In order to give impart to the ceramic powder of the rheological properties desired and cohesion during the step of shaping it becomes necessary to control the choice of a number of parameters to be used which include: The granulometric properties and morphological state agglomeration. The physical characteristics of a powder are the agglomeration phase, shape, particle size, density and specific mass (Bertrand and Edward, 1995). Granulometry ceramic particles can vary depending on the purpose of products.
The average size of the final particles depends on the used technique, the characteristics of the grinding body (material, shape, size), the milling time, and the medium (dry grinding, in an aqueous or non-aqueous medium, with or without dispersant and charge ratio). The disintegration and dispersion of the ceramic particles are fundamental steps in the methods of shaping by way, liquid, plastic or dry. A good dispersion technique allows minimizing the final quantity of defects in the uncooked body and the final ceramic pieces.
The rheological properties of the suspensions are very sensitive to the particle size distribution and their specific surface. The minimum viscosity can be reached if a mixture contains a fine/coarse ratio 36/64. The adaptation of the physical characteristics is mainly performed by grinding, granulating and selection (Boch, 2001). The wet grinding may have some advantages over dry grinding, although, wear of the balls can be much higher for the same material and requires 30% less energy than dry grinding for size reduction equivalent (Hausonne et al., 2005)
The study is carried on the use of the following raw materials: clay, kaolin, feldspar and sand. The elementary chemical analyses were carried out in an X-ray fluorescence spectrometer type PW2540 Vrc Change Sample Dy-1189. The mineralogical analyses were carried out in diffractometer X 'Pert PRO, Dy-type 2233-0525. Granulometric analysis was performed by pipette "Robinson". The mechanical strength was determined with a bending device Gabrielli type - Model: 424 CRAB. The used process is wet way process. Raw materials (plastic not plastic) were ground in a wet medium with humidity of 40% in earthenware jars "Gerhards," type TPR, with grinding body in alumina beads and with grinding times of up to 1 h 30 min and 2 h. The resulting powders was humidified at 7 and 8%, and then sieved, with a mesh opening (0.25 - 1 mm and 1.25 mm) sieve. Sieving of the powders were obtained after drying the slurry made in a sieve Haver & Becker type. The pressing is done using a hydraulic press semi-automatic Gabrielli 262367 type. Drying the ceramic tiles is done in a laboratory oven Memmert type with a temperature of 110°C. The raw shard cooking is performed in a rapid-firing oven model: N200A, type NR: 55269 with a temperature of 1180°C within 50 min.
Chemical analysis
Chemical analysis of raw materials is listed in Table 1. The predominant elements in the clay, kaolin and feldspar are SiO2, Al2O3, K2O and CaO. In the clay case, the rate of CaO is in the range of 6 to 20%, the clay is "marly". The mass ratio of Al2O3 to Fe2O3 of all considered materials is equal to 5; this will give a broken pink because it is between 3 and 5%. The deformation and removal should be taken into consideration because of the low refractory nature of raw materials (Boch, 2001).
A high concentration of SiO2 means that it is present in the raw material which is in two forms. The origin of the silica is in the mantle, zone of the heat convection and zone transformation of the substances. It is located in the peripheral areas of granites (pegmatites, for example), in hydrothermal lodes, in metamorphic and sedimentary rocks. The Al2O3 is present under low content 45%. This allows the deduce of clay and kaolin with both having a low refractory nature, and low plasticity.
The Fe2O3 is much more present in the clay which gives it the properties of clay which is slightly ferruginous. The iron acts as a melting element during firing by forming eutectic melting at lower temperatures. CaO, whose presence indicates that the mineral contains plagioclase and comes from the carbonates. It acts as a melting element, and combines it with the silicates during cooking.
The Na2O, K2O, are alkaline oxides, which comes mainly from feldspars, illites, micas and smectites. They play the role of fondants and also act as melting. Their associations with iron oxide, occurs during firing the sinterizing reactions, which gives the products, their definitive qualities.
TiO2 is an accessory mineral present in many metamorphic rocks (gneiss, mica schist, granulite, eclogite, etc.) in magmatic rocks (granites, syenites, etc.) in pegmatites and quartz lodes. In alluvial sands, it is much more responsible for the yellow color (Tarassoff et al., 2006). The high value loss on ignition of clay comes from the release of CO2 due to carbonates decomposition and the SO3 release gas.
Mineralogical analysis
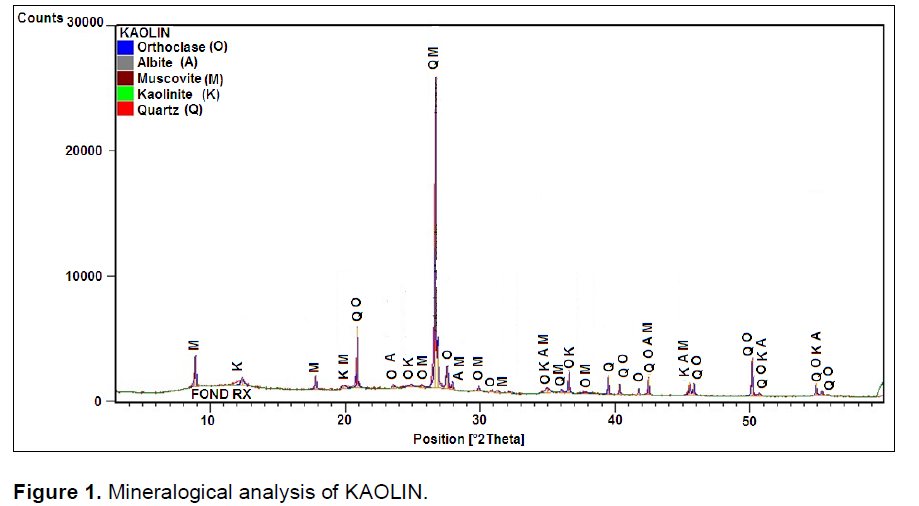
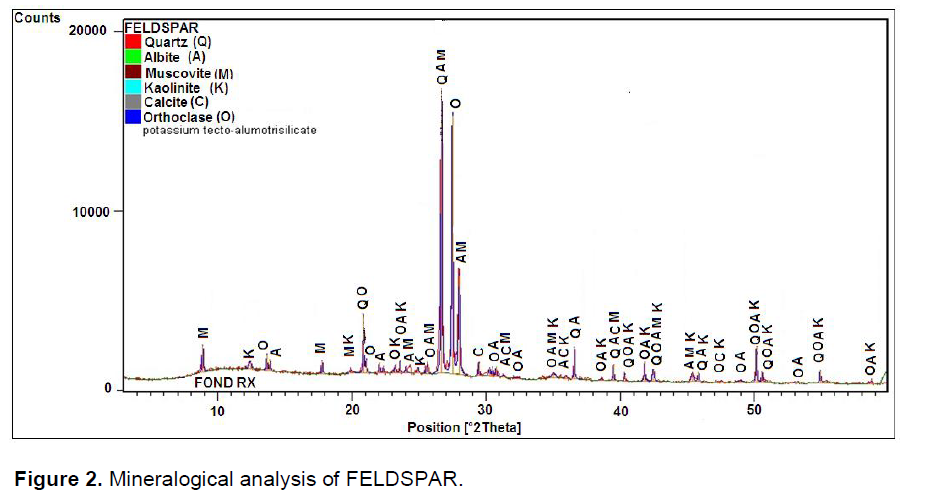
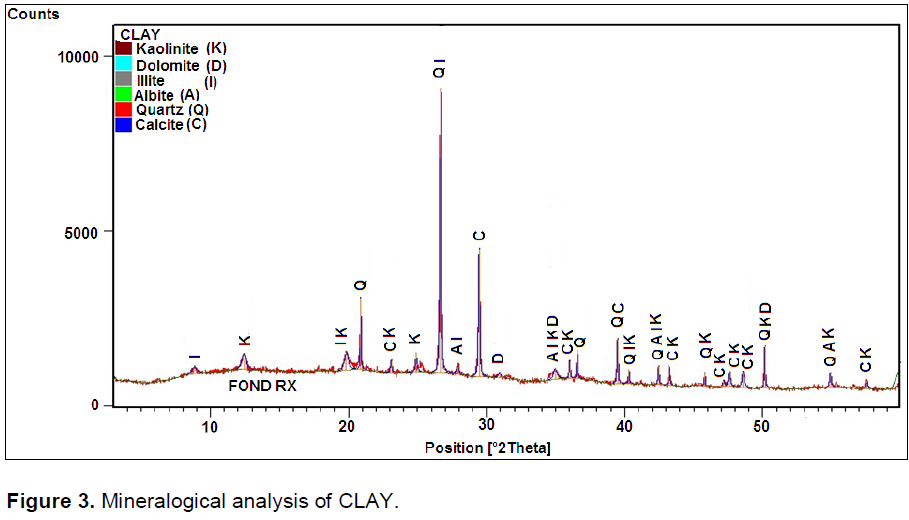
Spectra of Figure1 show that the kaolin is characterize by a predominance of quartz and muscovite elements. Figure 2 Feldspar shows a predominance of peaks belonging to quartz, feldspar and calcite. Elements were added during cooking for the formation of liquid phase which decreased the porosity of product. The mineral muscovite is also present; belonging to the family of mica which has the ability to resist chemical attack, thermal and participates dielectric properties of materials (
Encyclopedie-gratuite/muscovite, 2013). The spectra of Figure 3 corresponding to the clay minerals show a predominance of calcite and illite. The high levels of iron in the clay comes from the illite which is a type of glauconite: illite, rich in iron (
Ctmnc, 2009). The spectra of sand analyze shows a predominance of quartz with spectra belonging to the feldspar accompanied by calcite trace. The grinding efficiency and the reduction of apparent size of the crystallites were also followed by X-ray diffraction (Naimi, 2006).
Granulometric analysis
Particle size analysis by the pipette "Robinson" has provided the following fractions rates: clayey particles 64, dust particles 30.73 and sand particles 5.27%. We notice that the clay particles are predominant, which enables the deduced of clay plastic enough to cause a delicate drying. However, adding a degreaser is necessary to avoid any sensitivity to drying.
Technological parameters
The mixtures of contents are presented in Table 2. The research protocol is based on 05 mixtures types (M1, M2, M3, M4 and M5). Kaolin substitution was made by feldspar. The contents of this latter are: 10, 12, 14, 16 and 18% respectively). The viscosity is determined using a Ford Cup of 4 mm diameter, by measuring the flow time with 100 ml of suspension. The suspension was dried in a drying oven for 24 h at 110°C and then ground into powder with three different grain sizes (0.25 to 1 and 1.25 mm).
Rheological parameters
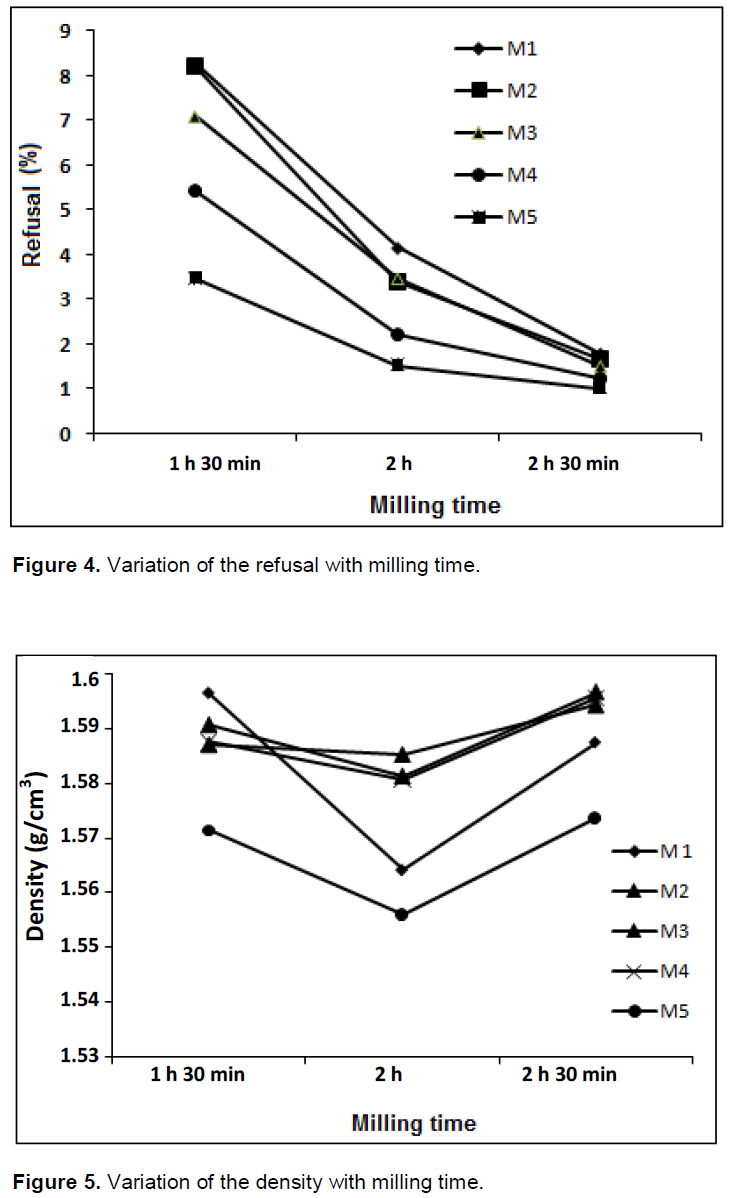
By considering values of refusal of each mixture, we found out that the last mixture decreases remarkably with increasing milling time. Only mixtures M3 and M4, respond to the requirements of manufacturing a ceramic tile for a milling time of 1 h 30 min. The values ??are 7.10 and 5.43% respectively. Refusal values ??are quite low due to amorphization of the material, followed by formation of particles of smaller sizes, structural changes and dislocations, case observed for M5 for a milling time was 2 h 30 min. Refusal values??, density, viscosity and thixotropy for 05 mixtures are presented in Figures 4 to 7 respectively. Refusal values are quite low due to amorphization of the material, followed by formation of particles of smaller sizes, dislocations and structural modifications case observed for C5. Refusal values, density, viscosity and thixotropy for 05 mixtures are presented in Figures 4, 5, 6 and 7.
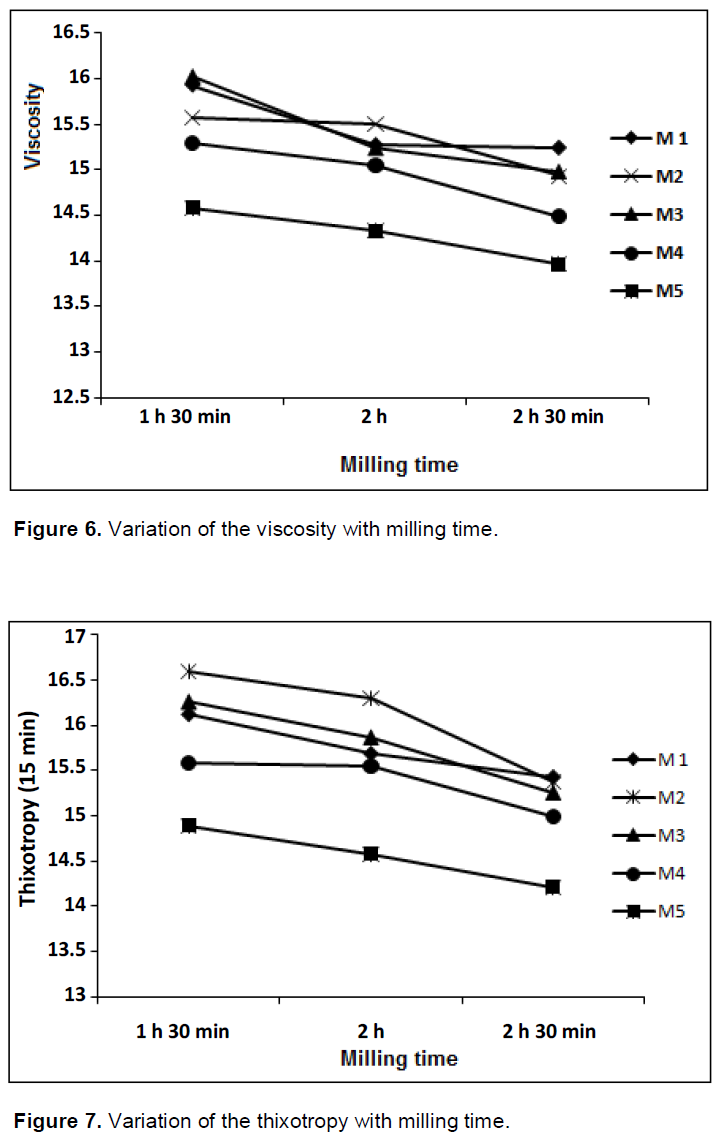
Figure 5 shows a decrease in density for a milling time of 2 h, then. A further grinding, provides finer grains which stick together giving hardly castable fluids. The decrease in density values is not significant and is within the range of standards for manufacturing ceramic tiles.
For more energy saving, the grinding time of 1 h 30 min is recommended. The viscosity values vary in a similar way to that of the density values. The minimum values are 14 s and maximum values are about 16 s. The suspensions obtained present good flows. Almost all argillaceous barbotines of casting used in the ceramic industry are more or less thixotropic. They present a flow facilitated by stirring (Hausonne et al., 2005).
Suspension is complete when no particle remains in the bottom of the tank or on the surface of the liquid. For a good stability and good suspension flow, the heavy coarse particles must be detached from the bottom, form deposit and total number of particles remains steady by time (Zwietering, 1958; Kendall and Stainton, 2001). In wet milling, the yield is much higher. An energy saving gain of at least 30% should be considered. If the material must be dried after grinding, it is clear that the labor cost by wet grinding will be much higher (Euroforum, 2009).
Physical and mechanical characteristics of dry and fired products
Considering the main physico- mechanical characteristics of M3, M4 and M5, we notice that the values of the open porosity for the mixture M3 vary proportionally in relation to the grain size. This case is observed for 03 grain sizes, because the amount of liquid phase produced is in theoptimum proportions which allow good wetting of the grains. For mixtures M4 and M5 porosity, there was an increase for a grain size of 1 mm and then decrease for a grain size of 1.25 mm. In this case, the liquid phase obtained as a function of addition is larger but does not permit a good wetting of the
grain size of 1 mm. For a little more advanced grinding (size 1.25 mm), the surface tension of grains increases, while the approximation mechanism up and connection is easily conducted. Also observed, is the decrease in porosity accompanied by decrease in absorption values. We must underline that the materials morphology obtained after sintering is largely conditioned by the powders size and their treatment. The porosity can take extremely variable forms. High porosity has a negative influence on the material strength (Kurz et al., 1995).
Chemical composition tesson of mixtures M3, M4 and M5
By considering the chemical compositions values of tesson fired mixtures M3, M4 and M5 in Table 3, we notice that mixture M3 contains more SiO2. The presence of SiO2 therefore, causes the breakdown of current capillaries causing a decrease in absorption values (Lemougna, 2004). This case is well justified by physicochemical mechanisms values obtained in this study. By a comparative study between the three mixtures, we notice that the best properties are provided by the mixture M3 for a grain size of 1.25 mm. Furthermore, we find that for the three types of grain, there is a decrease of absorption values which is due to the increase of gresification rates. The shrinkage values on products (dried, cooked), the values of porosity, absorption and flexural strength of the products (raw products, dry and cooked) are respectively as follows: [(2.65 to 4.29%) - (14.24 to 7.09%) - (10.69 Kgf / cm2, 46.08 kgf / cm2 - 234,47Kgf / cm2)]. By virtue of their durability, their enamel, particularly scratch resistant, low porosity, and feldspathic ceramics are well adapted to applications in the domain of ceramic tiles. The feldspar rate in the mixture is 3 to 14%. The mineralogical source of feldspar is a factor determining the technological and mechanical properties of sintered products. The composition M3 answers this (Boch, 2001).
The main results provided by this study are:
(a) Feasibility. It is possible by this technique in a few hours of grinding, to obtain a powder with grains of several tens of millimeters from a mixture intended to elaborate ceramic tiles having grain initially enough coarse .
(b) Identification of parameters governing this process are three forms: the duration of milling, grain size and the solid charge of barbotines. Thus, it is possible to prepare the necessary barbotines to shape, right in the middle of implementation, that is, the size reduction is performed directly in the medium (liquid and adjuvant) used for the shaping. The rheological parameters study shows that the best values ??are obtained with mixtures M3, M4 for a grinding time of 1 h 30 min. Moreover, one notices a considerable decrease in the value of refusal, for mixing M4 relative to the mixture M3. This is due to amorphization of the milled powder. To this effect, we carry our gaze on the results of the mixture M3 compared to the results of the rheological properties. The study of physico-mechanical properties of uncooked, dried and cooked for its products shows that the best values are those obtained with the mixture M3 having a grain size of 1.25 mm. Granulometry of studied samples is excellent for use in the ceramic tiles manufacture.