ABSTRACT
Tissue trauma induces migration and activation of neutrophils through specific mediators. Vacuolated neutrophils in peripheral blood smear of septic patients correlated with mortality. However, scarce data exist with respect to findings in hemorrhagic shock (HS) trauma patients. The aim of this work was to evaluate the number and size of cytoplasmic and nuclear vacuoles in polymorphonuclear neutrophil (PMN) obtained from a peripheral blood smear stained with the May-Grunwald-Giemsa method in trauma patients with hemorrhagic shock. Seven sequential blood samples were taken from 20 patients with severe hemorrhagic shock and 20 patients who sustained mild thoracic trauma (control group). The first sample was obtained shortly after admission to the hospital followed by new samples taken at 6, 12, 18, 24, 48 and 72 h. Blood smears from both groups were processed to assess vacuolization and vacuole morphology in one hundred PMNs at each time point. The number and the area of vacuoles in the nucleus and the cytoplasm were determined using the program Image-Pro Express version 4.0 for Windows (Media Cybernetics, Bethesda, MD, USA). The number and the area of vacuoles in the cytoplasm and nucleus were significantly different (p <0.05) between shock and control groups. Moreover, serum lactate and heart rate correlated directly with the number (r=0.634) and the area (r=0.624) of cytoplasmic vacuoles as shown by multivariate analysis (p<0.05). Severe hemorrhagic shock induces greater vacuolization of PMNs as compared to mild trauma. PMN vacuolization has direct correlation with serum lactate, a known marker of severe shock.
Key words: Hemorrhagic shock, trauma, lactate, inflammatory response, blood smear, neutrophils, vacuolization, apoptosis.
Abbreviation:
HS, Hemorrhagic shock; PMN, polymorphonuclear neutrophil; bpm, beats per minute.
Normal neutrophils are highly homogeneous cells. In a blood smear observed under light microscopy, the normal diameter of a neutrophil ranges from 12 to 15 µm. It is classically accepted that neutrophils survive for 8 to 12 h in the circulation (Gutierrez et al., 2004; Dancey et al., 1976). However, recently published data suggest that under physiologic conditions human neutrophils may remain in the circulation for up to five days
(Tofts et al., 2011; Pillay et al., 2011). However, neutrophil turnover can be accelerated in inflammatory responses. The belief that neutrophils exert their function solely as pathogen killers lacks current support. Several interactions with the immune system, including macrophages, dendritic cells, cells of the adaptive immune response, and inflammatory response unrelated have been described (Mantovani et al., 2011; Amulic et al., 2012; Kolaczkowska and Kubes, 2013). Unstimulated neutrophils exhibit a smooth round cell shape with uniform cytoplasmic granularity, whereas irregular cell shape, toxic granulations, and cytoplasmic vacuolization can be observed in trauma-induced neutrophil activation (Bain, 2005). Tissue trauma induces migration and activation of neutrophils through specific mediators. Furthermore, this condition can also lead to local and systemic release of mediators capable of inducing a systemic inflammatory response syndrome (SIRS) (Bone et al., 1992; Hensler et al., 2002; Rotstein, 2003). A positive correlation between the presence of those mediators and the severity of inflammatory response has been described (Donnelly et al., 1993; Donnelly et al., 1994; Martin et.al., 1997). Nevertheless, diagnostic testing to rapidly identify harbingers of SIRS is scarce. The aim of this study was to evaluate the number and
size of both cytoplasmic and nuclear vacuoles in PMNs of hemorrhagic shock trauma patients, obtained from peripheral blood smears, stained with the May-Grunwald-Giemsa method, and to correlate the findings with clinical and laboratory inflammatory markers data.
This study was approved by the Medical Ethics Committee of the Hospital Universitario Risoleta Tolentino Neves under the Protocol number 30/2011. Appropriate consent had to be signed by the patient or their next of kin prior to enrollment in the study. A preliminary analysis to determine the number of individuals to be allocated in each group was performed. Considering an expected mortality rate of 10% in severe trauma and using an alfa value of 0.05 and a beta value of 0.20, a sample size of 20 patients was determined for the study. Forty polytrauma male patients, 18 to 45 years old treated at the Hospital Universitário Risoleta Tolentino Neves (HURTN) between January 1, 2011 and June 30, 2013 were allocated into two groups, based on the severity of trauma. Group I – control patients with mild trauma to the chest with no need for chest tube thoracostomy. Group II – patients with blunt or penetrating trauma who presented in hemorrhagic shock and a Glasgow score ≥ 14.
Only patients brought directly to the trauma center from the scene were enrolled in the study. Inclusion criteria for patients of the group II were the presence of at least one of the following parameters: Systolic blood pressure < 90 mmHg and heart rate>100 bpm, unresponsive to an initial fluid bolus of 1 L of crystalloid solution (0.9% sodium chloride); Massive hemothorax (≥ 1500 ml) as demonstrated by a plain x-ray or by a computed tomography scan during the initial assessment; cardiac tamponade; massive hemoperitoneum or retroperitoneal hematoma (blood in 3 or more quadrants) at abdominal ultrasound or computed tomography scan; severe pelvic fractures associated with the hemorrhagic shock; need for more than 4 L of crystalloid bolus to maintain systolic blood pressure > 90 mm Hg; need for 4 or more units of packed red blood cells in the first 6 h following the trauma. The exclusion criteria for both groups were: Patient presenting co-morbidities, such as, diabetes, arterial
hypertension, chronic renal, hepatic or lung failure, cardiovascular or other chronic condition; Fever or signs of infection in the first 72 h; Positive blood culture samples obtained during the first 72 h of admission to the hospital.
Procedures
Blood samples were obtained from 20 patients in each group as part of the assessment in trauma for routine laboratory tests, using 10 mL syringes, and then transferred to vials containing EDTA (Becton Dickinson). Blood smears were prepared, in duplicates, on slide glasses (Precision Glass) laid over each smear and sealed with transparent nail polish after staining with the May Grünwald-Giemsa method (Woronzoftf-Daskoff, 2002). The first sample was obtained shortly after patient’s admission followed by subsequent samples taken at 6, 12, 18, 24, 48 and 72 h as per routine assessment of trauma patients. Stained slides were stored in a slide glass box until analyzed under a light microscope. The mounted glass slides were photographed under immersion light using an ABX 35 (Olympus®) microscope with a 1000X magnification lens.
The optical microscope images were captured with a resolution of 1392 × 1040 pixels and transferred from a video color camera (Cool/Snap Proof Color; Media Cybernetics, Bethesda,MD, USA) to a video system attached to a computer using the program Image-Pro Express version 4.0 for Windows (Media Cybernetics, Bethesda, MD, USA). All visual analyses on image acquired using a 100 objective were performed using the freeware ImageJ 1.48. (version 1,47f, Wayne asband/National Institutes of Health, USA) available online from the site: http://rsbweb.nih.gov/ij/download.html.
Nuclear and cytoplasmic vacuoles from 100 polymorphonuclear neutrophils were counted and their areas measured in both groups using a photography camera. Images were processed with Java image processing program (Software Image J® version 1.44). The remaining blood sample was used in routine laboratory examinations. Statistical analysis was performed using a statistics software (IBM SPSS statistics, Armonk, NY, USA). Mann-Whitney’s U test was used to detect differences between control and the hemorrhagic shock groups. Data were expressed as Mean ± SEM of the number as well as the area (µ2) of cytoplasmic and nuclear vacuoles/neutrophil in both groups. Spearman’s rank correlation coefficient was used to detect any correlation among the variables, and multivariate linear regression analysis was performed thereafter.
Statistical significant differences were set at p<0.05 .
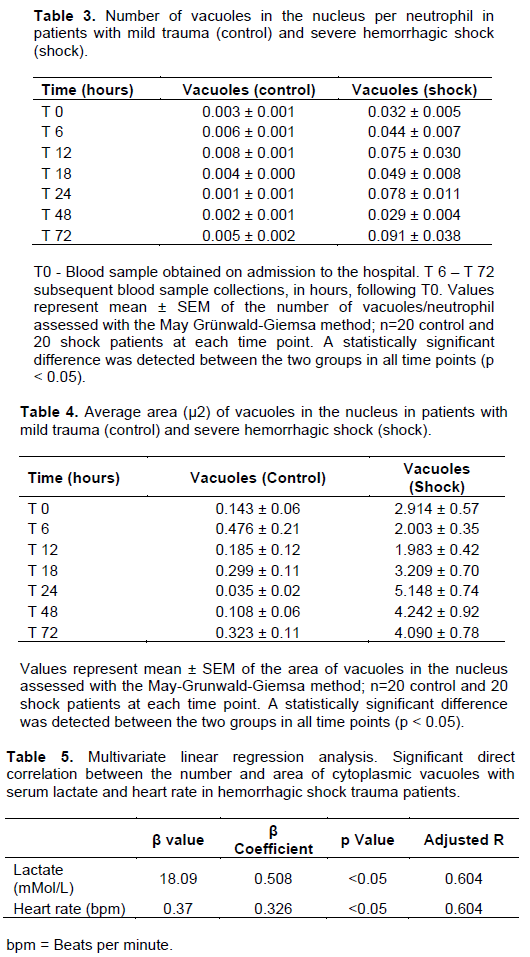
Penetrating mechanism (gunshot wounds) was the cause of injury in 19 out of 20 patients in the hemorrhagic shock group. A single patient sustained blunt trauma secondary to motor vehicle accident. The average number of vacuoles in the PMNs of hemorrhagic shock patients (Group II) was significantly higher (p< 0.05) with larger vacuoles in the cytoplasm in all time points investigated (Figure1). The number and area of cytoplasmic vacuoles/ neutrophil in both control and shock groups are depicted in Tables 1 and 2, respectively.

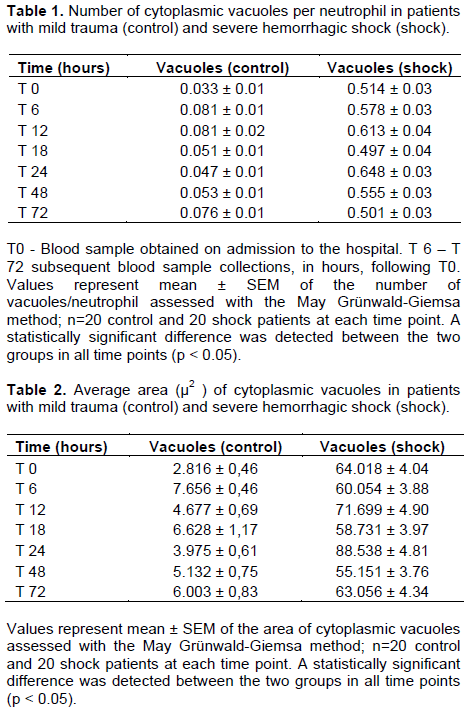
Similarly, the average number and the area of the vacuoles in the nucleus of the PMNs of hemorrhagic shock patients were also significantly greater than in the PMNs of control group patients (p<0.05) when compared with the HS group during all time points (Tables 3 and 4). More importantly however, was the direct correlation shown in multivariate linear regression analysis, between the severity of shock, assessed through serum lactate levels and heart rate, and cytoplasmic vacuolization of PMNs (Table 5).
The findings showed that severe hemorrhagic shock provokes more vacuolization of neutrophils in both the cytoplasm and the nucleus as compared to the minor trauma. Moreover, the study presented herein also demonstrated a direct positive correlation between the severity of shock and the number and area of cytoplasmic vacuoles in the PMNs of hemorrhagic shock patients. This finding has important clinical implication, considering the current unavailability of methods to predict potential overwhelming SIRS in the setting of traumatic hemorrhagic shock.
Overwhelming SIRS triggered by severe trauma involves the activation of several mediators belonging to both humoral and cellular-mediated responses. Those mediators are for the most part responsible for end organ damage and ultimately multiple organ failure (MOF) observed in the later stages of overwhelming SIRS (Schlag et al., 1991). PMNs have an important role in SIRS, given their activation by pro-inflammatory mediators such as TNFα, IL-1β, IL-6, IL-8 and macrophage migration factor (MMF) (Schlag et al., 1991; Botha et al., 1995). Activation of PMNs can be detected by morphological in their resting state (Schlag et al., 1991; Fujishima and Aikawa, 1995). Accordingly, cytoplasmic vacuolization is a known marker of cell degeneration and apoptosis (Fujishima and Aikawa, 1995). Previous study showed that elevated systemic release of pro-inflammatory mediators crucial to the activation of macrophages/neutrophils were detected shortly after the beginning of hemorrhagic shock (Ayala et al., 2002). Moreover, nuclear fragmentation and vacuolization have also been demonstrated in that setting and represent irreversible apoptosis (Wyllie et al., 1980). Ischemia/ reperfusion and hypoxemia in septic shock patients provoke inflammatory response that leads to cytoplasmic vacuolization and lysis of cellular organelles in PMNs thorough a mechanism involving reactive oxygen species (Mihalache et al., 2011). Vacuoles generated in that setting seem to be the result of the fusion of endosomes containing CD44 with auto-phagosomes and secondary granules (Mihalache et al., 2011).
Considering the fact that ischemia/reperfusion process releases pro-inflammatory mediators, and excessive generation of reactive oxygen species are also present in severe hemorrhagic shock, it is hypothesized that the vacuoles observed in the PMNs of the patients described in our study could have been generated by a similar mechanism. Although, this specific hypothesis was not investigated, it also finds support from previous investigation, wherein in vitro priming (activation) of human neutrophils with 2 µM of platelet activating factor (PAF) led to the formation of toxic granulation and cytoplasmic vacuolization similar to what was shown in the current study (Sheppard et al., 2002). Furthermore, it was recently demonstrated that PAF mediated pro-inflammatory response is in part caused by the release of reactive oxygen species (Klabunde and Anderson, 2002).
The role of apoptosis is also important to consider in this finding, given that this is the most common mechanism for neutrophil death under physiological and inflammatory conditions (Mihalache et al., 2011; Klabunde and Anderson, 2002). In both conditions, the presence of vacuoles in the cytoplasm and in the nucleus is a characteristic sign (Klabunde and Anderson, 2002). Likewise, the apoptosis pathway in PMNs also involves the generation of reactive oxygen species (Mihalache et al., 2011; Simon, 2003). Furthermore, tissue exposure to lactate can increase reactive oxygen species formation, presumably through elevations in NADH dehydrogenase produced by the lactate dehydrogenase reaction (Wolin et al., 1999). Several reports have shown that lactate clearance independently predicts mortality in trauma patients (Odom et al., 2013). This is highly relevant to findings with respect to a prognostication potential given that hemorrhagic shock increased serum lactate levels and a positive correlation was found between lactate and neutrophil vacuolization. This observation definitely deserves further investigation.
The authors declare that there is no conflict of interest.
REFERENCES
|
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A (2012). Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30:459-489.
Crossref
|
|
|
|
Ayala A, Chung CS, Lomas JL, Song GY, Doughty LA, Gregory SH, Cioffi WG, LeBlanc BW, Reichner J, Simms HH, Grutkoski PS (2002). Shock-Induced neutrophil mediated priming for acute lung injury in mice. Divergent Effects of TLR-4 and TLR-4/FasL Deficiency.
Crossref
|
|
|
|
|
Am. J. Pathol. 161(6):2283-2294.
Crossref
|
|
|
|
|
Bain BJ (2005). Diagnosis from the blood smear. New England J. Med. 353(5):498-507.
Crossref
|
|
|
|
|
Bone RC, Sibbald WJ, Sprung CL (1992). The ACCP-SCCM consensus conference on sepsis and organ failure. CHEST J. 101(6):1481-1483.
Crossref
|
|
|
|
|
Botha AJ, Moore FA, Moore EE, Kim FJ, Banerjee A, Peterson VM (1995). Postinjury neutrophil priming and activation: an early vulnerable window. Surgery 118(2):358-365.
Crossref
|
|
|
|
|
Dancey JT, Deubelbeiss KA, Harker LA, Finch CA (1976). Neutrophil kinetics in man. J. Clin. Investig. 58:705-715.
Crossref
|
|
|
|
|
Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Pollok AJ, Grant IS (1993). Interleukin-8 and development of adult respiratory distress syndrome in at risk patient groups. Lancet 341(8846):643-647.
Crossref
|
|
|
|
|
Donnelly TJ, Meade P, Jagels M, Cryer HG, Law MM, Hugli TE, Shoemaker WC, Abraham E (1994). Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit. Care. Med. 22:768-776.
Crossref
|
|
|
|
|
Fujishima S, Aikawa N (1995). Neutrophil-mediated tissue injury and its modulation. Intensive Care Med. 21:277-285.
Crossref
|
|
|
|
|
Gutierrez G, Reines DH, Wulf-Gutierrez ME (2004). Review: Clinical review: Hemorrhagic shock. Crit. Care 8(5):373.
Crossref
|
|
|
|
|
Hensler T, Sauerland S, Bouillon B, Bertil MD, Raum M, Rixen D, Helling HJ, Jonas A, Neugebauer E (2002). Association between injury pattern of patients with multiple injuries and circulating levels of soluble tumor necrosis factor receptors, interleukin-6, anti-interleukin-10, and polymorphonuclear neutrophil elastase. J. Trauma Acute Care Surg. 52(5):962-970.
Crossref
|
|
|
|
|
Klabunde RE, Anderson DE (2002). Role of nitric oxide and reactive oxygen species in platelet-activating factor-induced microvascular leakage. J. Vasc. Res. 39(3):238-245.
Crossref
|
|
|
|
|
Kolaczkowska E, Kubes P (2013). Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13(3):159-175.
Crossref
|
|
|
|
|
Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11(8):519-531.
Crossref
|
|
|
|
|
Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL (1997). Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit. Care Med. 25:1813-1819.
Crossref
|
|
|
|
|
Mihalache CC, Yousefi S, Conus S, Villiger PM, Schneider EM, Simon HU (2011). Inflammation-associated autophagy-related programmed necrotic death of human neutrophils characterized by organelle fusion events. J. Immunol.186:6532-6542.
Crossref
|
|
|
|
|
Odom SR, Howell MD, Silva GS, Nielsen VM, Gupta A, Shapiro NI (2013). Lactate clearance as a predictor of mortality in trauma patients. J. Trauma Acute Care Surg. 74:999-1004.
Crossref
|
|
|
|
|
Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L (2011). Response: the in vivo half-life of human neutrophils. Blood 117:6053-6054.
Crossref
|
|
|
|
|
Rotstein OD (2003). Modeling the two hit hypothesis for evaluating strategies to prevent organ injury after shock resuscitation. J. Trauma 54(Suppl):S203-S206.
|
|
|
|
|
Schlag G, Redl H, Hallstriim S (1991). The cell in shock: the origin of multiple organ failure. Resuscitation 21:137-180.
Crossref
|
|
|
|
|
Sheppard FR, Moore EE, Ryder J, Harken AH, Rezende-Neto JB, Banerjee A, Silliman CC (2002). "Primed" human neutrophils on a standard peripheral blood smear. J. Am. College Surg. 195(5):731.
Crossref
|
|
|
|
|
Simon HU (2003). Neutrophil apoptosis pathways and their modifications in inflammation. Immunol. Rev.193:101-110.
Crossref
|
|
|
|
|
Tofts PS, Chevassut T, Cutajar M, Dowell NG, Peters AM (2011). Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood 117:6050-6052.
Crossref
|
|
|
|
|
Wolin MS, Burke-Wollin TM, Mohazzab HKM (1999). Roles of NAD(P)H oxidases and reactive oxygen species in vascular oxygen sensing mechanisms. Resp. Physiol. 115:229-238.
Crossref
|
|
|
|
|
Woronzoftf-Daskoff KK (2002). The Wright-Giemsa stain. Secrets revealed. Clin. Lab. Med. 22:15-23.
Crossref
|
|
|
|
|
Wyllie AH, Kerr JR, Currie AR (1980). Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251-306.
Crossref
|
|