ABSTRACT
The influence of ecological factors on the behavioral pattern in animals is attracting research interest, particularly for drill monkeys. The aim of this study was to check if ecological changes influence behavior of captive drill monkeys. The study was carried out in Limbe Wildlife Center (LWC) from May 15th 2016 to August 15th 2016 while scan and focal samples were collected in mixed strategy. Continuous sampling started from 6:00 in the morning and ended at 6:30 in the evening, where the following behavioral categories were recorded: feeding, foraging, movement, resting, socialization, grooming, play, aggression and vocalization. Simultaneously, data were recorded on weather changes. Data analysis in this survey comprised of the descriptive and inferential statistics. The study showed a significant difference for the daily activity at different time ranges (X2=172.282 df =24 P<0.05). There was no significance between 6:8.59 am to 12:2.59 pm for foraging (X2=0.103 df =1 P=0.749). In addition, there was significance on foraging from 12 am to 2.59 pm and 3 to 6:30 pm (X2=9.607 df =1 P<0.002). The drills spent more time resting between 6 to 8.59 am and 12 to 2.59 pm (X2=6.164 df =1 P<0.013). There is a significance for feeding between 6 to 8.59 am and 3 to 6.30 pm for (X2=85.63 df =1 P<0.05). The results show that the drills spent more time feeding in the morning period than in the evening period of the day, but correlated with foraging behavior. There is a positive correlation between resting and weather changes (rain and sun) R2=0.11 P<0.05. All the age sex class categories spent less time foraging. (X2=12 df =1 P<0.05) is for time resting during the wet and (X2= 94.6 df=1 P<0.05) for sunny weather. This study revealed that ecological factors like weather and photo-period influence the behavior of drill monkeys in captivity.
Key words: Weather changes, wildlife, vocalization, habitat, behavior.
The behavioral study in wildlife is a key element for proper management and conservation purposes in captivity. It plays an eminent role in understanding both the causes and solutions to threaten species (Eadie et al., 1998). Understanding the ways in which animals sense and respond to their environment can provide crucial contexts for the preservation of viable populations in captive habitats. Studies of behavioralecology can provide significant contributions to conservation through evolutionary and ecological perspectives of how animals adapt to their environment (Krebs and Davies, 1993). Captive studies can also aid in understanding aspects of species-specific behavior, especially when behaviors are difficult to observe in the wild.
Zoos provide advantages to researchers by allowing longitudinal studies of behavior and reproduction, as well as opportunities for gathering data on all aspects of life history (Hardy, 1996). More so, preserving the behavioral and developmental diversity of animals maintained in captivity allows zoos to achieve their full potential in conservation. Captive propagation efforts and reintroduction programs in particular are dependent on captive animals, exhibiting normal reproductive and behavioral repertoires.
Primates attract attention of many researchers because they are closely related to human in terms of human social behavior. According to Chalmers (1979), primates are social animals and most of them interact with each other in their species. Social behavior refers to any behavior that involves another person (Else, 1991). Studies have shown that primate social behavior is more or less similar to human behavior such as eating, playing, fighting, keeping the baby and others (Rod, 1992).
Activity budgets for primates in disturbed areas such as human settlements are different from those in their natural habitat (Krebs and Davies, 1993). Many serious ecological changes had occurred due to the increasing human population and development of agricultural areas (Krebs and Davies, 1993). Primates have to change their daily behavior according to the environment to ensure their survival. Many studies have shown that the activity budgets vary by several environmental factors include diet, distribution and food sources (Passamani, 1998). It has been shown that the activity budgets of the species are influenced by several environmental factors, such as weather season as well as distribution and food sources (Passamani, 1998; Sato, 2012; Oates and Butynski, 2008). Studies of activity budgets in drill monkeys have shown that, the time spent in different activities vary diurnally and seasonally within age sex class groups in the rainforest and shrub land habitats.
The knowledge of the proportion of time that individuals spend on different activities during a day is important for understanding ecology and life-cycle of the studied species. The activity budget of the drill monkeys indicates how the species group interacts with environment and shows the investment of time, necessary for the understanding of its survival strategy (Defler, 1995). The variation in time budgets between primate species has been shown to fit certain physical traits and environmental conditions; for example the proportion of the time spent foraging is positively correlated to body weight and negatively correlated to the proportion of foliage in the diet (Clutton-Brock and Harvey, 1985). Time budgets are influenced by group size, habitat quality and proximity to human settlements (Singh and Vinathe, 1990). Very little research has been done on the activity budget of drill monkeys and mandrills in the wild and captivity. Social behavior in mammals is governed by bonding relationship, which consist mostly of affiliative interactions and dominant relationship that are established and maintained with agonistic interactions used in determining social status (Sachser et al., 1998).
The behavioral ecology of the drill monkeys may be quite different in their captive environment as compared to the wild. This is important for zoo biologists and conservationists to understand if the preservation of a species in a wild state is the main purpose that is for reintroductions. To preserve behavioral diversity among animals maintained in zoos, it is important for captive animals to develop normal behavioral repertoires (Carlstead, 1996). Environmental factors including weather changes and photoperiod have been suggested as proximate factors influencing primate behavior. The role played by these environmental factors on the group behavior of social drill monkeys in captivity is not fully understood (Harrison and Dukelow, 1973).
The main aim of this study is to find evidence on, the roles that these two environmental variables may play on the activity budget of the drill monkeys in Limbe Wildlife Center, Moreso. This study is important in developing our knowledge on the behavioral ecology of the drill monkeys that live in captivity, to clearly understand the daily activity budgets of this primate species which leads to its effective management.
Description of the study area
The Limbe Wildlife Center (LWC) is located in the center of the city Limbe (SW Cameroon, 4.1°27.12°N, 9.12°53.64°E), which was established in 1993 by the Cameroon’s Government and the Pandrilus Foundation. The location is crossed by roads, and situated near Limbe City Council. All species harbored in this place had been donated by the local hunters or confiscated by the Government of Cameroon. The LWC primarily helps to rescue these species and reintroduce them to natural environment in mount Cameroon national park. The climate of Limbe area including the reintroduction site, Mount Cameroon National Parkis characterized by a period of heavy rains occurring from the months of June to October, and a dry period extending from November to May. At lower altitude, the annual rainfall ranges from 1,000 mm3 at Cape Debundscha to less than 2,000 mm3 in the north-east around Munyenge area (Figure 1).
The mean annual rainfall decreases with altitude to approximately 4,000 mm3 at 1000 m and less than 3,000 mm3 above 2,000 m (Payton, 1993).The temperature falls with increasing elevation where mean air temperature is 26.78°C, with monthly values ranging from 24.98°C in August, the rainiest month. Payton (1993) points out that, the humidity remains at 75 to 85% due to the influence of the marine ecosystem.
Zoo is enclosed with a strong wire-net fence with an estimated height of 10 m and a circumference of 400 m. In the heart of this enclosure is the drill monkey cage occupying an estimated area of 900 m2.The wire-net cage with 100 has a few trees and a constructed woody stand device serving the 100 drill monkeys in the cage for climbing.
Data collection
The research data on the drill monkeys was collected during four months (from 15th May to 15th August, 2016), six days each month. Preliminary non formal observation was carried out to determine the behavior categories of the subjects (Md-Zain et al., 2008b).
Preliminary, observation is critical for the observer to be familiar with the subjects and their behaviors, thus enabling them to choose the right measures and recording methods (Martin and Bateson, 1993). The enclosure was divided into seven observational areas called zones; each zone had its distinctive point for clear identification. Behavioral observations began in the morning between 6:00 and 6:30 am and ended at 6:30 pm each day of the study. Data were collected using instantaneous scan sampling at predetermined intervals. Martin and Bateson (2007) define “instantaneous scan sampling” as when “a whole group of subjects is rapidly scanned, or “censured,” at regular intervals and the behavior of each individual at that instant is recorded.”
Behavioral data can be collected in several ways (Altmann, 1974). In categorizing these methods, Martin and Bateson (2007) distinguish between sampling rule (whose behavior is watched and when) and recording rules (how the behavior is recorded). Hence, the scan sample data for this survey was collected after every 10 min (Altmann et al., 1993). Between the 10 min of scan, sampling a focal sample was conducted for 5 min. All the scan observations were done from right to left throughout the study. The focal animal was randomly selected for the day, based on the age sex classes. The drill behaviors were recorded during scan and focal. The following behaviors were recorded; feeding, foraging, locomotion, social behaviors and resting.
The frequency data generated were analyzed by the use of exploratory statistical distribution tool for each observed behavior in the study. Perason chi-square was also used to compare the different activity budget for the behavior of each sex age class in the drill group.
From Figure 2, feeding behavior has the highest frequency at 10 am and 4 pm while foraging has its peak at the hours of 7 am and 5 pm. Resting behavior was observed with a significant drop at 4 pm and observations were done each day between 6 am and 6:30 pm as shown in Figure 3.There is a significance for the daily activity at different time ranges (X2=172.282 df =24 P<0.05). There was no significance between 6:8.59 am to 12:2.59 pm for foraging (X2=0.103 df =1 P=0.749) however, there was a significant difference on foraging from 12 am to 2.59pm and 3 to 6:30 pm (X2=9.607 df=1 P<0.002) where the drills spent more time resting from 6 to 8.59 am and 12 to 2.59 pm (X2=6.164 df=1 P<0.013). Also, there was a significance for feeding between 6 to 8.59 am and 3 to 6.30 pm (X2=85.63 df =1 P<0.05). The drill group spent more time feeding in the morning than in the evening period of the day (Table 1).
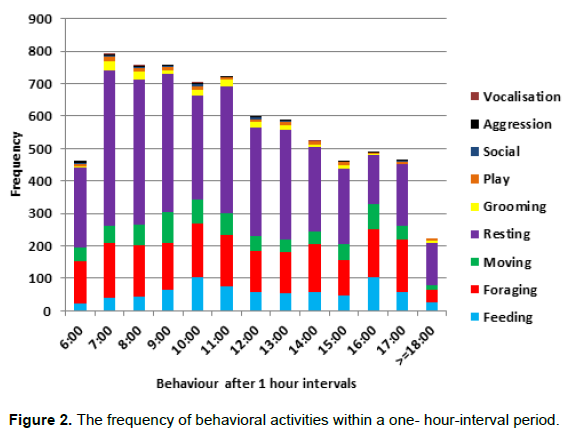
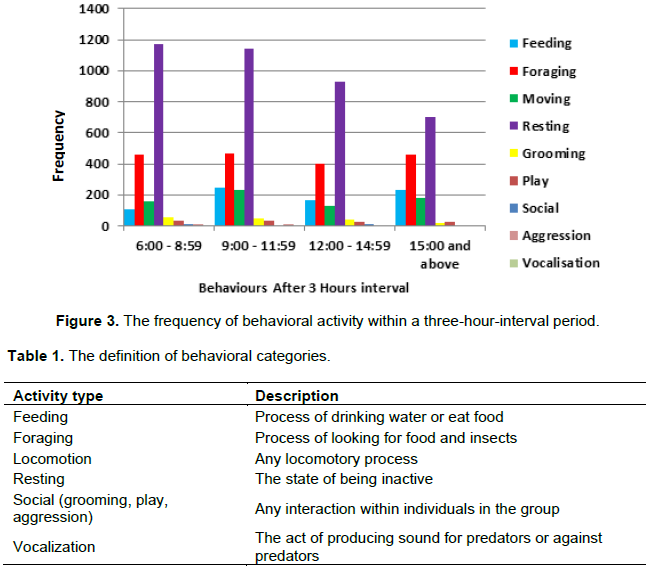
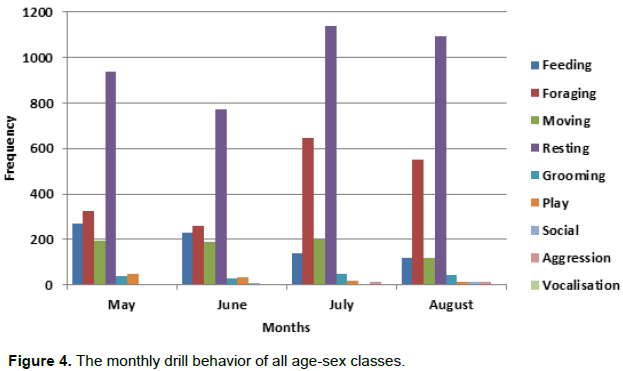
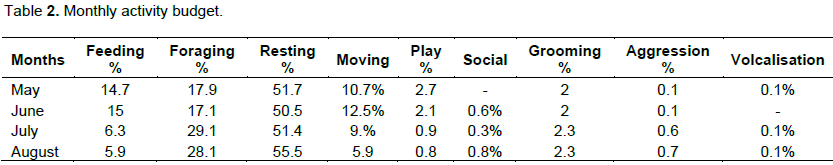
Table 2 shows the monthly time budget for the behaviors in which resting was the highest throughout the months with a mean value of (51.7%) followed by foraging with a mean value of (22.2%), feeding with a mean value of (10.25%) and movement with (9.5%). Furthermore, there is a significance between movement in the month of May and August (X2=27.52, df = 1, P< 0.05). There also exist no significance between resting in the month of May and August (X2=6.82, df = 1, P = 0.009) and no significance in the Months of May and June (X2= 0.464, df = 1, P > 0.05).
Resting behavior of the drill group was dominant and peaked in the month of July and August as shown in Figure 4. Foraging also was recorded high in July and August as compared to the other months. Movement behavior was reduced in August as compare to other months due to heavy rains that would always cause the drill group to rest. Other social behaviors like aggression, play, grooming and vocalization were also varied during this period of time.
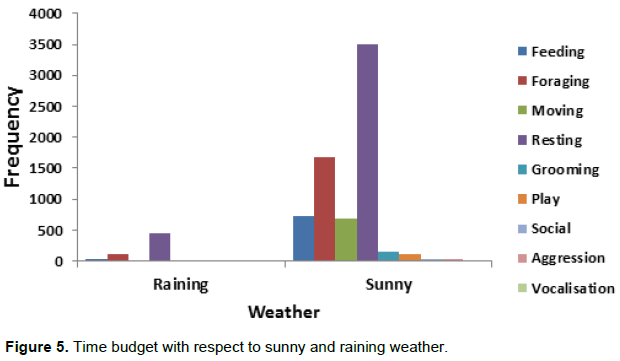
Rain fall actually brought a change in the time budget, much time was spent resting the reason for which it recorded the highest activity budget (Figure 5). Table 3 has shown a positive correlation between resting for raining and sunny weather (R2=0.11 P<0.05). All categories of age-sex class spent less time foraging (X2=12 df =1 P<0.05) and more time resting during raining than sunny weather (X2= 94.6 df=1 P<0.05). There was no significance for grooming and playing on weather changes (P>0.05).
Activity budget in the drill monkeys
Primates can flexibly adjust their activity budgets to deal with environmental changes. The amount of time that primate individuals allocated to each activity is affected by ecological and social factors (Gursky, 2000; Di and Fiore Rodman, 2001). This survey reveals that, the time budget for different behaviors differed significantly in daily observations. This agrees with study of Umapathy and Kumar (2000) on Macca silenus that showed fluctuation in daily activity budget.
Resting
Resting was the dominant behavior in this study. The group was observed spending resting time in sitting postures, quadrupedal standing and lying postures. During this period, the hind limbs were placed in a variety of position while the fore limbs often rest on the knees or between the hind limbs. Resting time however, is an unlimited source of additional time not only an absolute limit which is imposed by the fact that, there are only 12 h of the day light in the tropical day but some resting time may be for some other purposes like vigilance, allowing tired muscles recover, digestion, or simply as time gaps between successive bouts of activity.
The use of resting time for digestion may be important in drill monkeys, especially after feeding on food rich in fibers or leaves in order to extract nutrients from plants structural elements (Gursky, 2000). In addition to time required for digestion, environmental conditions may improve limitation on the amount of time that the animal can be active. This is particularly relevant in habitats characterized by high temperature where animals may be obliged to rest in the shade during the hottest part of the day. Furthermore, the animal groups’ feeding was programmed two times a day only causing the group to rest for longer periods.
Monthly time budget for the drill monkeys
This study shows the month of May with the highest activity while the August has the least. This supports the study of Di Fiore and Rodman (2001) who found that primate activity, feeding, foraging, resting, grooming, movement, vocal, social, aggression, and play are affected by rainfall intensity in the tropics. Following strategy two above, their results suggest that rather than increasing time dedicated to foraging or feeding, the monkeys rested more when fruit availability was lower.
In contrast to Isbell and Young (1993), study of Cercopithecus feeding and foraging time increased significantly in the season, in which the animals’ diets were limited almost entirely to the seeds of single plant species with presumed high handling time. Numerous studies have demonstrated that primate activity patterns change over time in response to seasonality Oates (1987) and Di Fiore and Rodman (2001). In consequence, there are seasonal peaks and troughs in the abundance of particular primate foods Oates (1987). When resource availability goes down, animals are expected to either increase time dedicated to feeding and foraging, thus an increase in energetic costs of finding food and reduced feeding is selective at the expense of resource quality which also generally minimize energy expenditure. The diets of drills in captivity do not depend on the seasons or monthly periods, where food is provided every day. This means that their time budget cannot be influenced by fruits scarcity or abundance.
Weather influence on the social behavior of drill monkeys
The maintenance of homeothermy in primates involves a combination of both autonomic and behavioral processes, where behavioral changes should be used first as a means to conserve the water and energy, required for autonomic processes (Dunbar, 1992). Direct weather constraints on activity budgets have received little attention. It is known that at high temperatures, some the animals tend to spend more time resting (Hill, 2006; Campos and Fedigan, 2009; Korstjens et al., 2010; Sato, 2012; Majolo et al., 2013), and that resting and shade-seeking which are critical for thermoregulation (Campos and Fedigan, 2009): high heat load can cause severe dehydration and potentially fatal hyperthermia. In contrast to the reduced demand for shade-seeking and resting in colder temperatures, the energetic demands of thermoregulation and digestion are higher in cold conditions, which mean that more time are needed to be spent on feeding (Satinoff, 2011; Majolo et al., 2013).
Grooming in drill monkeys is a very prominent form of social activity that was observed in this group early in the day, during the mid-day quiet period, and when the animals approach the sleeping site in the evening period of the day. The adult females with juveniles were observed groomed more by the adult males. This agrees with the findings of Silk et al. (2009) which states that grooming in primates is more frequent with lactating adult females. Majority of social primates spend time in maintaining their grooming relationships with conspecifics, which may detract them from their available feeding and resting time.
Captivity remains a key place to restore the decrease population of the endangered species in the wild. Drill scheduled their daily time into particular activities while foraging is the most intensified at the morning and evening resting at 12 to 14h.
The associations of these groups resemble that of any primate, where affiliative and agonistic interactions always keep individuals apart and some closer. The present decline of drill in the wild, paints a picture of the world in which the most second sexually dimorphic species to mandrills shall be found extinct. Wild drills habituate thick forest, usually foraging on the forest floor which possessed litter leaves, rotten logs of wood; where termites, insects are founds with other arthropods. Resting was the dominant activity recorded in the study, due to their relatively small enclosure with a two times feeding daily programme. Little had been seen on this species climbing the platform which may be considered as trees, male adult are regular individuals on the floor resting. Even though drill monkeys show high sexual dimorphism, male drills jump across trees with little stress looking for stable branches (Astaras, 2009).
The survey revealed a deviation in the activity budget for this group of captive drills, while their natural behaviors are being affected in captivity. Foraging, feeding and vocalization are key activities for their survival in a natural habitat, but when these behaviors are altered the changes of surviving in the wild might be limited. In case of any reintroduction or release programme, I strongly recommend a soft, semi-provisioned food release before a hard release. Drill will exhibit all their social behavioral repertoires which constitute agonistic and affiliative interactions. Certain behaviors like movement/resting should frequently be checked on age/sex classes because so much resting may be due too much food intake by male adults, which can result to diseases.
According to the results obtained from the study; resting, foraging, feeding and moving were the most dominant behaviors while grooming, playing, social, aggression, and vocalization were the least dominant behavior. This study is important in order to understand clearly the daily activity budgets of drill monkeys on ecological changes in captivity, leading to the effective management and conservation of this species in the future.
The authorities should budget and provide enough food and medical care for the drill monkeys in the zoo to normalize some of the social behaviors like resting believed, which will be high unprecedentedly. The zoo enclosure and cages should be equipped climbing, playing, feeding and resting facilities to simulate the wild environment where these animals are being prepared for reintroduction.
Animals should be kept whenever possible, in social groups that are most reflective of natural social systems. Keepers and collection managers will have to closely monitor newly placed males and remove them, where the males experience higher than normal levels of aggression. To ensure that captive animals are experiencing an environment that best represents the wild condition, animals should not be housed together with other species unless there is natural overlap in their ranges. Species with overlapping range are less likely to promote stress responses (assuming we are not housing prey with predators).
The authors declared no conflict of interest.
REFERENCES
|
Altmann J (1974). Observational study of behavior: sampling methods. Behavior 48:227-265.
Crossref
|
|
|
|
Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM (1993). Body size and fatness of free-living baboons reflect food availability and activity levels. Am. J. Primatol. 30:149-161.
Crossref
|
|
|
|
Astaras C (2009). Ecology and Status of the Drill (Mandrillus leucophaeus) in Korup National Park, Southwest Cameroon: Implications for Conservation. PhD thesis, Georg-August University of Gottingen pp. 36.
|
|
|
|
Campos FA, Fedigan LM (2009). Behavioral adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. Am. J. Phys. Anthropol. 138:101-111.
Crossref
|
|
|
|
Carlstead K (1996). Effects of captivity on the behavior of wild mammals. In: Kleiman D, Allen M, Thompson K, Lumpkin S, editors. Wild Mammals in Captivity: Principles and Techniques. Chicago: The University of Chicago Press. Pp. 317-333.
|
|
|
|
Chalmers N (1979). Social Behavior In Primates. London: Edward Arnold (Publisher) Limited.
|
|
|
|
Clutton-Brock TH, Harvey PH (1985). Species differences in feeding and ranging behaviour in primates. In: Clutton-Brock TH, editor. Primate Ecology: Studies of Feeding and Ranging Behaviour in Lemurs, Monkeys and Apes. London: Academic Press. Pp. 557-584.
|
|
|
|
Defler TR (1995). The time budget of a group of wild woolly monkeys (Lagothrix lagotricha). Int. J. Primatol.16(1):107-120.
Crossref
|
|
|
|
Di Fiore A, Rodman PS (2001). Time allocation patterns of lowland woolly monkeys (Lagothrix lagotricha poeppigii). Int. J. Primatol. 22(3):449-480.
Crossref
|
|
|
|
Dunbar RIM (1992). Time: a hidden constraint on the behavioural ecology of baboons. Behavioral Ecology and Sociobiology, 31, 35–49.
Crossref
|
|
|
|
Eadie JM, Semel B, Sherman PW (1998). Conspecific brood parasitism, population dynamics, and the conservation of cavity-nesting birds. in T. Caro, ed. Behavioral ecology and conservation biology. Oxford University Press, New York. Pp. 306-340.
|
|
|
|
Else JG (1991). Nonhuman primates as pest. In: Box, H.O. (ed). Primates Responses to Environmental Change. Pp. 115-165.
Crossref
|
|
|
|
Gursky S (2000). Effect of seasonality on the behavior of an insectivorous primate Tarsius spectrum. Int. J. Primatol. 21:477-495.
Crossref
|
|
|
|
Hardy D (1996). Current Research Activities in Zoos. In: Kleiman D, Allen M, Thompson K, Lumpkin S, editors. Wild Mammals in Captivity: Principles and Techniques. Chicago: The University of Chicago Press. Pp. 531-536.
|
|
|
|
Harrison RM, Dukelow WR (1973). Seasonal adaptation of laboratory maintained squirrel monkey (Saimiri sciureus). J. Med. Primatol. 2:277-283.
|
|
|
|
Hill RA (2006). Thermal constraints on activity scheduling and habitat choice in baboons. Am. J. Phys. Anthropol. 129:242-249.
Crossref
|
|
|
|
Isbell LA, Young TP (1993). Seasonal and ecological influences on activity budgets of vervet monkeys and their implications for group living. Behav. Ecol. Sociobiol. 32:377-385.
Crossref
|
|
|
|
Korstjens AH, Lehmann J, Dunbar RIM (2010). Resting time as an ecological constraint on primate biogeography. Anim. Behav. 79(2):361-74.
Crossref
|
|
|
|
Krebs JR, Davies NB (1993). An introduction to behavioral ecology. Blackwell Scientific Publications, London.
|
|
|
|
Majolo B, MacFarland R, Young C, Qarro M (2013). The effect of climatic factors on the activity budgets of Barbary macaques(Macaca sylvanus). Int J Primatol 34:500–514
Crossref
|
|
|
|
Martin P, Bateson P (1993). Measuring Behavior: An Introductory Guide. 2nd Edn., Cambridge University Press, Cambridge, ISBN: 978-0521535632, Pp. 238.
Crossref
|
|
|
|
Martin P, Bateson P (2007). Measuring behavior: An introductory guide( 3rd Edition). Cambridge: Cambridge University Press.
Crossref
|
|
|
|
Md-Zain BM, MY Yen, IA Ghani (2008b). Daily activity budgets and enrichment activity effect on Chimpanzees (Pan troglodytes) in captivity. Sains Malaysiana, 37:15-19.
|
|
|
|
Oates JF (1987). Food distribution and foraging behavior. In "Primate Societies" (B.B. Smuts, D.L. Cheney, R.M. Seyfarth, R.W. Wrangham, T.T. Struhsaker, Eds.), pp. 197-209. University of Chicago Press, Chicago.
|
|
|
|
Oates JF, Butynski TM (2008). Mandrillus leucophaeus. IUCN Red List of threatened species.
View
|
|
|
|
Passamani M (1998). Activity budget of geoffroy's marmoset (Callithrix geoffroyi) in an Atlantic forest in Southeastern Brazil. Am. J. Primatol. 46:333-340.
Crossref
|
|
|
|
Payton RW (1993). Ecology, altitudinal zonation and conservation of tropical rainforest of Mt Cameroon. Report to the Overseas Development Administration, London. Pp. 251.
|
|
|
|
Rod PMK (1992). Primates of the World. London. Blandford Villiers House.
|
|
|
|
Sachser N, Dürschlag M, Hirzel D (1998). Social Relationships and the Management of Stress. Psychoneuroendocrinology 23:891-904.
Crossref
|
|
|
|
Satinoff E (2011). Behavioral thermoregulation in the cold. Compr. Physiol.14:481-505.
Crossref
|
|
|
|
Sato H (2012). Diurnal resting in brown lemurs in a dry deciduous forest, E8 GUAN, et al. Zoological Research www.zoores.ac.cn northwestern Madagascar: implications for seasonal thermoregulation. Primates 53(3):1-9.
|
|
|
|
Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL (2009). The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R Soc. Lond. B Biol. Sci. 276:3099-3104.
Crossref
|
|
|
|
Singh M, Vinathe S (1990). Inter Differences in the Time Budgets of Bounet Monkeys (Macaca radiata). Primates 31:589-596
Crossref
|
|
|
|
Smuts B, Cheney D, Seyfarth R, Wrangham R, Struhsaker T (1987). Primate societies. Chicago: The University of Chicago Press.
|
|
|
|
Umapathy G, Kumar A (2000). The demography of the lion-tailed macaque (Macaca silenus) in rain forest fragments in the Animalai Hills, South India. Primates 41:119-126.
Crossref
|