were measured using complexometric titration. For Calcium concentration, EDTA was used alongside Patton’s and Reeder’s indicator with Sodium Hydroxide as buffer solution (pH-12). The concentration of Magnesium ion was determined using a standard solution of EDTA and Erichrome Black T as indicator with Ammonium Chloride and Ammonium Hydroxide as buffer solutions (pH 10).
RESULTS AND DISCUSSION
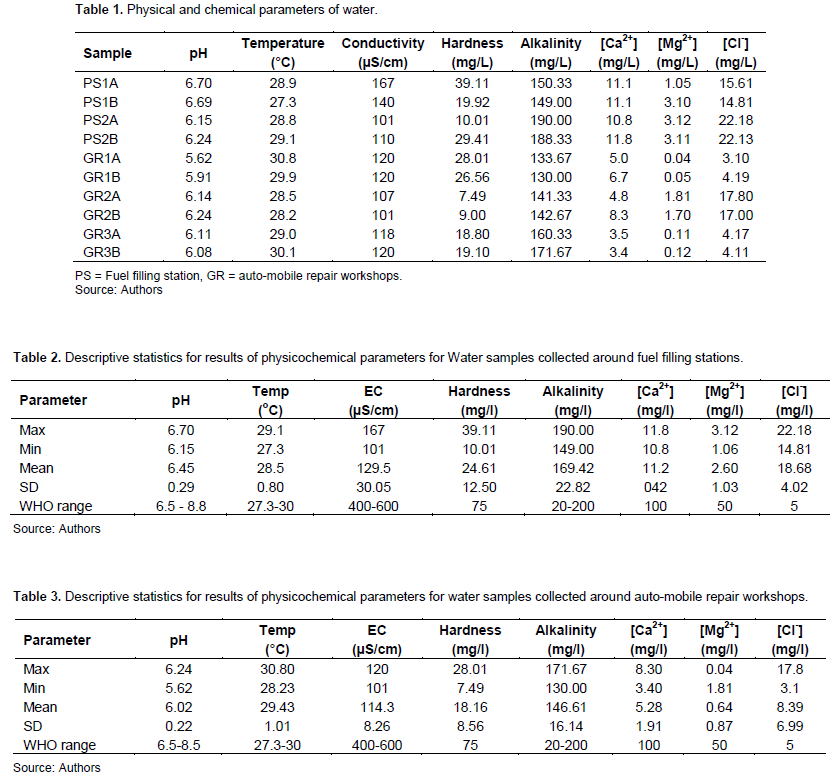
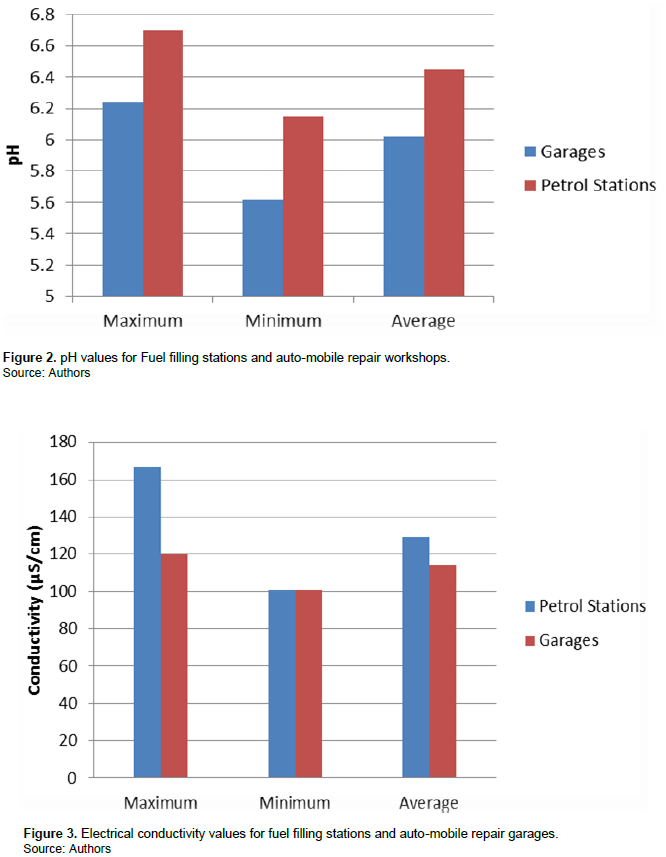
The results obtained from analysis of water samples are presented in Table 1 and statistical analysis presented in Tables 2 and 3 for fuel filling stations and auto-mobile repair workshops, respectively.
Potential hydrogen (pH)
pH is referred to as “potential hydrogen” and is the measure of the acidic or basic state of the water. It is calculated as the logarithm of the reciprocal of the concentration of hydrogen ion (pH = Log 1/H+). The results obtained shows that pH values ranged from 5.62 to 6.24 for auto mobile repair workshops, and 6.15 to 6.70 for fuel filling stations (Figure 2). pH values are considered optimum for water and aquatic species in the range of 6.5 and 8.5 (WHO, 2008).
The presence of hydrocarbons and heavy metals from spilled oil may be an indication for the reduced pH level of water. The values of pH lower than 6.5 can cause the corrosion of metal pipes and subsequent release of toxic metals such as lead, cadmium, and zinc (Buridi and Gedala, 2014). The pH values thus indicated that the water within the selected study areas is not suitable for domestic use or consumption. In a similar study conducted by Vincent-Akpu et al. (2015) in Delta State Nigeria, it was noted that the pH values of surface water resources near spilled sites were within permissible limits which is not in line with the findings of the study. The differences in pH values of surface water resources in the spilled sites may have resulted from the differences in the quantity of spilled oil discharged in the nearby surface water resources.
Conductivity
Conductivity is the measure of the ionic concentration in the water. In other words, it is the ability of water to conduct electrical current due to its ionic content.
Increasing the salt content in water increases its conductivity (Oyem and Oyem, 2013). The mean electrical conductivity recorded for fuel filling stations was 129.5±15.03 while that for auto-mobile repair workshops stood at 114.3±3.37 (Figure 3). The electrical conductivity values of samples were generally lower than WHO range (400-600 µS/cm).
The low values recorded within the study area may be attributed to the increased intensity of rainfall thus rainwater runoffs causing reduction of salts in water. However, an excessive increase of the surface water electrical conductivity will generate modifications of the bacterial ecosystem and also influence the survival of aquatic fauna and flora (Demakoye et al., 2017). The findings of this study are similar to the findings of Odipe et al. (2020) on the effects of filling stations and auto mobile repair workshops on groundwater quality in Ilorin metropolis where authors noted that the conductivity values were within permissible limits in their study area.
Temperature
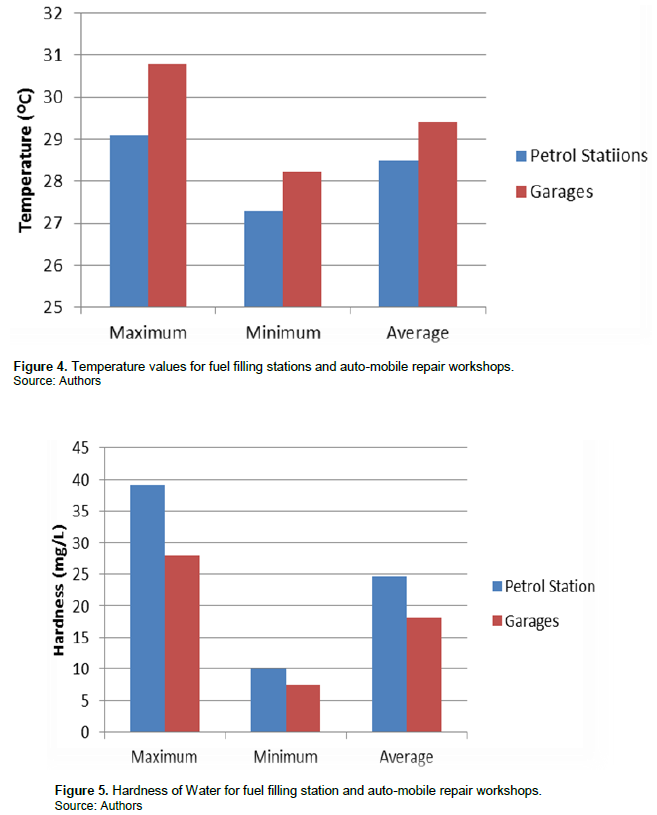
The mean value of temperature recorded for fuel filling stations was 28.5°C±0.40, and that of auto-mobile repair workshops stood at 29.43°C±0.41 (Figure 4).
However, acceptable temperature values range from 27.3 to 30.0°C for portable water (WHO, 2008). This indicates that temperature values were all within the acceptable limit. The high temperature values recorded may be attributed to high atmospheric temperature within the study area. It should be noted that temperature of water controls the rate of all biological process and thus affect aquatic systems.
Hardness
The mean value for water hardness was 24.62 mg/L ±12.50 for fuel filling station samples and 18.16 mg/L ± 8.56 for auto-mobile repair workshops (Figure 5).
Hardness of water is a function of the concentrations of magnesium and calcium ions present in the samples as well as other ions like sulphate, chloride and hydrocarbonate ions. The presence of very high concentrations of anions in water indicates the water may either be permanently or temporally hard. However, concentrations in the samples collected did not exceed the maximum allowable concentration of 75 mg/L as recommended by the WHO. The results of the present study are similar to the findings of Vincent-Akpu et al. (2015) who noted that hardness of surface water near fuel filling stations in Delta State Nigeria were within permissible limits
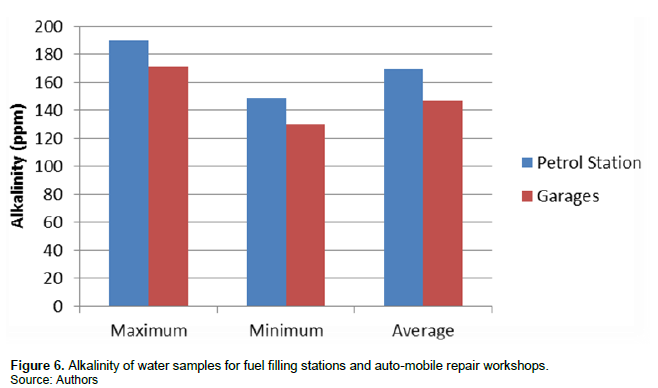
Alkalinity
Alkalinity is water acid-neutralizing capacity and is influenced by the presence of carbonate, hydroxide and bicarbonate ions in water. Alkalinity acts as a stabilizer for water (Patil et al., 2012). Alkalinity values concentration recorded mean values of 169.42 ppm ± 11.41 for fuel filling stations and 146.61 ppm ± 6.95 for auto-mobile repair workshops (Figure 6).
High values of alkalinity will cause a bitter taste of water and adversely affects the human digestive system. It will also lead to scale formation in water due to conjunction of bicarbonate and calcium. The extremely low alkalinity of water may be the reason for the low pH values of water. It should be noted that, alkalinity values are however lower for auto-mobile repair workshops compared to fuel filling stations in this study.
Ionic concentration
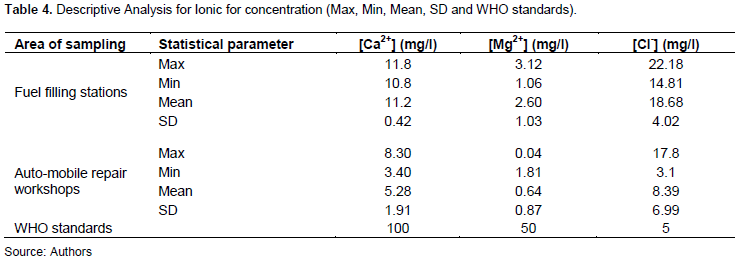
Concentration of Ca+ had a mean value of 11.64 mg/L ± 0.04 and 5.28± 1.91 values for fuel filling stations and auto-mobile repair workshops, respectively. With a MAC of 100 mg/L, the results indicate that these values were within limits in both sites. Mg2+ values were recorded at 2.06± 1.03 mg/l and 06.4± 0.87 mg/l while Cl was recorded at 18.68 ± 4.02 mg/l and 8.39 ± 6.99 for fuel filling stations and auto-mobile repair workshops, respectively. With a MAC value of 50 mg/l for Mg2+, the present results show that Mg2+ were within permissible limits for both sides. However, Cl concentration was out of MAC in both sides since the mean values for these sites were above 5 mg/l (Table 4).
High concentration values of chloride may render water unsuitable for consumption and will affect the aesthetic properties of water. It may equally cause body irritation in humans, stomach discomfort and increase the corrosiveness of water (Adefemi and Awokunmi, 2010). The concentration of chlorine is the study area requires attention as the values are above WHO allowable limits (5 mg/l).
CONCLUSION
The findings of this study noted that temperature, alkalinity, conductivity and hardness were within the WHO acceptable ranges for surface water sources around fuel filling stations and auto-mobile repair workshops. pH values were however lower than expected thus causing the alteration of other parameters like conductivity of surface water resources in both sites. The pH of water affects other parameters in water thus “out-of-limit” values of these parameters indicate that the water is not suitable for domestic use. Ca+ and Mg2+ concentration was within limits while Cl was above WHO recommended limits. It was concluded that the amount of waste oil generated at fuel filling stations and auto mobile repair workshops is significant to cause negative effects on the environment. This study also suggests proper wastemanagement of used oil and the relocation of these facilities from environmentally sensitive areas such as surface water systems to prevent the harmful effects of spilled oil on environment and human health.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Adefemi SO, Awokunmi EE (2010). Determination of physico-chemical parameters and heavy metals in water samples from Itaogbolu area of Ondo-State, Nigeria. African Journal of Environmental Science and Technology 4(3). |
|
|
Adelekan BA, Abegunde KD (2011). Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria. International Journal of the Physical Sciences 6(5):1045-1058. |
|
|
Buridi KR, Gedala RK (2014). Study on determination of physicochemical parameters of groundwater in industrial area of pydibheemavaram, Vizianagaram District, Andhrapradesh, India. Austin Journal of Public Health and Epidemiology 1(2):1-2. |
|
|
Demakoye V, Martine K, Yacouba O, Seydou G, Siméon S (2017). Evaluation of the Quality of Household Waste Leachate Treated by Settling Ponds at the Ouagadougou Waste Treatment and Valorization Center. Journal of Environmental Protection 8(12):1567-1582. |
|
|
Epa U (2001). United States environmental protection agency. Quality Assurance Guidance Document-Model Quality Assurance Project Plan for the PM Ambient Air 2:12. |
|
|
Hilpert M, Mora BA, Ni J, Rule AM, Nachman KE (2015). Hydrocarbon release during fuel storage and transfer at gas stations: environmental and health effects. Current Environmental Health Reports 2(4):412-422. |
|
|
Law G (2010). Administrative subdivisions of countries: a comprehensive world reference, 1900 through 1998. McFarland. |
|
|
Nganje TN, Edet AE, Ekwere SJ (2007). Concentrations of heavy metals and hydrocarbons in groundwater near Fuel filling stations s and mechanic workshops in Calabar metropolis, southeastern Nigeria. Environmental Geosciences 14(1):15-29. |
|
|
Odipe OE, Sawyerr HO, Adewoye SO (2020). Filling stations and their effects on groundwater quality in Ilorin metropolis. International Journal of Environmental Protection and Policy 8(1):11. |
|
|
Oyem ILR, Oyem L (2013). Effects of crude oil spillage on soil physico-chemical properties in Ugborodo community. International Journal of Modern Engineering Research 3(6):3336-3342. |
|
|
Patil PN, Sawant DV, Deshmukh RN (2012). Physico-chemical parameters for testing of water-a review. International Journal of Environmental Sciences 3(3):1194. |
|
|
Vincent-Akpu IF, Tyler AN, Wilson C, Mackinnon G (2015). Assessment of physico-chemical properties and metal contents of water and sediments of Bodo Creek, Niger Delta, Nigeria. Toxicological and Environmental Chemistry 97(2):135-144. |
|
|
World Health Organization (WHO) (2008). Guidelines for drinking-water quality: second addendum. Volume 1, Recommendations. World Health Organization. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0