Although the mortalities from malignant otitis externa (MOE) have greatly reduced, it is still a potentially fatal clinical condition. This study was undertaken to review the treatment outcomes and prognostic factors in MOE and to compare the behavioral pattern of cases caused by pseudomonas and non-pseudomonas organisms. A retrospective chart review of patients diagnosed with MOE in a tertiary institution over a 13 year period was conducted. Treatment outcome was divided into survival and mortalities groups. Demographic and disease factors were analyzed regarding mortalities using univariate and multivariate analysis. Seventeen of 22 cases were analysed. Nine (53%) were diabetic while 5 were HIV positive. After average of 7 weeks of antibiotic therapy ± surgical debridement, the disease resolved in 59%. Mortality was 41%. Diagnostic delay, poor blood sugar control, and extensive disease were found to predict mortality (P = 0.051, 0.048, and 0.006 respectively). Age, sex, causative organism, HIV infection, facial nerve and other cranial involvement did not significantly predict mortality. Pseudomonas aeruginosa was isolated in 11 patients. The rest had atypical organisms, Staphylococcus aureus and Proteus spp. There was no significant difference in the disease extension, mortality, duration of treatment and facial nerve involvement between pseudomonas and non-pseudomonas groups. However the pseudomonas group were predominantly diabetic (p = 0.03). It is concluded that malignant otitis externa still has a significant mortality despite aggressive therapy. Extensive temporal bone/intracranial disease, poor blood sugar control, and diagnostic delay portend a poorer prognosis. S. aureus is an increasingly important causative organism in MOE especially in non-diabetic patients.
Malignant otitis externa (MOE) is a rapidly progressive infection of the external ear characterized by invasive inflammation of the external auditory canal, marked by necrosis of surrounding cartilage and bone tissues with tendency to extension along sub-temporal fat planes (Walton and Coulson, 2014; Lasisi and Nwaorgu, 2001). Pathologically, MOE was divided by Benecke into necrotizing otitis externa, in which only soft tissues and cartilage undergo necrosis, and skull base osteomyelitis, in which temporal or skull base bones are progressively destroyed (Benecke, 1989; Peleg et al., 2007). Pseudomonas aeruginosa is the predominant causative organism in most reports, but increasing number of reports have implicated non-pseudomonas organisms including Aspergillus fumigates and Staphylococcus aureus as the isolated causative agents (Walton and Coulson, 2014; Hobson et al., 2014). The disease is mostly seen in elderly diabetes and immunocompromised individuals, but it has also been reported in apparently healthy non-immunosuppressed individuals (Nguyen et al., 2010). Although the mortality figures from MOE have been shown to be improving in last 2 decades, it is still a potentially fatal clinical condition with high mortalities ranging from 20 to 60%, still being reported especially from developing countries (Lasisi and Nwaorgu, 2001; Lee et al., 2011; Loh and Loh, 2013). Although studies have attempted to examine prognostic factors for survival, there still remains a lack of consensus regarding the identifiable prognostic factors to guide treatment. This is compounded by its unpredictable clinical course. The study aim to review the treatment outcomes in a tertiary institution, as well as compare the behavioral pattern of MOE caused by pseudomonas and non-pseudomonas organisms, and examines its mortality risk factors.
A retrospective charts review of 22 consecutive patients with diagnosis of MOE at the Department of Otorhinolaryngology in a tertiary institution between 2004 and 2016 was performed. The study was approved by the ethical review committee of the institution.
The following data were assessed: Presenting symptoms and clinical signs, patients’ underlying medical conditions, diagnostic delays, bacteriological culture results, radiographic features, disease extensions, cure rate, complications, and mortalities. Diagnoses were based mainly on clinical history of ear discharge, ear ache, facial nerve palsy, headache, as well as finding of necrotic debris in the ear canal, in addition to computed tomography temporal bone findings. Extensive disease was defined by infratemporal fossa involvement, temporal bone/petrous apex involvement, and intracranial involvement. The initial antibiotic therapy for most of the patients included intravenous administration of ciprofloxacin and/or ceftriaxone. The antibiotics were changed according to the antimicrobial sensitivity pattern of subsequent ear culture results. In resistant cases, gentamicin was added. Surgical debridement was carried out for removal of extensive necrotic debris and sequestrum.
Those patients with inconsistent and/or insufficient/incomplete data were excluded from analysis. Data was analyzed with the SPSS statistical software (version 16.5; IBM Corp) Chi-square or Fisher exact test were used to find the significance of study parameters on categorical scale. The potential risk factors were tested with logistic regression analysis. The criterion for statistical significance was set at P value of < 0.05.
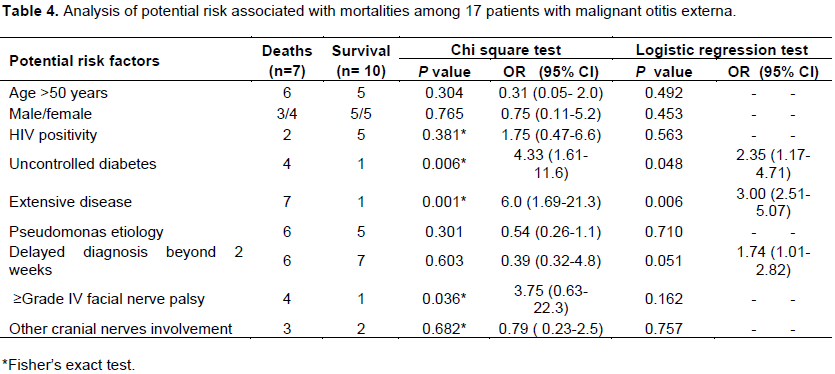
Twenty two patients with MOE during the study period were identified. Five were excluded due to insufficient or inconsistent clinical data, and 17 were analyzed. There were 8 males and 9 females. Fifteen were adults with age range of 24 to 80 years and mean of 56.7±16.3 years. The remaining 2 patients were children aged 2 and 5 years respectively. Table 1 outlined the patients’ characteristics and the disease attributes. Nine (53%) were diabetic, whereas 5 of the non-diabetics were HIV positive, while the rest were neither diabetic nor HIV positive. The two children in the study were HIV positive. Among the diabetics, 5 had poorly controlled blood sugar with their fasting blood sugar exceeding 16.5 mmols per litre. The microbiological culture was positive for bacteria in 15 (88%) patients. P. aeruginosa was the predominant organism which was isolated in 65% of the patients. The atypical organisms were S. aureus (18%) and Proteus spp. (6%). P aeruginosa was isolated in all the diabetics except one patient that had negative culture, whereas all the atypical bacteria were isolated in the non-diabetic patients (Tables 1 and 2).
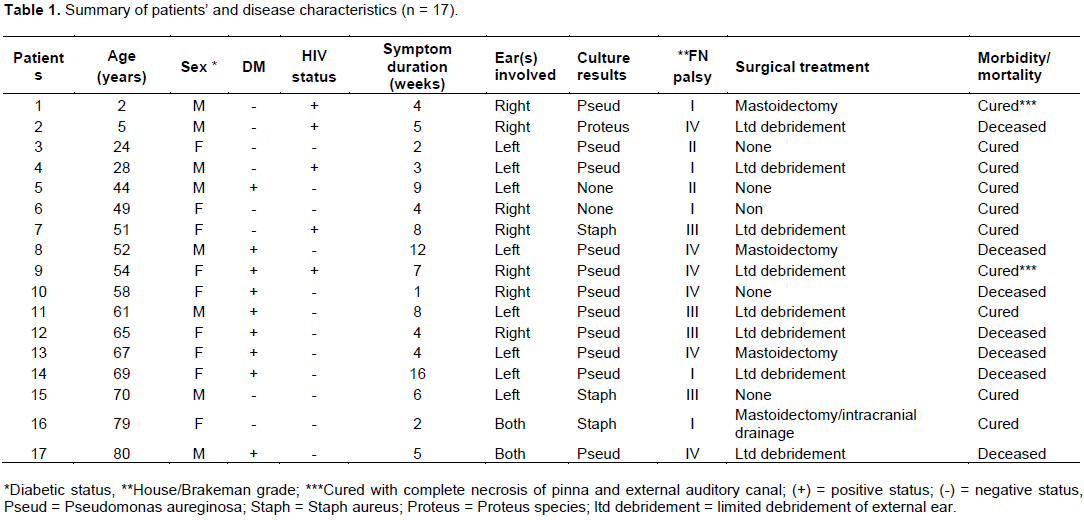
Facial nerve palsy occurred in 70% of the patients with 50% of them being severe (grade IV) palsy (Table 1). Other cranial nerve palsy documented were V and VI in 2 patients respectively and IX in one patient. Table 3 outlined the pattern of disease extension from clinical and radiological assessments. The disease was limited to the external auditory canal in only 35% of the cases, with the mastoid being the most common site of disease extension.
The disease resolved in 10 (59%) after an average of 7 weeks of antibiotic therapy with surgical debridement in some. Two of the survivors had significant morbidity from extensive destruction of pinna and external auditory canal. The rest did not survive despite treatment with antibiotics and surgical debridement after varying time frames. The overall mortality rate was 41%. The mean antibiotic course was 6.9 ± 2.9 weeks. All the patients received ciprofloxacin and/or ceftriaxone either as initial antibiotic therapy or as definitive treatment following antibiotic sensitivity results. Surgical debridement was performed in 65% of the patients, and this included bedside limited debridement of soft tissue necrosis in 8 patients, mastiodectomy in 4 cases, and additional drainage of cerebella abscess in one patient.
Comparison of the pseudomonas and non-pseudomonas infected malignant otitis externa was outlined in Table 2. The pseudomonas group were predominantly diabetic than the non- pseudomonas group (P = 0.03). The pseudomonas group also had more propensity for extensive tissue destruction and mortality although not statistically significant (P = 0.064 and 0.092 respectively). The non-pseudomonas MOE were treated for an average of 2.2 more weeks of antibiotics than the pseudomonas group (P = 0.241). Other factors such as HIV status and rate of facial nerve palsy were not significantly different between the two groups.
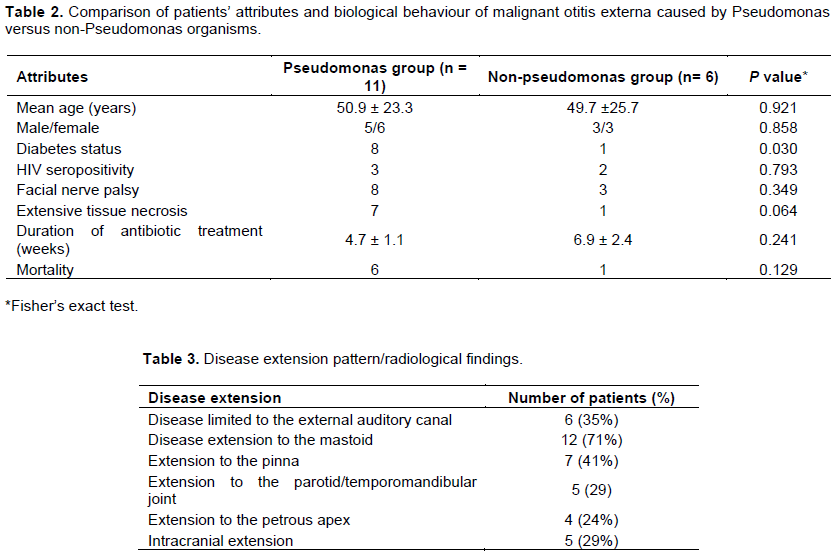
Significant mortality risk factors identified for MOE in univariate and logistic regression analysis included poor diabetic control with blood sugar exceeding 16.5 mmols per litre, extensive disease (defined by extensive temporal bone destruction and/or intracranial extension), and diagnosis delayed beyond 2 weeks (P = 0.048, 0.006, and 0.051 respectively)(Table 4). Mortality was neither significantly related to the aetiological organism nor to HIV status. Mortality was significant for severe grade of facial nerve palsy in univariate analysis but not so in logistic regression test.
Malignant otitis external is a relatively uncommon but severe disease of the external auditory canal which has been associated with mortality of 40 to 60% (Lasisi and Nwaorgu, 2001; Lee et al., 2011; Stevens et al., 2015; Kwon et al., 2006). The development of anti-pseudomonal antibiotics such as fluoroquinolones and ceftazidime, has reduced mortality significantly with increasing number of reports indicating better mortality figures at <10% (Franco-Vidal et al., 2007; Pulcini et al., 2012). A number of studies have examined potential prognostic factors such as clinical presentation, imaging findings, microbiology and facial nerve involvement (Lee et al., 2011; Loh and Loh, 2013; Stevens et al., 2015; Kwon et al., 2006; Soudry et al., 2011; Soudry et al., 2007). However, conclusive prognostic factors are yet to be identified. The emergence of fluoroquinolones-resistant and multidrug resistant pseudomonas organisms have created difficulty in developing consensus on the optimal choice of antibiotics and duration of therapy (Pulcini et al., 2012; Berenholz et al., 2002).
The study found diabetes as the underlying debilitating disease in 53% of the patients, whereas 29% were immune depressed due to HIV infection similar to other reports (Franco-Vidal et al., 2007; Martel et al., 2000). However higher diabetic figures of 80 to 95% have been reported (Lee et al., 2011; Loh and Loh, 2013; Pulcini et al., 2012; Chen et al., 2011).
Pathological basis of MOE propagation among diabetics has been attributed to microangiopathy which often occurs in diabetes mellitus. It was suggested that this microangiopathy may decrease local blood flow, and results in a low concentration of antibiotics in target tissue (Lee et al., 2011).
P. aeruginosa was identified as the causative organism in 65% of the patients, predominantly among diabetics, with atypical non-pseudomonal organisms constituting 24%, predominantly among non-diabetics, similar to other studies (Loh and Loh, 2013; Franco-Vidal et al., 2007). However higher figures of 85 to 95% pseudomonas isolation have been reported (Pulcini et al., 2012; Gehanno, 1994). In contrast to the findings, lower rates of pseudomonas isolation <50% have also been reported (Hobson et al., 2014; Chen et al., 2011). In one series, pseudomonas was isolated in only 27% of the 19 patients studied. The increasing reports of atypical organism isolation in MOE specifically imply that S. aureus is an increasingly important organism leading to MOE. Diagnosis should not be center on isolation of pseudomonas, rather a high index of suspicion for atypical organisms, should be maintained especially in patients with signs and symptoms of MOE who do not have diabetes.
Comparison of the behavioral pattern of MOE caused by pseudomonas and non- pseudomonas organisms in this study revealed that the pseudomonas infected cases were more likely to have diabetes mellitus. Although the pseudomonas group tended to develop more extensive tissue destruction and have more mortality, these observations were not significant. Hobson et al. (2014) similarly reported significant association between pseudomonas infected group and diabetes mellitus than the non-pseudomonas cases. They however reported no difference between the two groups regarding rate of bony erosion and extensive disease. This underscores the need to adopt aggressive treatment protocol for all cases of MOE regardless of organism involved.
Overall the mortality rateof the study was 41% in line with other reports (Lee et al., 2011; Stevens et al., 2015; Kwon et al., 2006). In contrast a number of reports indicated lower mortalities than the results of the study at less than 10% (Franco-Vidal et al., 2007; Pulcini et al., 2012). This apparent difference in the mortality figures between the present study and the aforementioned reports may be attributed to likely differences in the severity patterns of the MOE in this study in comparison with that in the aforementioned studies. Although the severity pattern of MOE was not specified in those studies, extensive temporal bone destruction in 47% of the patients was documented in this study. Some studies that carried out systematic stratification of the disease severity showed that the severe sub-group of MOE had more significant mortality than the less severe MOE (Peleg et al., 2007; Stevens et al., 2015; Soudry et al., 2011). However, the relatively smaller number of patients in this study compared to the aforementioned studies, which analyzed 46 and 32 patients in their series respectively, may have also contributed to poorer mortality figures.
Potential significant mortality risk factors identified in this study were extensive disease with extensive temporal bone/intracranial involvement, poorly controlled blood sugar exceeding 16 mmol/L, and diagnostic delay/commencement of definitive treatment. Quite understandably many previous reports are in agreement with the present results regarding significant association between extensive disease and poorer survival rate (Lee et al., 2011; Loh and Loh, 2013; Stevens et al., 2015; Soudry et al., 2011). Similarly, poor diabetic control with diabetic complications has been shown to lead to shorter survival in line with the study findings (Joshua et al., 2008). However, Loh and Loh (2013) found that diabetic control did not affect the prognosis in contrast to the data of this study. It is plausible that the design in Loh and Loh (2013) series and this study did not apply similar parameter in defining the level of diabetic control. The poorer prognosis observed among the poorly controlled diabetics may be attributed to microangiopathy which may have decreased local blood flow to the diseased tissues, resulting in a low concentration of antibiotics in these tissues with resultant poor responses.
Contrary to a report in which delay in the commencement of intravenous antibiotics did not show adverse outcome (Loh and Loh, 2013), Guevara et al. (2013) demonstrated significant association of poor prognosis with diagnostic delays and delays in commencement of treatment similar to the data of this study. The average diagnostic delay in this study was 5.9 weeks, in line with other reports, which ranges from 6 to 13 weeks (Loh and Loh, 2013; Guevara et al., 2013). The reasons for diagnostic delays and commencement of definitive treatments are often related to the indistinguishable nature of the initial symptoms from simple otitis externa. It is usually only after multiple failed treatment attempts that MOE is suspected.
Although severe facial nerve palsy seemed to be significantly associated with increased mortality in the univariate analysis, it was not significant on logistic regression test. Most reports are in agreement with the data of this study on the lack of significant correlation between rate of facial nerve involvement and mortality (Lee et al., 2011; Soudry et al., 2007). Facial nerve involvement has been shown to represent a sign of progression of MEO although it does not, by itself, worsen prognosis (Soudry et al., 2007). Moreover, severer forms of facial nerve involvement also represent significant morbidity which often lingers after infection has been controlled. The statistical significance of this study was limited by the small number of cases recruited.
In conclusion the results indicate that MOE still presents as potentially fatal disease of the external auditory canal and temporal bone with significant mortality and morbidity despite aggressive antibiotic and surgical treatments. Mortality was significantly influenced by extensive temporal bone/intracranial involvement, poor blood sugar control, and diagnostic delay. The study also highlights S. aureus as an important causative organism in MOE especially in non-diabetic patients.
The authors have not declared any conflict of interests.