Known for its diversity and high endemicity, half of Madagascar's floral richness is sheltered in the Island's eastern evergreen dense rainforest; including key, threatened and socio-economically valuable species. The vulnerability of such habitat, combined with overexploitation of timber, threatens the livelihoods of forest communities and conservation of biological diversity. Up till date, the main actions regarding Madagascar’s biodiversity are leading at the ecosystem level through protected area systems, without necessarily ensuring real conservation of all key species. These are rarely considered as a criterion for effective protected area management. Although decisions on long-term conservation and genetic improvement require detailed scientific and economic knowledge of species, very limited data are available. This research study sets out to promote the development of scientific knowledge of six key endemic species belonging to lowland rainforest ecosystems, to propose a variant of models for the conservation and sustainable use of their genetic resources. The research was conducted using several methods including ecological studies of the habitats of these key species, demographic analysis, and monitoring of their biological and phenological behaviors. The results obtained made it possible to identify three priority groups of key species for which specific conservation and sustainable use measures are proposed.
The six key species have been chosen in such a way to represent the most conservation status according to IUCN, in addition to their socio-economic values and the ecological roles they play. This serves to highlight proposals for specific conservation and sustainable use measures (Table 1 and Figures 1 to 4).
Five protected areas distributed throughout the eastern part of Madagascar, from North to South, were chosen as study areas to better identify intraspecific variability at different latitudes across the natural range of key species distribution (Figure 5). The choice of protected areas relates to the general objective of the project, which is to ensure complementarity between the species-based conservation approach and the ecosystem-based approach. The northeast part includes 3 sites: Pointe à Larrée, Tampolo and Betampona. The first two are New Protected Areas (NPA) established since 2015, covering 770 ha and 675 ha, respectively; while Betampona is an Integral Nature Reserve (INR) established in 1927 and covers 2228 ha. In the south-eastern part, 2 sites belonging to the Atsimo Atsinanana Region have been selected: Manombo, which is a 5320 ha Special Reserve created in 1962; and Mahabo Mananivo, which is a New Protected Area established in 2015 on 2745 ha.
The research topics addressed in this study include the following: limited scientific understanding of key species, vulnerability of their habitat, risk of species loss with the usual ecosystem-based conservation approach, and the total absence of silvicultural practices in natural forests. In order to address this issue and achieve the research objective, the following hypotheses are put forward: "Changes in ecological habitat conditions affect the viability of species"; "Species adopt particular behaviors in relation to changes in the ecological conditions of their environment"; "The reproductive biology of target species can be assisted by artificial measures".
For this purpose, ecological types were defined across the study sites by combining the following factors: plant community type, habitat degradation status, toposequence and soil type. Key species belonging to each ecological type, thus defined, have been subjected to demographic, phytosociological, phenotypic and biological analysis. To this end, sampling systems were adopted based on the Braun-Blanquet (1965) plot method: 30 square 20 m x 20 m plots were delimited by ecological type at each study site. The choice of plot location is of a rational type, based on the presence of at least one individual of a key species. In each plot, first, the inventory work concerned individuals of all the key species encountered. Then, with the adapted classification of Blaser and Rakotomanana (1990), inventoried trees were categorized into three groups according to their development stages (Table 2) and the values of the following dendrometric parameters: diameter at breast height (DBH), total height (TH), and biovolume (Vi). This is in order, firstly, to characterize the demography of each target species, reflected by the specific density per ecological type, expressed by the number of individuals present per unit area (Smith, 1963); secondly, to establish the total population structure per species, determined by the distribution curve of the number of individuals according to diametric classes (Rollet, 1969), thus allowing to define their silvicultural comportment in terms of light requirement, called “temperament.” The projection of the crown and the shape of the barrel were additional phenotypic characteristics.
Then, 5 seed trees (ST) per species by ecological type were selected, numbered and geolocated to monitor their phenological behavior. The choice was made for trees of good phenotypic quality, vigor and in good sanitary condition. On each selected individual-tree, the followings were documented: different phenophases (period, frequency and duration) defined by Comps et al. (1987), foliation, flowering and fruiting. Fruit and/or seed production capacity and natural seedlings (wildings) availability under the crown of parent plants were estimated during the fruit ripening phenophase to determine the natural reproduction potentiality of each key species. To this end, the following parameters were considered and evaluated: number of fruits, seeds and wildings per unit area (N m-2) on 1 m × 1 m square plots around the seed tree along a given axis (Andriambelo, 2007).




During the fruit ripening phenophase, the estimation of the fruit and/or grain production capacity and the availability of natural seedlings (wild) under the crown of the seed plants made it possible to determine the natural reproductive potential of the species. For this purpose, the following parameters were considered and evaluated: number of fruits, seeds and wildings per unit area (N m-2) on 1 m x 1 m square plots around the seed tree along a given axis (Andriambelo, 2007). It was also an occasion to determine the dissemination modes of target species, based on the state of seed dispersal and/or seedling establishment (random, regular, aggregated), as well as their natural regeneration capacity (Rothe, 1964).
Finally, the data collected were analyzed using a series of scientific methods to test research hypotheses, establish the variability of key species across their natural range and identify the differentiation factors in order to propose model variants of conservation and sustainable use. First, the use of a cross-classification table (contingency table) made it possible to identify the ecological preferences of the 6 key species, considering the specific density (indiv. ha-1). To this end, the existence of a relation between the qualitative variables "key species" and "defined ecological types" was detected by “Chi-square” independence test. Then, an interdependence between these two variables led to a “Correspondence Analysis” (CA) to identify affinities between key species and different ecological types. Subsequently, the “Analysis of variance” (ANOVA) and the “Kruskal-Wallis” non-parametric test were applied to study the variability of target species across their natural range. The quantitative variables to be explained were the dendrometric parameters (DBH, TH, and Vi). The explanatory ones were constituted by the ecological types where the target species was encountered. ANOVA is a parametric statistic requiring normally distributed data; when this was not the case, or if it concerns discrete qualitative or quantitative variables, such as phenotypic (crown and bole shape) and biological variables (number of fruits, seeds, wildings per unit area), the non-parametric Kruskal-Wallis test was used (Labreuche, 2010). For the ANOVA and Kruskal-Wallis tests, the significance level was set at p ≤0.05. The identification of a “significant difference” among the variables at the end of the two types of tests led to an additional test of multiple pairwise comparisons (“post-hoc analysis”) to determine the explanatory factors for differentiation and which levels of the independent variable differ from each other level.
Asteropeia amblyocarpa (Asteropeiaceae)
The key species A. amblyocarpa was found in Tampolo. The Correspondence analysis (CA), using “symmetric plot” of the rows (“key species”) and columns (“defined ecological types”) of the contingency table, identified its ecological preferences as well as those of the other target species (Figures 6 and 7).
At the "seed tree" (ST) development stage, A. amblyocarpa adopts a particular preference for littoral forest ecosystems on coastal plains. At these locales, the types of alluvial, hydromorphic and sandy soils are favorable for its development. Generally, if the sand drains easily, therefore dries out and quickly depletes nutrients, one of the forms of adaptation to these habitat types is the presence of mycorrhizae living in symbiosis with this species. For Dupuya haraka, ST individuals are isolated and distant from the ecological types defined according to a symmetric plot (Figure 6). This did not allow the ecological preference of the species to be identified. However, the literature suggests that Dupuya haraka occurs in humid, and also in deciduous forests, in the Northeastern to the Eastern of Madagascar, mainly between 10-598 masl (Ramanantsialonina, 2019) on sandy, sandy-silt or well-drained limestone soils (Louppe et al., 2008). The two Dalbergia species, D. baronii and D. chapelieri, seem to have the same ecological preferences at the ST development stage. They adapt to several types of formations: littoral forests on coastal plains, low and medium altitude evergreen humid forests, degraded environments, and even in "non-forest" habitat types such as tree-lined agricultural fields (food crop mosaics). These agricultural fields would probably have been ancient forest ecosystems but have kept living individuals of Dalbergia. Seed trees of Faucherea tampoloensis and Tina thouarsiana form a group with a particular affinity for the ecological types of coastal forests on coastal plains, and seem to adapt well on two types of soils: sandy soils and temporarily flooded alluvial soils.

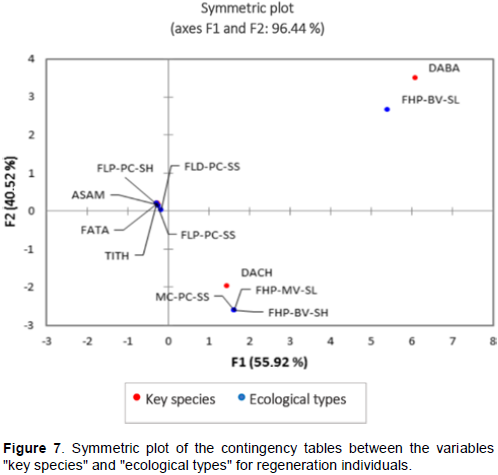
For natural regeneration individuals (RG), Asteropeia amblyocarpa, F. tampoloensis and T. thouarsiana constitute an isolated group with a particular affinity for littoral forests on coastal plains (Figure 7). Regeneration is absent for Dupuya haraka. This species seems to have a difficulty in regeneration, and a low potential for reproduction in its natural state. Dalbergia chapelieri's juvenile trees have the same ecological preferences as the ST development stage, which adapts to almost all types of formation: it can be found in humid valleys as well as on drier crest, on lateritic or sandy soil, and even may survive as a shrub after resprouting in secondary vegetation (Contu, 2012). However, Dalbergia baronii regenerations are very demanding, adopting a strict preference for low-elevation humid forest types on bottom slopes (Figure 7).
The population of Asteropeia amblyocarpa is characterized by a high abundance of natural regeneration compared to adult individuals (Table 3). The potential for regeneration of the species in its natural state is quite good with a rate above 500% according to the Rothe scale (1964). Inventoried trees generally have the same phenotypic characteristics: a tolerable crown shape, more or less circular in plan with some deficiencies in symmetry, and a good conformation of the bole shape; i.e., straight, round and solid, cylindrical, without serious defects. Trees up to 50 cm of DBH have been recorded, but the average DBH of the key species remains quite low (DBHa = 10.77 cm).
The high reproductive potential of the target species A. amblyocarpa is partly explained by its phenological behavior, characterized by a year-round observation period of flowering and fruiting phenophases. Seed trees produce abundant flowers and fruits during these phenophases (average production per parent plants Nm = 42 mature fruits m-2). The continuity and regularity of the phenological rhythm, spread over most of the year, is announced by Sabatier and Puig (1986) as characteristic of small trees in the forest understory that thrive under particular edaphic conditions such as hydromorphic soils that reduce climatic seasonality. Indeed, the distribution by diametric class of the trees inventoried in exponential form (Figure 8), according to the Rollet curve (1969), gives the species a sciaphilous character temperament of the undergrowth. As a result, the opening of the canopy due to windthrow and anthropogenic disturbances significantly affects the installation and development of natural seedlings. In disturbed environments, the natural regeneration rate or NRR (Rothe, 1964) can be as high as 1000%. This high rate in disturbed areas can be explained either by the low proportion of adult individuals in relation to regeneration due to their exploitation, or by the fact that the species needs a certain luminosity condition to regenerate. This was explained by Whitmore (1989), that even "shade-tolerant" species, able to survive and even grow in the shaded undergrowth, have responded positively to a certain opening of the canopy, a condition allowing them to move on to the next stages (thicket, saplings, young pole stands).
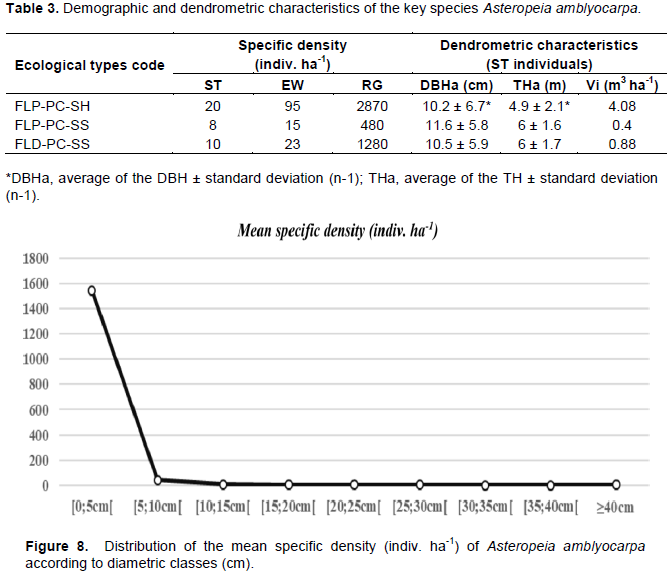
A. amblyocarpa could therefore be a semi-sciaphilous to sciaphilous species. However, with good germination capacity in the natural state, abundant availability of wildings under the crown Na = 36 seedlings/ m2/ indiv. ST, and a high NRR% (NRR > 500%), many regenerative individuals (RG) cannot survive and develop to the “young” and “large poles stands” stages of development (EW and ST) (Table 3). The aggregate dispersal pattern of seedlings suggests that the high density of wildings per unit area (m2) has favored natural selection to reduce competition between individuals. But several factors can damage young regrowth during this stage of development, the most important of which are: pathogenic fungi (Augspurger, 1983), insects and vertebrates feeding on leaves or stems (Angulo and Aide, 2000), mortality due to falling debris (Clark and Clark, 1991), water reserve in the soil (Wright, 1992), light exposure (Baraloto, 2001).
Dupuya haraka (Fabaceae)
The species has been found in Tampolo and Betampona. The distribution of individuals recorded in the inventory plots according to diametric classes showed a low abundance of seedlings and the absence of natural regeneration (Table 4).
Over its natural distribution area as a whole, the demographic characteristics of the species reflect its difficulty in regenerating and its very low potential for reproduction in the natural state, with a NRR of 0% (Rothe, 1964) at both sites. Several factors could explain this behavior. During the phenological monitoring of the ST individuals, no flowering or fruiting phenophase was observed. It is therefore assumed that this species exhibits discontinuous and irregular phenological behavior, which, according to Sabatier and Puig (1986), is characteristic of large trees in the forest. Effectively, Dupuya haraka's seed trees share the same phenotypic characteristics throughout its natural range; that is, large individuals of up to 80cm of DBH and up to 30m in TH; high average exploitable wood volume of 13.8m3 /ha; good conformation of the tree characterized by a topless, circular, planar, symmetrical, dense and extended crown with a straight, round, cylindrical, flawless and branchless bole. However, the variation in ecological types has no effect on these phenotypic characteristics (ANOVA; DBH: p = 0.95, TH: p = 0.90, Vi: p = 0.84). The species thus has an interannual alternation of production which, according to Normand (2014), results from a year of high production followed by one or two years of low or no production. Production alternation can be influenced by climatic or endogenous (tree-specific) factors (Goldschmidt, 2005). When fruiting takes place, the conditions of regenerations installation are strongly associated to the physiological behavior of the seeds. Indeed, D. haraka seeds have a delayed germination process whose dormancy removal is conditioned by several parameters. They adopt partial dormancy, which after collection and storage for about 6 months has shown a low germination rate of 30-40%, while those stored away from heat and humidity after 1.5-2 years have reached 84% germination rate (Louppe et al., 2008). Thus, the variability of environmental conditions such as soil moisture, litter type and thickness, light quality and temperature has a direct influence on the continuation or termination of the process (Dalling et al., 1998). While the erratic aspect (Figure 9) of the distribution curve of individuals according to diameter classes (Rollet, 1969) determines a pioneering heliophilous temperament (“shade intolerant”) for this species; and natural young seedlings prefer full sunlight (Louppe et al., 2008). The high density of vegetation cover and associated species, as in the case of the Betampona site, tend to compromise survival and the passage of regeneration to more advanced stages of development (regrowth, sapling). In addition, D. haraka is an endozoochorous species, and there is a need for intestinal seed transit, after ingestion by an animal, to stimulate seed germination. Louppe et al. (2008) argue that it is the lemurs that eat and disseminate the seeds of Dupuya haraka. The considerable decrease in the number of lemurs, especially in the Tampolo protected area, due to poaching and habitat destruction, could have significant effects on the species' reproductive capacity in the natural habitat.

D. baronii (Fabaceae)
This key species has been found in Pointe à Larrée, Tampolo and Manombo. Whereas in the past, the species was found in the Mahabo Mananivo site with a specific abundance of 34 indiv. ha-1 for EW and ST stems with a DBH ≥ 5cm (Ralambomanana, 2006), currently no individuals have been found. According to Razafintsalama (2016), D. baronii is among the highly exploited rosewood species, so that even the seed trees have disappeared, resulting in the absence or rarity of juvenile individuals and regeneration (Table 5).
In addition to the influence of anthropogenic disturbances on the demographic characteristics and reproductive potential of the species, they are also influenced by ecological parameters, such as variability of the habitats throughout its distribution area (Kruskal-Wallis; fruit production: p <0.02; availability in wildings: p < 0.01). The fruit and seed production capacity of the ST individuals is low on the coastal plains of the northeastern part (Na = 6 mature fruits/ m2 /indiv. ST) compared to those of the southeastern part (Na = 13 fruits/ m2/ indiv. ST) situated on the low-elevation humid forest. This is influenced by the phenological shift of seed trees in these two areas: the fruiting and maturing phenophase lasts two months (October - November) in the North and four months (November - February) in the South. This difference appears to be explained by climatic variability between sites, constituting the external factors of phenological variability including local daytime and nighttime temperatures, precipitation and photoperiodism. Climate is not only variable on a temporal scale but also on a spatial scale that could vary within a region and even at the local level (Rasamimanana, 2011). In the face of this climate variability and the adverse effects of climate change, the species may have adopted a particular form of adaptation. According to Kramer (1995), this adaptation generally takes the form of a compromise between the different needs of the plant by moving forward, backward or reducing the onset and duration of a phenophase. This phenomenon is often found in species that are most vulnerable to rainfall variability, such as those in the sub-humid regions of southern Madagascar: Acacia bellula, Rhigozum madagascariensis, Grewia franciscana, Commiphora sp., Uncarina grandidieri and Terminalia fatrae (Rasamimanana, 2011). This ecological and phenological variability between sites significantly affects the installation and development of natural regeneration. The natural regeneration rate is zero (NRR = 0%) in littoral forests on the coastal plain of the northeastern part of Madagascar. Similar results were found by Razafintsalama (2016) confirming that Dalbergia baronii appears to have a regeneration problem in this region (NRR < 100%). Soil characteristics were identified as another factor of variability conditioning the germination of seeds in their natural state and the availability of natural seedlings. In coastal plains on sandy soils, the thin and highly permeable layer, often low in organic material and humus, is not favorable to seed germination and natural regrowth development (Na = 3 wildings/ m2/ indiv. ST). In temporarily flooded areas with hydromorphic soils, the fruit ripening period coincides with the period of heavy rainfall leaving light-weight fruits and seeds washed away by the floods (Na = 2 wildings/ m2/ indiv. ST). On the other hand, in the southeastern part, with an average seedling availability under the crown of Na = 16 wildings/ m2/ indiv. SM, the natural regeneration capacity, according to the Rothe scale (1964), is good with a NRR = 133%. In this region, natural regenerations and juvenile individuals adopt a particular preference and form an isolated group at lowland humid forest types on bottom slopes (FHP-BV-SL) where the germination potentiality of seeds in the natural state, young seedlings development and growth are favored by lateritic soils characterized by a very rapid podzolization phenomenon (Figure 7).
As for the phenotypic characteristics of the species, they are little affected by the variation in the ecological parameters of the habitat throughout its natural range (ANOVA; DHP: p = 0.77; HT: p = 0.5; Vi: p = 0.08); so one could assume that they are determined by their genome. The inventoried plants are generally of poor conformation, characterized by a strongly asymmetrical, open crown, with an irregular, tortuous, partly defective shaft. The bell-shaped, spread-out appearance of the stem distribution curve depending on diametric classes (Figure 10), according to Rollet (1969), gives the species a heliophilous temperament. This statement is consistent with the characteristics of the species according to Blaser et al. (1993): Dalbergia baronii has a nomadic temperament and is used in silvicultural enrichment.
Dalbergia chapelieri (Fabaceae)
D. chapelieri was met in Pointe à Larrée, Manombo and Mahabo. Considered to be a widespread species (Du Puy et al., 2002), variation in ecological types has little effect on the following phenotypic characteristics: crown and bole shape, DBH and adult tree biovolume. In relation to these variables, all individuals share common characteristics across their range: a tolerable crown shape (more or less circular in plan with some deficiencies of symmetry or with some dead branches), a poorly shaped bole (irregular, tortuous, partly defective) with a DHPa = 14.2 cm. On the other hand, in relation to the variable total height TH, the tree found in low and medium altitude humid forests have higher heights (THa = 7.8 m) than those inventoried on coastal forests (THa = 6.5 m) (ANOVA; p < 0.035). These variations can be explained by various factors, including species temperament, density and structure of associated vegetation, and competition for light (Rakotondrasoa et al., 2013) (Table 6).
Like D. baronii, the demographic characteristics and natural reproductive potentiality of the target species are highly dependent on the ecological variability of the habitat. A phenological shift between the seed-tree ST in the northeast and southeast has also been observed: the fruiting and maturing phenophase lasts two months (December - January) in the north and up to three months (November - January) in the south. This made it possible to identify two significantly different groups in terms of fruit and seed production capacity (Kruskal- Wallis; p < 0.001). On the one hand, individuals characterized by a relatively low average production of Na = 11 mature fruits/ m2/ indiv. ST were found in the North. And on the other hand, in the South, trees were found with an abundant fruit production capacity of up to Na = 30 mature fruits/ m2/ indiv. SM; even in agricultural wooded fields. But unlike Dalbergia baronii, no significant difference was observed between the availability of wildings under the crown of selected parent plants across its natural distribution area (Kruskal-Wallis; p = 0.103). The germination capacity of the species in its natural state is average, with an availability of Na = 20 wildings/ m2/ indiv. ST. However, the installation and development of young regrowth in the upper stages (thicket, saplings, young pole stands) varies according to habitat types. Soil type has been identified as the main differentiating factor. The installation of natural regenerations is more favored in the ecological type of low and medium altitude humid evergreen forests, on temporarily flooded alluvial soils on which natural regenerations are relatively good reaching a NRR of 255% on the Rothe scale (1964). In the sandy coastal plains of littoral forests, the density of natural regenerations is low, especially in disturbed environments and agricultural fields (NRR < 80%). This also seems to be linked to the semi-sciaphilous builder type temperament of the species, resulting from the negative exponential aspect of the curve (Figure 11) of stem distribution by diametric class (Rollet, 1969), where a significant opening of the canopy linked to the destruction of its habitat compromises the development and installation of young seedlings.

Faucherea tampoloensis (Sapotaceae)
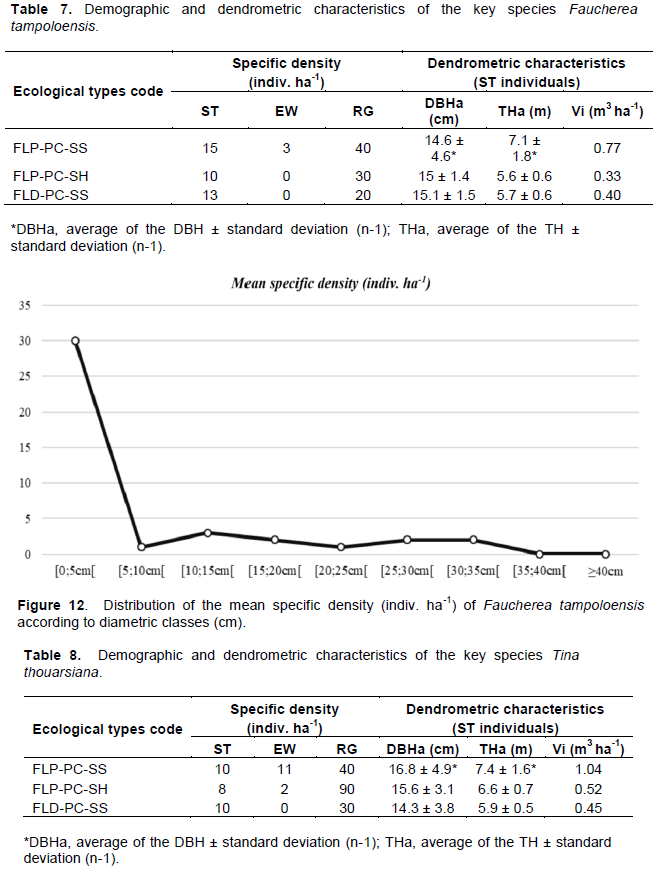
F. tampoloensis was found at two sites in the northeastern part: Pointe à Larrée and Tampolo. Throughout the species' natural distribution area, the variation in phenotypic characteristics of the inventoried trees, particularly the total height and timber potential, is strongly influenced by ecological types (ANOVA; TH: p < 0.003; Vi: p < 0.04). Two significantly different groups were identified with respect to these two dendrometric variables. The stems inventoried at Pointe à Larée have a higher total height (TH) and wood volume (Vi) (THa = 8.2 m; Vi = 0.88 m3 ha-1) than those found at Tampolo (THa = 5.6 m; Vi = 0.38 m3 ha-1). In contrast, the DBH does not vary according to habitat types with an average of DBHa = 14.9 cm over its entire distribution area (ANOVA; DBH: p = 0.78). Similar cases have been observed in the target species D. chapelieri, and other endemic species such as Uapaca bojeri, where trees with the same DBH have significantly different total heights TH (Rakotondrasoa et al., 2013). However, it should be noted that the state of habitat degradation is more significant in Tampolo than in Pointe à Larrée, which is the main factor differentiating the timber potential between these two sites.
The demographic behaviors of the target species are characterized by a small number of individuals in all diameter classes (Table 7). Among the main factors that influence this distribution is the phenological behavior of the species during flowering and fruiting phenophases. They are spread over about 4 months and are marked by the abundance of flowers. Aubreville (1974) had described that flowering in this species occurs from January to April; while phenological monitoring work at study sites showed that flower bud formation begins in October, followed by fruit bud appearance from December. This phenological lag could be considered as a form of adaptation of the species to climate change phenomena (Kramer, 1995). The species has advanced the onset of flowering and fruiting phenophases to meet its various needs (Kramer, 1995). However, this time lag coincided the period of evolution of floral buds to the stage of fruit bud formation, with the cyclonic periods when Madagascar's eastern coastal forests are subjected to repeated passage (Lisan, 2015). The flowers and fruits, small and very slight, do not resist strong winds. Very few bud fruits reach the ripening stage. The potential for fruit and seed production is, therefore, low throughout its natural range. The distance and location of the seed plants from the sea has a negative influence on their ability to produce fruit, and varies significantly according to the environment (Kruskal-Wallis; p < 0.006). Individuals located along the seaside are directly exposed to strong winds during cyclonic periods and have a low production capacity of Na = 5 mature fruits/ m2/ indiv. SM. On the other hand, trees further inland, at transect level, have a higher production potential of Na = 15 mature fruits/ m2/ indiv. SM. But, this variation does not affect germination capacity in the natural state where the availability of natural seedlings under the crown of the parent plants remains low throughout the species' range (Nm = 4 wildings/ m2/ indiv. SM). Consequently, over its entire range, the species has a low germination and regeneration capacity in its natural state (NRR < 80%). This low potentiality is particularly marked in the types of degraded forest formations (NRR = 40%). This is partly explained by the semi-sciaphilous character temperament (“sciaphilous builder type”) (Figure 12) of the species, where the large opening of the canopy tends to compromise seed germination, survival and development of natural seedlings to higher stages.
Tina thouarsiana (Sapindaceae)
The species has been found in three sites: Pointe à Larrée, Tampolo and Mahabo Mananivo. The ecological environment types have very little effect on the variation of the following phenotypic characteristics: crown and bole shape, DBH and biovolume (ANOVA; DHP: p = 0.43; Vi: p = 0.08). With an average DBH of 15.5 cm and a biovolume of 0.67 m3 ha-1 over its entire natural range, adult trees generally have a good crown shape: free from above, circular in plan, symmetrical and extended. As for the shape of the bole, it is tolerable: partially straight, conical and without serious defects. However, three significantly different groups were identified with regard to the total height TH variable (ANOVA; p < 0.02). In other words, as in the case of the D. chapelieri and F. tampoloensis species, for the same diameter, some inventoried trees are significantly higher than others (Table 8).
The state of degradation of forest formation types has been identified as a differentiating factor. Individuals in disturbed environments are smaller in size compared to those found in less-disturbed formations. This is related to the heliophilous character temperament of the species (bell-shaped appearance according to the Rollet distribution curve) (Figure 13); where trees in undisturbed areas, with a density of vegetation that is much higher, seek to prioritize growth in height for light competition.
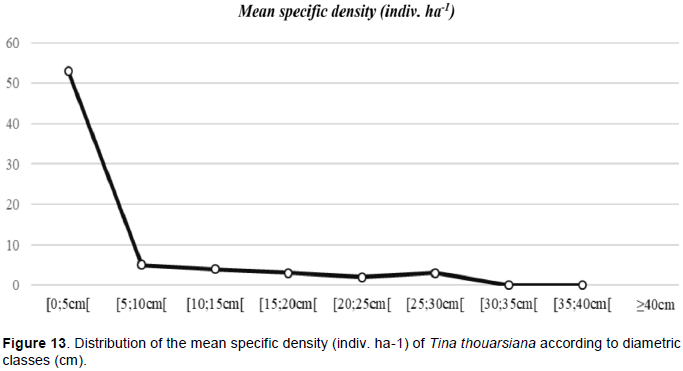
The flowering and fruiting phenophases of the target species are spread throughout the year, with a fruit ripening frequency of 3 to 4 times per year. During fruit ripening periods, the production capacity depends on ecological parameter variations (Kruskal-Wallis; p < 0.04). Production is abundant for seed trees in littoral forests with little disturbance, on sandy soils (FLP-PC-SS) with an average density of Na = 46 mature fruits/ m2/ indiv. ST. It is average for the other ecological types with an abundance of Na = 14 mature fruits/ m2/ indiv. ST. The availability of wildings under the crown of the seed trees, on the other hand, does not depend on the ecological types (Kruskal-Wallis; p = 0.06). Over its entire range, it appears that the species has good germination capacity with a high abundance of natural seedlings of Na = 20 wildings/ m2/ indiv. ST. But, the installation and development of these young regrowths are conditioned by the ecological conditions of their habitat. The installation of natural regeneration is favored by the types
of alluvial and temporarily flooded soils with a good NRR of 225%. In contrast, many natural seedlings (regrowth) fail to survive and develop to the next stages (thicket and saplings) on sandy soils; the natural germination rate is low (NRR < 100%). Also, the degree of degradation of the habitat has a significant influence on the conditions under which natural regenerations are installed. On the same type of soil (sandy), the NRR% is higher in degraded habitat types (NRR = 75%) than in low-disturbed areas (NRR = 50%). This variability is explained by the heliophilous character temperament of the species requiring the opening of the canopy and a less dense vegetation cover to promote optimal conditions for seed germination and the survival of young regrowth.
Actions for the conservation and sustainable use of key species
Several factors were taken into account in the elaboration of conservation and sustainable use models for the 6 key target species (Figure 14). The conservation status of these species according to the IUCN and CITES lists is one of the central elements allowing a hierarchization in regards to their vulnerability, from which the priorities for conservation actions have mainly been proposed.
The categories "Critically Endangered (CR), Endangered (EN) and Vulnerable (VU)" include so-called "threatened" species, which are at high risk of extinction in the wild and deserve special attention (Barneix and Gigot, 2013). A. amblyocarpa (CR), T. thouarsiana (EN), D. haraka (VU), D. baronii (VU) and D. chapelieri (VU) belong to this category. The two species of the genus Dalbergia are also listed in Appendix II of CITES, the trade in specimens of which must be regulated and all forms of exploitation must comply with the laws in force and use methods that are not detrimental to the survival of the species.
The taxon in the category of "Insufficient Data (DD)" may include unknown species that would be classified as threatened if a minimum of information on the status of their populations were available. F. tampoloensis belongs to this category. However, the IUCN and CITES statutes alone are not sufficient to define conservation and sustainable use priorities. They require the integration of complementary or associated factors. For the 6 key species, their demographic characteristics and the variability of their biological behaviors were chosen as additional factors to provide more supplementary information needed to propose and prioritize conservation measures. The sites most representative of the variability of these factors have been chosen as high priorities for conservation actions in order to better preserve the genetic resources of the species.
In relation to all these factors, species with the same characteristics have been grouped together (Figure 14) as listed in the following groups: high priority species (1), characterized by low fruit and seed production potential, low natural germination capacity and difficult natural regeneration; medium priority species (2), with relatively abundant fruit and seed production, good germination capacity in the natural state, but difficult natural regeneration; and low priority species (3) characterized by abundant fruit and seed, good germination and natural regeneration capacity. Each group thus formed was the subject of proposals for specific conservation and sustainable use measures in order of priority (grouping by multi-species approaches).
Species conservation actions are focused on two types of measures: "in-situ conservation", which aims to maintain the survival, increase and protect the population of target species against extinction by restoring their habitats and conducting natural regeneration or reproduction; and "ex-situ conservation", which focuses on the reproduction of species outside their natural habitats. As for sustainable use actions, they consist in improving the potential of key species in terms of production (wood and non-wood forest products) and reproduction.
Conservation actions
With a conservation status of "CR", the populations of Asteropeia amblyocarpa are characterized by a very restricted distribution throughout its natural range and have only been found in one site at the NPA Tampolo. In order to ensure the conservation and sustainability of the species, the extension and translocation of existing populations to an additional area of potential habitat is recommended, the installation conditions of which should meet the ecological requirements of the species. The same applies for the restoration of population size and distribution of species at risk to their historical level. If D. baronii has previously been encountered in Mahabo, it is essential to restore this species by establishing a new population. However, the recovery of species at their historic stations should not be considered as a replacement for the protection and management of existing stations.
Thus, priority conservation measures essentially consist in maintaining existing populations through "in- situ" actions. The first step is to restore the current and future habitats of key species, but also of fauna species that play an important role in their natural reproduction, through enrichment and forest restoration actions by well-adapted and valuable species; then to maintain the current populations of the target species through assisted natural regeneration (ANR) techniques. It consists in materializing and protecting young seedlings from natural formations, and in complementing natural regeneration with artificial regeneration. These actions concern in particular highly (1) and medium (2) priority species.
To this end, the production of seedlings in nurseries meets these various objectives, as a support for "in-situ" conservation, but also for the strengthening of "ex-situ" populations. However, the establishment of nurseries and plant production activities require the supply of seeds and vegetative fragments, and should take into account the propagation and reproduction techniques, sexual or vegetative, adapted for each target species. Seed germination tests in experimental devices are recommended for species with low natural germination capacity, namely D. haraka, D. baronii and F. tampoloensis. At the same time, vegetative propagation tests (cuttings, layering) are also recommended for these groups of species, but especially those with low fruit and seed production potential. On the other hand, for groups of species where fruit and grain production are abundant (A. amblyocarpa, D. chapelieri, T. thouarsiana), the "ex-situ" conservation of reproductive material in seed banks is one of the best ways to prevent the loss of genetic biodiversity and thus guarantee a future for threatened species (Bacchetta et al., 2006).
For example, not only they make it possible to save seeds of species with delayed germination and whose germination requires a conservation period to emerge, such as D. haraka, but they also provide and preserve breeding material from potential new sites for population expansion through domestication and reintroduction. The aim is, therefore, not only to keep a large number of seeds in the bank; but to know the plant material in various aspects in order to guarantee the conservation of a site's biodiversity. However, transfer and relocation should only be considered to increase the size of existing stations, create new populations or restore the areas of occurrence and occupation of target species. In such cases, donor populations should be closely monitored to ensure that they do not decline as a result of the harvesting of individuals or propagating material.
Sustainable use actions
Concerning the priority actions to improve the production and reproduction potential of the target species, specific measures are recommended for parent trees and juveniles. But first, the habitat of these key species must be maintained to satisfy and promote their installation conditions. For this purpose, passive and active restoration actions are recommended. The main techniques of so-called “passive restoration” aim to promote natural self-restoration and regeneration through individual silvicultural treatments, and by reducing pressures and habitat degradation factors (e.g., firewalls, protection of species' areas of occurrence, and traditional social convention). As for “active restoration”, it involves planting and/or enrichment actions in the strict sense of the term, with the aim of restoring the ecological connectivity of forests or enriching fallows with species of interest that are often overexploited by populations.
Different actions may be necessary: temporarily defending the areas of occurrence of seed trees against various biological and anthropogenic threats; promote seed germination and seedling survival of target species and reduce competition through depressing natural seedlings (wildings and/or natural regeneration), selective thinning, cutting trees or shrubs, brushing work for the benefit of target species with light-demanding character such as D. haraka, D. baronii and T. thouarsiana; and through enrichment planting for shade-tolerant species like A. amblyocarpa, D. chapelieri and F. tampoloensis, in order to make the associated vegetation more dense. They also consist in choosing objective trees (“elite trees”) which are individuals of good phenotypic quality, vigorous, in good health and dominant on which is concentrated the production of quality and sufficient production (reproductive material, wood and non-wood forest products). The aim is to select a limited number of trees early among the best subjects in a stand and to carry out silvicultural operations for their benefit. These silvicultural operations, such as artificial pruning, should optimize the use of resources by improving the phenotypic quality of juvenile and adult trees in order to enhance the quality of the wood. The forest technique to be implemented is also part of a dynamic tree silviculture, ensuring that the selected trees are placed in optimal growing conditions. For example, sufficient early thinning is needed for the objective-trees to allow priority removal of dominant bothersome associated trees.