ABSTRACT
Malaria and septicaemia, both major causes of infant and early childhood morbidity and mortality in Nigeria, often co-exist and are difficult to differentiate. This study was designed to test the hypothesis that C-reactive protein (CRP) levels could differentiate between malaria and malaria coexisting with septicaemia. One hundred and fifty-one children aged 6 to 60 months with fever without localising signs and 141 aged/sex-matched controls were studied. C-reactive protein levels in all the children were determined while the febrile children had their blood cultures done. ANOVA and students‘t’ test were used to determine the difference between groups. Sensitivity, negative predictive and positive predictive values for malaria coexisting unit septicaemia were calculated for various levels of CRP. One hundred and thirty (86.1%) of the subjects had malaria alone while 21 (13.9%) had malaria coexisting with septicaemia. Organisms isolated were mainly Enterobacteriaceae (7), Staphylococcus aureus (9), Salmonella spp. (4) and Streptococcus pneumoniae (1). The mean serum CRP levels in subjects with malaria alone and malaria coexisting with septicaemia were 82.16 ± 44.94 mg/l and 108.44 ± 55.65 mg/l respectively (P=0.0176). At the diagnostic level of 90 mg/l (value just greater than the mean for malaria alone), CRP was highly sensitive (sensitivity 76.2%) in detecting septicaemia in 21 subjects with co-morbidity while specificity (36.9%) was low. It is concluded that CRP can differentiate between malaria and malaria with septicaemia. In children with malaria, antibiotics should be started at the CRP level of ³ 90 mg/l.
Key words: Malaria, septicaemia, C-reactive protein, diagnosis.
Malaria infection, and septicaemia defined as the presence of infection (in the blood) together with systemic manifestations of infection (Levy, 2003; Dellinger, 2013), are major causes of infant and early childhood morbidity and mortality in Nigeria (Adepoju et al., 2017; Akpede et al., 1993). Both often co-exist (Akpede et al., 1993; Tupchong et al., 2015). While each has different modality of treatment, clinical differentiation can be difficult. This causes delays in treatment and worsens outcome. In addition, attempt to use laboratory parameters like white blood cell count, absolute neutrophil count and erythrocyte sedimentation rate to differentiate malaria from malaria coexisting with septicaemia have not been sufficiently reliable (Rasmussen and Rasmussen, 1982; Enyuma et al., 2015). However, C-reactive protein CRP, an acute phase reactant, has been found useful in differentiating bacterial from non-bacterial infections in various circumstances (Liu et al., 2013; Sabel et al., 1974). Though malaria infection is known to stimulate CRP production, we hypothesized that malaria co-existing with septicaemia would stimulate even more CRP production. This work was designed to determine the value of C-reactive protein levels in differentiating between malaria alone and malaria with septicaemia in children.
Consecutive studies of 292 (151 febrile subjects without localizing sign/signs and 141 age/sex matched healthy controls) children, aged 6-60 months were carried out. These were attending the Children Emergency Room (CHER) and the Child Welfare Clinic (CWC) of the University of Calabar Teaching Hospital (UCTH) Nigeria. All had their blood films (thick and thin) examined for malaria parasite after staining with Giemsa stain and read under ´100-power microscope (WHO, 1991). In addition, blood culture (not done on controls), was done and antibiotic sensitivity of isolated organisms was determined by the Disk Diffusion Method (Tendencia, 2004). Erythrocyte sedimentation rate (ESR) (Westergren method) was also done. Total white blood cell count (WBC) absolute neutrophil count (ABNC) were also done within 2 h of collection of sample by an Automated Haematology System Cell Counter (ADVIA(g) 60 Closed Tube (CT) manufactured by Bayer Cooperation, New York, United States of America. Serum CRP was estimated for both subjects and controls using High Sensitivity Enzyme Immunoassay (EIA) kit – Kalon Biological Ltd., U.K. Data were analysed in groups using EPI info version 6; 2002. ANOVA and Students t-test were used to determine the significance of the difference between three and two groups respectively. A p-value of < 0.05 was regarded as significant. Sensitivity, specificity, positive predictive value and negative predictive value for malaria coexisting with septicaemia were calculated for various levels of CRP. Ethical clearance was obtained from the Reasearch Ethics Committee of the University of Calabar Teaching Hospital, Calabar, Nigeria.
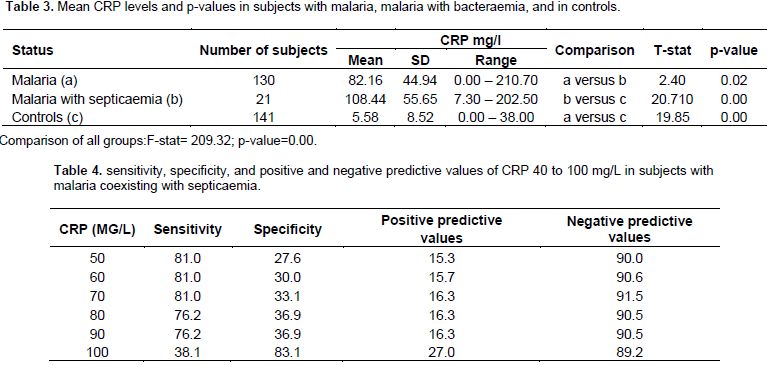
One hundred and fifty one subjects were recruited. Eighty-six (57.0%) were males while 65 were females (43.0%) giving a male to female ratio of 1.3: 1. One hundred and thirty (86.1%) subjects had malaria alone while 21 (13.9%) had malaria coexisting with septicaemia. Organisms isolated were Staphylococcus aureus (9), Enterobacteraeciae (7), Salmonella spp. (4) and Streptococcus pneumoniae (1) most of which were sensitive to gentamycin and ceftriaxone (Table 1). The mean age of those who had malaria with coexisting septicaemia was 20.28 ± 9.27 months with a range of 6 to 38 months. The means of WBC count, absolute neutrophil count, and ESR could not differentiate subjects with malaria alone from those with malaria coexisting with septicaemia (Table 2a and b). The range of CRP levels for children with malaria alone was 0.00 – 210.70 mg/L and for children with malaria and septicaemia 7.30-202.80 mg/dL. The respective means were 82.16 ± 44.94 mg/l and 108.44 ± 55.65 mg/L respectively. This difference was statistically significant at p<0.0176 (Table 3). At a diagnostic level of 90 mg/l (value greater than the mean for malaria alone), CRP was highly sensitive (sensitivity=76.2%) in detecting septicaemia in 21 subjects with co-morbidity while the specificity was low (36.9%) (Table 4). Out of 141 aparasitaemic controls, 37 had serum CRP values above normal (0.2 to 6.0 mg/l).
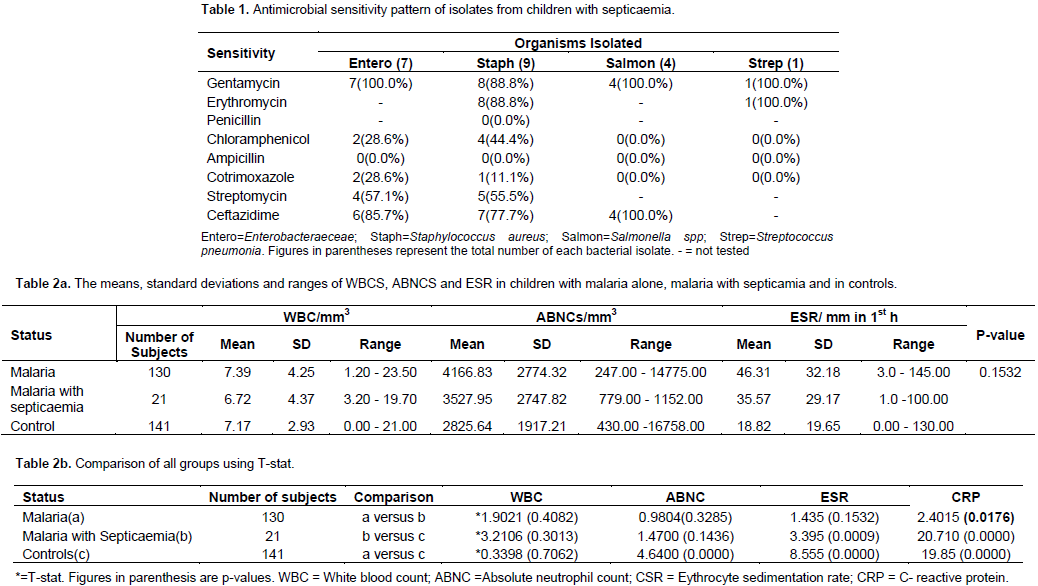
This study has confirmed a high prevalence (13.9%) of septicaemia coexisting with malaria in young children. The main isolates were Enterobacteriaceae, S. aureus and Salmonella spp. Most (88.8%) of these isolates were sensitive to gentamycin and ceftriaxone. The high prevalence of septicaemia in children with malaria has been attributed to possible impairment of the capacity of monocytes that have ingested erythrocytes to phagocytose and kill bacteria that invade the blood stream. Also, the immune system of children with Plasmodium falciparium malaria is thought to be further impaired by a transient loss of B-cell function and reduction in T-lymphocytes (Weinstein and Swartz, 1974; Whittle et al., 1984). In addition, cytoadherence of infected erythrocytes to vascular endothelium occurs in P. falciparum malaria (Warrell et al., 1990). This may lead to obstruction of blood flow and capillary damage with resultant vascular leakage of protein and fluid, and oedema and tissue anoxia in the brain, heart, lungs, intestines, and kidneys. Damage to the intestinal mucosa due to tissue anoxia makes it possible for enteric organisms to escape into the blood stream (Warrell et al., 1990; John, 2016). This may explain why enteric organisms were the main isolates in this study.
The means of WBC count, absolute neutrophil count, and ESR could not differentiate subjects with malaria alone from those with malaria coexisting with septicaemia. Several factors including haemolysis (common in malaria), endogenous steroid, and catecholamines lead to increase in WBC and absolute neutrophil count, while female sex, anaemia, dilutional problem, increased temperature of specimen especially in the tropics, and a tilted measuring tube lead to increase in ESR. These factors might come into play during acute illnesses thereby rendering WBC, absolute neutrophil count, and ESR less appropriate for use in the detection of septicaemia in children with malaria (Philips et al., 1986; McLellan and Giebink 1986; Bouree et al., 2000; Enyuma et al., 2015). While malaraia alone stimulated CRP production in this study, those with malaria coexisting with septicaemia had higher mean serum CRP levels (108.44 mg/l) than those with malaria alone (82.16 mg/l). This difference was highly significant (p<0.0176). Whether this high mean level in subjects with co-morbidity was as a result of a synergistic or summation effect is unclear.
It is probable that their combined activation of mononuclear cells resulted in the production of higher levels of inflammatory cytokines (tumour necrosis factor, interleukins 1, 2 and 6) that stimulated hepatic synthesis of higher inflammatory proteins including CRP (Bouree et al., 2000). It has been observed that patients with bacterial infections tend to have higher peaks of CRP than those with other conditions (Landry et al., 2017). Thus, serum CRP could distinguish between malaria and malaria co-existing with septicaemia. When this was done at CRP value of 90 mg/l, it gave a sufficiently high sensitivity of 76.2% and a negative predictive value (NPV) of 90.5% but a low specificity of 36.9% and positive predictive value (PPV) of 16.3%. Despite the low specificity and low positive predictive value, CRP has several advantages over ESR, WBC`, and absolute neutrophil count.
It is known to rise 6-12 hafter onset of illness (Kohli et al., 1993) and not affected by immunological status of the child, age, sex, or anaemia (Black et al., 2004). It returns to normal within a week of successful treatment while it takes three to six weeks for ESR to do so (McLellan and Giebink, 1986; Kohli et al., 1993). It is cost effective and is adapted for easy technique of measurement (Kohli et al., 1993). In India, Chhatriwala et al. (2014) demonstrated the prognostic value of CRP in children with malaria, though they did not explore the role of septicaemia in this response. Septicaemia is rapidly fatal in young children if not treated promptly. Therefore, it is recommended that at CRP levels of ≥ 90 mg/l antibiotics should be started. We recommend in our environment that gentamycin and ceftriaxone be used. These can be withdrawn if preliminary blood culture result is negative.
The authors have not declared any conflict of interests.
We are grateful to nurses and staff of the Children Emergency Room and Child Welfare Clinic who participated in the management of these children. Finally, we thank the Medical Laboratory Scientists for their assistance in the conduct of the various tests.
REFERENCES
|
Adepoju KA, Akpan GE (2017). Historical assessment of malaria hazard and mortality in Nigeria – cases and deaths: 1955-2015. Int. J. Environ. Bioener. 12(1):30-46.
|
|
|
|
Akpede GO, Abiodun PO, Sykes MR (1993). Malaria with bacteraemia in acutely febrile pre- school children without localising signs: Coincidence or association/complications. J. Trop. Med. Hyg. 3:146-50.
|
|
|
|
|
Bouree P, Botterel F, Lancon A (2000). Comparative study of ESR and CRP in acute malaria. Available at:
View
|
|
|
|
|
Chhatriwala M, Patel B, Shah R, Shaikh N, Gokani R, Nilawar A (2014). Prognostic value of serum C - reactive protein in malaria IJBAR. (10):513-515.
|
|
|
|
|
Enyuma COA, Ekanem EE, Udo JJ, Asindi AA (2015). Haematological indices in febrile neonates with malaria in parasitaemia in Calabar. Niger. Med. J. (5):323-326.
|
|
|
|
|
John CC (2016). Malaria (Plasmodium). In: Behrman R E, Kliegman R M, Jenson H B (2000). eds. Nelson Textbook of Paediatrics. 20th ed. Philadelphia: W B Saunders Company (Publishers). pp. 1709-1721.
|
|
|
|
|
Kohli V, Singhi S, Sharma P, Ganguly, KN (1993). Value of serum C- reactive protein concentrations in febrile children without apparent focus. Ann. Trop. Pediatr. (4):373-378.
|
|
|
|
|
Landry A, Doherty P, Qullette S, Carter LJ. (2017). Causes and outcomes of markedly elevated C-reactive protein levels. Can. Fam. Phys. 63(6):e316-e323.
|
|
|
|
|
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003). 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference. Crit. Care Med. 29(4):530-538.
Crossref
|
|
|
|
|
Liu S, Ren J, Wu X, Ren H, Yan D, Wang G, Gu G, Li J, Xia Q, Han G (2013). Prelimminary case-control study to evaluate diagnostic values of C-reactive protein and erythrocyte sedimentation rate in differentiating active Crohn's disease from intestinal lymphoma, intestinal tuberculosis Behcet's syndrome. Am. J. Med. Sci. 346(6):467-472.
Crossref
|
|
|
|
|
McLellan D, Giebink GS (1986). Perspectives on occult bacteraemia in children. J. Pediatr. 109(1):1-8.
Crossref
|
|
|
|
|
Phillips RE, Looareesuwan S, Warrell DA, Lee SH, Karbwang J, Warrell MJ, White NJ, Swasdichai C, Weatherall DJ(1986). The importance of anaemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. QJM: An Int. J. Med. 58(3-4):305-323.
|
|
|
|
|
Rasmussen NH, Rasmussen NL (1982). Predictive value of white bloodcell count and differential cell count to bacterial infections in children. Acta Pediatr. 5:775-778.
Crossref
|
|
|
|
|
Sabel KC, Hanson LA (1974). The clinical usefulness of C-reactive protein (CRP) determinations in bacterial meningitis and septicaemia in infancy. Acta Paediatr. 3:381-388.
Crossref
|
|
|
|
|
Tendencia EA (2004). Disk Diffusion In: Laboratory Manual of Standardized Methods for antimicrobial tests for bacteria isolated from aquatic animals and environment. Tigbavan, Ibilo; Aquaculture Dept, Southeast Asian Fisheries Development Centre. pp.13-29.
|
|
|
|
|
Tupchong K, Koyfman A, Foran M. (2015). Sepsis, severe sepsis, and septic shock: A review of the literature. Afr. J. Emergency Med. 5(3):127-135.
Crossref
|
|
|
|
|
Warrell DA, Molyneux ME, Beales PF (1990). Severe and complicated malaria: march 1988. Trans. Royal Soc. Trop. Med. Hyg. P 8.
|
|
|
|
|
Weinstein L, Swartz MN (1974). Pathophysiologic changes due to localization of Infections in Specific Organs. In: Weinstein L, Swartz MN, eds. Pathophysiologic (physiology) Mechanism of Diseases. 5th edn. Philadelphia: W.B Saunders (Publishers). pp. 389-510.
|
|
|
|
|
Whittle HC, Brown J, Marsh K, Greenwood BM, Seidelin P, Tighe H, Wedderburn L (1984). T-cell control of Epstein–Barr virus-infected B cells is lost during P. falciparum malaria. Nature 312(5993):449-450.
Crossref
|
|
|
|
|
World Health Organization (WHO) (1991). Basic malaria microscopy. World Health Organization. pp. 19-20.
|
|