Physico-chemical analysis of groundwater samples from Ankpa, north central Nigeria was carried out to investigate the quality of the water for drinking and irrigation purposes. The results revealed water of average low pH, indicating slightly acidic conditions of the water. The water is soft, and it is characterized as Na++K - HCO3 type. It is good for drinking and other domestic uses. Four indices were evaluated from the groundwater chemistry in order to establish the suitability of the water for irrigation purposes. They are the sodium adsorption ratio (SAR), the percentage sodium (%Na), the magnesium hardness (MH) and the permeability index (PI). The SAR values range from 0.86 to 11.19, with an average of 5.68. The interpretation indicates that the groundwater is good to excellent for irrigation. It further shows that over 90% of the samples can be classified as S1 waters, with no alkali hazard anticipated for the crops grown with the water. The range of the %Na in the groundwater in this study is between 16.28 and 73.97, with an average value of 51.27. This shows that the water ranges from excellent to doubtful for irrigation. The magnesium hardness (MH) in the groundwater ranges from 2.05 to 41.04%, with an average of 20.35%. This indicates that the entire water is safe for use for irrigation purposes. The chloride and sulphate concentrations in the groundwater indicate that the water is excellent for irrigation. However, the permeability index shows completely opposite conditions in the area, with all samples with values above 75.
The chemical composition of groundwater and the water types found in an environment are determined greatly by the composition of the water of precipitation, local geology, types of mineral found in the environment through which the recharge and groundwater flows. Other determinants include anthropogenic activities such as mining and waste disposal, as well as climate and topography. The quality of water in turn determines its usability for domestic, industrial and agricultural purposes. The inability of the state-owned WaterBoard to supply clean, treated water has led the residents of Ankpa to resort to other water sources, such as hand dug-wells and untreated surface water sources, especially the Mabolo River (the local name for the Anambra River).
The population of Ankpa town, the political and administrative headquarters of Ankpa Local Government Area has greatly grown since the creation of Kogi State in 1991. Rise in population has often had its inherent adverse consequences on the environment, of which among the chief is the generation of large volumes of domestic waste which are difficult to manage. This is the case in Ankpa. Domestic wastes are disposed either in open dumps or in the Mabolo River due to absence of sanitary waste disposal systems within the town. These conditions place a great concern on the quality of the water used for domestic purposes in Ankpa. The poor sanitary conditions in the town have continued to expose both groundwater and surface water to possible contamination, consequent from the direct washing of waste materials by seasonal rain water into the River Mabolo to the leaching of dissolved matter leaching into the groundwater system. The Mabolo (Imabolo) river serves as an important source of water supply in Ankpa, because of lack of reticulated public water supply. Obeta et al. (2015a, b) have reported the poor quality of the Mabolo (Imabolo) river for consumption and other domestic purposes. The river might be making a very important contribution to the recharge of the shallow sandy aquifer, thereby contributing further to the degradation of the groundwater. Leachate from open dumps has been found to contribute significantly to the quality of water in some Nigerian cities with shallow groundwater systems (Onwuka et al., 2004, 2013a; Ehirim et al., 2009; Omonona et al., 2014). Obeta and Ocheja (2013) had reported unwholesome quality of groundwater in Ankpa, which is attributable to conditions related to poor hygienic conditions in the area.
Evaluation of groundwater for irrigation purposes has been carried out by many researchers, and several standards for the suitability of irrigation water have been set by many bodies. Examples include the works by Doneen (1964), Stuytzand (1989), Hagras (2013), Bhuiyan et al. (2015), and Mirza et al. (2014).
Location and geomorphology of the study area
Ankpa township is located within latitude 7° 23’ N to 7° 25’ N and longitude 7° 37’ E to 7° 39’ E in the central part of Ankpa local government area of Kogi State, north central Nigeria (Figure 1). The town lies on a gently undulating plateau bisected by the River Anambra valley. The river drains most of the area flowing from north to south of Ankpa and abuts the eastern side of the town. This may have been responsible for developments in the area, tending towards the western part of the river valley as residential and infrastructural developments as well as commercial activities are more concentrated in the western flank of the river.
Climatic conditions in the area
The climate of Ankpa is that of tropical hinterland of Nigeria (Iwena, 2012). The area experiences two main seasons, namely, wet and dry season. The wet season commences in May and lasts till October/November. Ankpa receives annual rainfall of up to 1000 mm. Dry dust-laden Harmattan wind blows from the north-east into the area during the dry season starting from November to April. Temperatures in the area range from 17 to 34°C. Average monthly relative humidity recorded in the area for the same year ranged between 65 and 94%. Ankpa belongs to the Guinea Savannah vegetation of Nigeria (Iwena, 2012). Cash crops such as palm trees and cashew (Anacardium occidentale) are commonly grown in areas directly overlain by the Ajali Sandstone, because of the pedological peculiarities of the areas (Nwajide, 2013); and particularly, Ankpa area is one of the largest producers of cashew in Nigeria.
Geology of the area
Ankpa lies within the northern flank of the Anambra sedimentary basin of Nigeria. The Anambra Basin is a funneled shaped basin (Figure 2) whose formation commenced with the Mid-Santonian deformation in the Benue Trough, displacing the major depositional axis westward, thereby leading to the formation of the basin (Obaje, 2009). The post deformational sedimentation in the Lower Benue Trough therefore constitutes the Anambra basin. Sedimentation in the basin commenced with the Campanian-Maastrichtian shales of the Enugu and Nkporo Formations, overlain by the coal measures of the Mamu Formation followed by the Cretaceous sandstones of the Ajali and Owelli Formations, which in turn are overlain by the Imo and Nsukka shale Formation deposited in the Paleocene. The uppermost layer consists of the Eocene Nanka Sandstone.
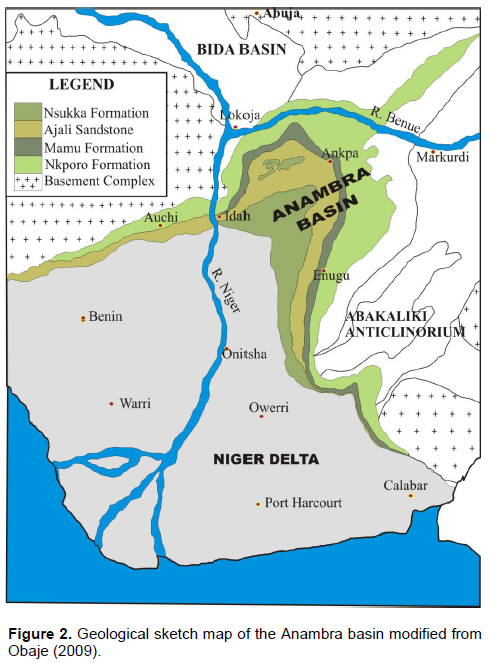
The Ajali Formation, which is also known as the Ajali Sandstone, is the most important aquiferous sandstone formation in the Anambra Basin, with thickness exceeding 300 m to about 450 m (Offodile, 2002). Ankpa area is located on the Ajali Formation. The Formation outcrops around Idah-Ankpa and Nsukka Plateaux, marking the Idah-Enugu Escarpment and covering most of Idah, Ankpa and Nsukka. According to Nwajide (2013), the characteristic scenery of most areas is underlain by the Ajali Sandstone is that of a gently undulating topography or flat plains, punctuated by inselbergs, which are lone mounds of erosional resistors. The vast terrain called the Ankpa plateau, which emerges southwards with the plains west of Enugu, exemplifies its characteristics. The formation consists of coarse grained sandstones with thin lenticular shales beds of grit and pebbly gravel. The aquiferous sands are friable, poorly sorted and typically whitish at depth. The Ajali sandstone tends to be ferruginized as a result of weathering (Kogbe, 1989) and laterite is virtually the topsoil in all areas underlain directly by the formation. Lateritic thicknesses up to 40 m have been reported in areas south of Ankpa (Ezeigbo and Ozoko, 1989).
The drainage density on terrains underlain by the Ajali Sandstone is generally low. This may be due to the ease of infiltration that greatly reduces overlain flow. Another perspective is that the water table is generally low, again due to ease and great depth of infiltration, such that springs are rare and rivers are therefore rarely generated (Nwajide, 2013).
The existence of several shallow perennial hand-dug wells in Ankpa is an evidence of the existence of shallow groundwater in the area. Some wells are as shallow as 10 m. This is not a reflection of the regional aquifer in the Ajali Sandstone, which has been reported to be up to 100 m in places (Ezeigbo and Ozoko, 1989). It is not the interest of this work to investigate the aquifer types in Ankpa. But the existence of shallow hand-dug wells that serve the water needs of the people portends health threat to the people because shallow aquifers are vulnerable to pollution (Onwuka et al., 2004, 2013a; Ofoma et al., 2008).
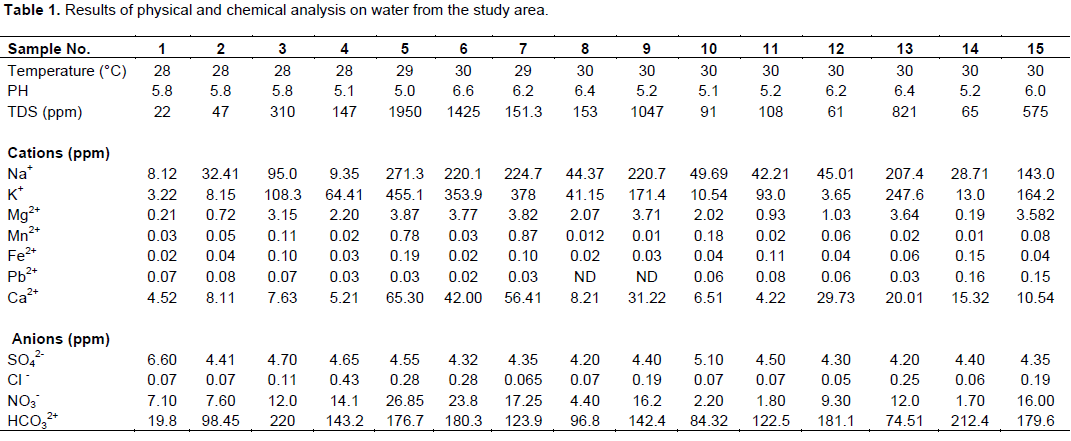
The investigation commenced with the utilization of global positioning systems (GPS) to mark out the locations of the fifteen (15) water wells within the study area (Table 1). This was followed by collection of the groundwater samples from the wells in pairs of plastic and glass bottles. One set of samples was used for the cation determination and the other set for anion determination. At the point of sampling, physical parameters such as acidity (pH), temperature and electrical conductivity that change rapidly with time were measured shortly after water withdrawal. Two (2) drops of concentrated Trioxonitrate (v) acid were added to each of the samples in the plastic bottles for homogenization and prevention of absorption/adsorption of trace metals to the walls of the container (Schroll, 1975). The treated samples were subsequently used to determine the concentration of the following cations and heavy metals: Na+, K+, Ca2+, Mg2+, Fe2+, Mn2+, and Pb2+. The samples in the glass bottles were used to determine the concentration of the following anions: SO42-, Cl-, NO3-, and HCO32+.
Determination of concentration of Ca2+, Fe2+, and Pb2+ was done using A.A.S. –Buck Scientific Model 210 VGP. Flame analysis was used to determine the concentrations of Na+ and K+. Concentration of SO42- was determined using the colometric method while concentrations of Cl- and HCO3- were determined using titrimetric methods. Determination of NO3- was done using a UV spectrophotometer. Total dissolved solids (TDS) were determined by evaporation technique involving gravimetric analysis, and this method has a detection limit of approximately 10 mg/L.
The results of the physico-chemical analysis of the water samples were evaluated to determine the quality of the water for both domestic and agricultural uses. For example, the potability of the water was determined by evaluating the hardness of the samples, and also by comparing the concentration levels of the parameters with the World Health Organisation (WHO) standards for drinking water. The parameters were also evaluated to determine and classify the water type (facies), and also to evaluate the corrosion and encrustation potentials of the water, as well as its acceptability for irrigation purposes. Groundwater characterization into facies was done by the use of Stiff pattern diagrams and Piper trilinear diagrams. Stiff pattern diagrams facilitate rapid comparison of results using absolute concentrations in milliequivalent per litre. The patterns were plotted by converting the measured absolute concentrations of the cations and anions to milliequivalent per litre followed by plotting of concentrations of the cations to the left of a vertical zero axis and the anions to the right of the same axis (Todd and May, 2005). For the Piper trilinear diagram, concentrations of major cations expressed as percentages of total cations in milliequivalent per litre were plotted as single plots on a left triangle while that of anions was plotted on a right triangle. The two plots were projected into a central diamond shaped area parallel to the upper edges of the central area.
Total hardness of the water, HT, was determined using the Todd and Mays (2005) relationship:
HT = 2.5 [Ca2+] + 4.1[Mg2+] mg/L (1)
The irrigation characteristics evaluated are the concentration of sodium in the water, the magnesium hardness (MH), and the permeability index (PI) of the soil. The concentration of sodium in the water was used to determine the sodium adsorption ratio (SAR) and the percentage sodium (%Na) in the water. The SAR was calculated using the expression of Todd and Mays (2005), as given in Equation 2:

(2)
T
The location of wells where samples collected and results of the field and laboratory measurements on the water samples are shown in Table 1. Table 2 shows the values of the chemical parameters in both mg/l and meq/l, along with some average values. The measured temperature of water (between 28 and 30°C), indicates that the area is warm at the time of measurement since temperature is a representation of the degree of hotness/coldness of the area at the time of sampling.
The pH values for groundwater within the area range from 5.0 to 6.6. These values indicate that water from the area is slightly acidic. This value agrees with Freeze and Cherry (1979) assertions on hydrogeochemical sequences and facies, that rainwater in non-urban, non-industrial areas have pH values generally between 5 and 6.
Some of the samples had slight taste which could be as a result of dissolved substances. For shallow wells, particles carried by rainwater while infiltrating underlying formations may not be well filtered out before making contact with the aquifer.
TDS in the groundwater from the area ranged from 22 to 1950 mg/L. Based on Carroll (1962) groundwater classification, 80% of the water samples from the study area with TDS between 22 and 821 mg/L can be classified as fresh water while 20% of the samples with TDS between 1047 mg/L and 1950 are brackish. In the course of movement, there may be some localized contact/dissolution of sodium salt (as indicated by the water type) in the area responsible for the 20% brackish water.
Though a detailed study of corrosion was not carried out, the few corrosion parameters measured in the groundwater were used to assess the corrosion potentials of groundwater in the area. The implications of the acidity (pH 5-6) and warm temperature of groundwater (between 28 and 30°C) in combination with TDS >1000 mg/L in about 20% of samples from the area is that groundwater from the area is capable of corroding metals, especially in the presence of other parameters like dissolved oxygen (not determined). A combination of any two corrosion parameters such as pH <7, TDS >1 000 mg/L, warm temperature, HCO3 > 50 mg/L and DO are required for corrosion to take place (Johnson, 1975).
Water type
Characterization of groundwater in Ankpa, using the Stiff pattern, Piper trilinear and Gibbs diagrams, are as shown in Figures 3, 4 and 5, respectively. These diagrams facilitate rapid comparison of results using the absolute concentration in Meq/l. The Stiff patterns can be a relatively distinctive method of showing water composition differences and similarities (Hem, 1985).
Those with similar qualities tend to plot together as a group.
The size of the pattern is approximately equal to the total ionic content (Hounslow, 1995). Apart from the pattern for sample 1, the Stiff patterns look similar and suggest no significant difference in the values of the TDS in the water samples, the shapes also suggest that the water samples are of the same source, with Na+ and HCO3- as the dominant cation and anion, respectively (Onwuka et al., 2013b).The order of dominance for cations is Na++K+ > Ca2+ > Mg2+, while that of anions is HCO3- > SO42- > Cl-.
Piper diagram can be used to determine water type, hydrochemical facies and ion exchange (Hounslow, 1995; Freeze and Cherry, 1979). The diamond part of Piper diagram may be used to characterize waters of different types (Hounslow, 1995). Water plotted at the lower corner of the diamond is primarily composed of alkali carbonates (Na++k+ & HCO3-+ CO32-) and can be classified as Sodium/Potassium Bicarbonate type. All the cations in the water samples plotted within the sodium and potassium section of the Piper trilinear plot, while the anions plotted within the bicarbonate section of the plot is as shown in Figure 4. This implies that the water is Na++K - HCO3 type and is in agreement with the results of the Stiff diagrams. This type of water can be described as juvenile water. It has low concentration of calcium and magnesium. The main source of such water is rainfall. The pH of the groundwater falls within the range of pH of water of precipitation.
Hydrochemical facies are dintinct zones that have cation and aniom concentrations described within defined concentration categories (Freeze and Chery, 1979); and designation of the facies is based on the manner suggested by Back (1961) and Back and Hanshaw (1965), in which facies are designated according to the domain in which they occur on the segments of a Piper diagram. On the basis of this subdivision, the groundwater in Ankpa belongs to the Sodium-Potassium facies and Bicarbonate facies. Clay minerals can have high cation-exchange capacities and may exert a considerable influence on the proportionate concentrations of the different cations in the water associated with them (Hem, 1985).
The Piper diagram clearly shows that cation exchange softening has increased the Na concentration at the expense of Ca and Mg concentrations. The Piper diagram also suggests that sulphate reduction may have taken place. Sulphate reduction is a kind of groundwater reaction that causes bicarbonate to increase, partly at the expense of sulphate. This reaction is caused by anaerobic bacteria (Hem, 1985).
The Gibbs (1970) diagram shows the mechanisms controlling groundwater chemistry, considering evaporation, dilution, weathering and precipitation. Most of the water samples from Ankpa plotted within the region of weathering, indicating more rock-dominated influence as opposed to evaporation, dilution and precipitation. This indicates that the water may have undergone possible extensive interaction with the geologic material underlying the area (Olayinka and Olayiwola, 2001; Tijani, 2003; Wang et al., 2004; Rao, 2006). Weathering is caused by the interaction of rocks with the atmosphere and hydrosphere, and can give rise to different products such as hydrolysates which are secondary products of the chemical breakdown of aluminosilicates, such as feldspars which are made up of clay minerals (Hounslow, 1995). Clay minerals are one of the major sources of cations like Na+, K+, Mg+ and Ca+ (Todd and Mays, 2005). These cations are common in the groundwater.
Hardness
The results of hardness of groundwater determined using divalent metallic cations responsible for hardness in groundwater in the study area, mainly calcium and magnesium is, shown in Table 3. Based on the Linsley et al. (1992) hardness classification of water (Table 4), only 3 samples, representing 20% of the water, are hard, with one (sample 5) tending to very hard water. The moderate hardness in Locations 9, 12 and 13 can be attributed to the closeness of the wells to a massive open refuse dump that has accumulated for over 20 years with leachate flowing into the sandy soil and possibly infiltrating into groundwater.
The water quality for irrigation
Quality of irrigation water is determined by its chemical composition and the conditions of use (Hussain et al., 2010). Two types of salt problems may exist in water: those associated with the total salinity and those associated with sodium (Fipps, 1969). Soils may be affected only by salinity or by a combination of salinity and sodium. Soil containing large proportions of sodium, with carbonate as the predominant anions is termed as alkali soil, whereas with chloride or sulphate as the predominant anions the soil is termed as saline soil. Both the spoil types will not support plant growth (Brindha et al., 2014). Saline soils are soils with high levels of total salinity. Saline conditions often result in “physiological” drought, where the soil is rendered impermeable, and though the field appears to have plenty of moisture, the plants wilt because the roots are unable to absorb water. Water with high salinity is toxic to plants and poses a salinity hazard (Fipps, 1969).
The other type of salt problem in water, known as sodium hazard, or alkalinity hazard, is the relative proportion of sodium to other principal cations. It is one of the most important characteristics in determining the quality of water for irrigation (Sadashivaiah et al., 2008). In this work, two aspects of sodium hazard of the
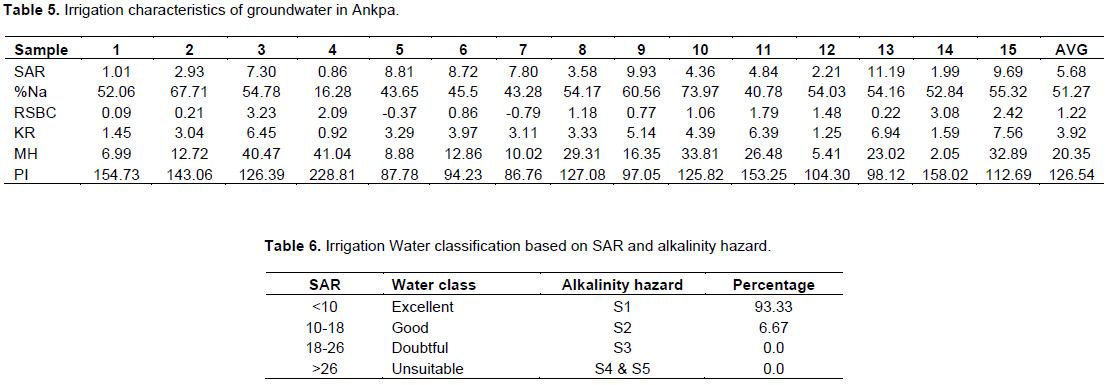
water in Ankpa area are considered as the Na% and sodium adsorption ratio (SAR). The sodium adsorption ratio is used to predict the potential for sodium to accumulate in the soil, which would result from continued use of sodic water (Al-Tabbal and Al-Zboon, 2012). It is a measure of the tendency of sodium ion (Na+) to displace calcium ion (Ca2+) in the irrigation water due to its high position on the table of electrochemical series (Uzoije et al., 2015). This implies that more of sodium ions are readily available in the water as considerable amount of calcium is displaced. This condition produces adverse effects on the soil’s physical conditions and consequently on the crops, leading to a situation reported as sodicity or sodium hazards (Ayers and Westcot, 1985, 1994; Uzoije et al., 2015). High SAR in irrigation water makes the soil hard, compact and impervious (George, 1983), and results in water-logged soil conditions (Misstear et al., 2006), with its related plant diseases which include stunted growth and leaf burnt (Uzoije et al., 2015). In contact with soils, sodium reacts and reduces the permeability of the soils thereby supporting little or no plant growth. The results of SAR calculated for groundwater in the study area are shown in Table 5. The SAR values range from 0.86 to 11.19, with an average of 5.68. Table 6 shows that apart from sample 13 whose water is just good, fourteen (14) of the samples, constituting more than 90%, are excellent for irrigation, with respect to the sodium absorption ratio classification (Richard, 1954). Table 6 also shows the classification of the alkalinity hazard (Al-Tabbal and Al-Zboon, 2012), which indicates that all the samples, apart from Sample 13 fall in the low sodium class (S1). Sample 13 falls in the S2 class. The implication is that no alkali hazard is anticipated to crops grown with the water. According to Singh et al. (2009), low sodium water (S1) can be used for irrigation on almost all soils with little danger of the development of harmful levels of exchangeable sodium; while medium sodium water (S2) will present an appreciable sodium hazard in fine textured soils with good permeability. High sodium water (S3) may produce harmful levels of exchangeable sodium in most soils and will require special soil management, including good drainage, high leaching and organic matter additions.
The range of the %Na in the groundwater in this study is between 16.28 (Sample 4) and 73.97 (Sample 10), with an average value of 51.27 (Table 5). Table 7 shows that the water ranges from excellent to doubtful for irrigation. No samples fall under water class “Good” and class “Unsuitable”. When the concentration of sodium is high in irrigation water, sodium ions tend to be absorbed by clay particles, displacing Mg2+ and Ca2+ ions. This exchange process of Na+ in water for Ca2+ and Mg2+ in soil reduces the permeability and eventually results in soil with poor internal drainage (Al-Tabbal and Al-Zboon, 2012). Deflocculation and impairment of soil, which reduce permeability due to high %Na in groundwater result in restricted air and water circulation during wet conditions, and such soils are usually hard when dry (Kranth, 1987; Collins and Jenkins, 1996; Saleh et al., 1999; Singh et al., 2008, 2009). Besides affecting the growth of plants directly, high levels of sodium (and other salts), which affect soil structure, permeability and aeration, indirectly also affect plant growth (Singh et al., 2008, 2009).
Sadashivaiah et al. (2008) observed that in waters having high concentration of bicarbonate, there is a tendency for calcium and magnesium to precipitate as the water is increased in the form of sodium carbonate. The concentration of magnesium in water would adversely affect the soil quality, rendering it unfit for cultivation (Venugopal et al., 2009). The high concentration of magnesium in groundwater can be attributed to dolomite, a chief mineral of sandstone and siltstone (Haritash et al., 2008). Waters with MH >50 are considered to be harmful and unsuitable for irrigation use (Szabolcs and Darab 1964; FAO/UNESCO, 1967; Singh et al., 2009).
Magnesium in water would adversely affect the soil quality, rendering it unfit for cultivation (Venugopal et al., 2009). Magnesium hardness (MH) in the groundwater in the study area ranges from 2.05 to 41.04%, with an average of 20.35% (Table 5). MH above 50 is considered to be unsuitable for irrigation (Singh et al.,2009; Brindha et al., 2014). This indicates that the entire water is safe for use for irrigation purposes. If magnesium is less than 50, then the water is safe and suitable for irrigation (Szabolcs and Darab, 1964; Al-Tabbal and Al-Zboon, 2012).
The permeability index (PI) of a soil is a function of the sodium, calcium, magnesium and carbonate in the soil (Doneen, 1964; Vasanthaiviger et al., 2010). The PI of groundwater in the study area ranges from 87.76 to 158.02, with an average value of 126.54 (Table 5). Doneen (1964), and also the World Health Organization (WHO) classified irrigation water quality into three: Class I, Class II and Class III (Brindha et al., 2014; Åžen, 2015). Water of Class I and Class II with 75% of maximum permeability in the Doneen chart are considered to be good and suitable for irrigation, while Class III water is unsuitable for irrigation (Brindha et al., 2014). With regards to the PI, all the water samples fall under Class III, and are therefore unsuitable for irrigation.
The Kelley ratio (KR) is the level of Na+ measured against Ca2+ and Mg2+ (Kelley, 1940; Paliwal, 1967). In this study, KR ranges from 0.92 to 7.56, with an average of 3.92 (Table 5). KR value more than one is generally considered unfit for irrigation (Al-Tabbal and Al Zboon, 2012). It is seen from Table 5, therefore, that the water, apart from sample 4, is unsuitable for irrigation.
A relation of alkaline earths with weak acids is expressed in terms of residual sodium carbonate (RSC) for assessing the quality of water for irrigation (Richards, 1954). It is determined as excess of CO32- and HCO3- over that of CA2+ and Mg2+ concentration in irrigation water. It gives an overview of the permeability problems of soil when irrigated with high sodium waters rather than normal irrigation waters (Hussain et al., 2010). When the weak acids are greater than the alkaline earths, a precipitation of alkaline earths occurs in soil, which damages the permeability of soil (Rao et al., 2012; Bhuiyan et al., 2015). Gupta (1983) suggested that residual sodium carbonate (RSC) should be calculated simply as residual sodium bicarbonate (RSBC) (Mirza et al., 2014). RSBC indicates the excess concentration of HCO3- over Ca2+ (Hussain and Hussain 2004). The RSBC for the groundwater samples ranges from -0.79 to 3.23, with an average of 1.22 (Table 5). The classification of irrigation water is as given in Table 8, after Gupta and Gupta (1987). Table 8 indicates that the groundwater is safe for use as irrigation water.

Chloride in irrigation water is the most common source of toxicity. Chloride is highly soluble in water; and because it is not absorbed or held back by soil, therefore moves readily with the soil-water, and is taken up by the crops, moves in the transpiration stream and accumulates in the leaves (Hussain et al., 2010). In the study area, chloride concentrations in groundwater vary between 0.001 and 0.008 meq/l, with evaluation rating of 1 (Christiansen and Olsen, 1973). This implies that the groundwater is excellent for irrigation, according to the classification of Scofield (1936). Again from the Scofield classification (Table 8), the sulphate content in the water also shows the water to be excellent for irrigation.