ABSTRACT
A field experiment was carried out to evaluate heavy metals and physiochemical concentration of tannery effluents from Kano metropolis. Fifteen samples from five selected tannery industries from Sharada and Challawa industrial estate were collected and determined for heavy metals using atomic absorption spectrophotometric method (AAS) and the result of the study showed that the range mean and standard deviation values for chromium (Cr), lead (Pb), iron (Fe) and copper (Cu) were found to be 3.33 ± 0.74 - 5.79 ± 0.96 mg/L, 0.67 ± 0.07 - 3.10 ± 1.07 mg/L, 3.53 ± 1.06 - 8.12 ± 0.69 mg/L, and 0.82 ± 0.53 - 1.51 ± 0.91 mg/L, respectively. The physiochemical values were pH 3.96 ± 1.22 - 10.60 ± 2.49, conductivity, 1554 ± 17.05 - 11410 ± 414.32 μs/cm; total suspended solid, (TSS) 1026.00 ± 170.01 - 3365.60 ± 112.63 mg/L and total dissolved solid (TDS) was 1585.00± 49.53 - 7250.00 ± 73.73 mg/L. With exception of Fe in all sites and Pb in S4, values for the other metals were above the maximum permissible limits of both the Federal Environmental Protection Agency of Nigeria (FEPA) and World Health Organization (WHO). The values for TDS for S4 and pH value for S1 to S3 were within allowable limits, but conductivity and TSS values were above the allowable limit. These findings render the effluents harmful to the environment, and there is the need to take practical steps to avoid pollution and impending health hazard.
Key words: Concentration, contamination, effluent, heavy metal, physiochemical, sewage, tannery.
Discharges from tanneries are part of the major causes of environmental contamination in Nigeria and the world over. In recent years, large scale usage of chemicals in various human activities has grown considerably, and pollution has assumed an escalating dimension due to the continual expansion of urbanization, industrial development and agricultural activities. These pollutants find their way to aquatic ecosystem such as rivers, ponds and lakes, which pose a risk to both the human health and environment (Rehman and Anjum, 2010).
Insufficient environmental monitoring and planning often result in discharging of industrial and sewage waste into rivers and lakes which lead to gradual pollution of the water resources. Many times such wastewater is drained to the agricultural land where this polluted water is used for irrigating crops including vegetables (Aklilu et al., 2013). The consequences of untreated industrial effluent has increased water bodies pollution, loss of aquatic life
and uptake of polluted water by plants and animals, which eventually get into the human body resulting in health related problems. The situation is compounded by the fact that the common man in most of these developing countries like Nigeria does not have access to portable water, and in many instances, untreated river water is used as source of drinking water (Dan’Azumi and Bichi, 2010).
Groundwater resources are experiencing an increasing threat of pollution coming from industrial effluents which contain high concentration of metals and other physiochemical pollutants, that may be toxic, mutagenic, carcinogenic, and teratogenic, especially chromium, copper cadmium conductivity and TDS (Malarkodi et al., 2007). These effluents released on the land or into the surface water will ultimately leach to ground water and lead to contamination. Heavy metals are not easily biodegradable and lead to their accumulation in human vital organs causing varying degrees of illness on acute and chronic exposure. The accumulation also results in a series of well documented problems in plants and animals because they cannot be completely degraded (Malarkodi et al., 2007; Mustafa et al., 2010).
Chromium is a major constituent of tannery effluent tends to accumulate in living organisms, causing serious diseases and environmental pollution. It exists in oxidation states of +2, +3, and +6. The trivalent oxidation state is the most stable form of chromium and is essential to mammals in trace concentration and relatively immobile in the aquatic system due to its low water solubility. The hexavalent chromium is much more toxic to many plants, animals, and bacteria inhabiting aquatic environments (America Public Health Association (APHA), 1998; Dan’Azumi and Bichi, 2010). Hence, tannery effluents offer a wide scope of environmental problems and health hazards and are becoming more complex and critical in developing countries.
Currently, plants and microorganisms are used to remove some heavy metals such as mercury (USEPA, 2000). Plants which exhibit hyper accumulation can be used to remove heavy metals from soils by concentrating them in their bio matter. In medical usage, heavy metals are loosely defined and include all toxic metals irrespective of their atomic weight: "heavy metal poisoning" (USEPA, 2000). Consumption of water with high concentrations of TDS and conductivity have been reported to cause disorders of alimentary canal, respiratory system, nervous system, coronary system besides , causing miscarriage and cancer (Reddy and Subba, 2001).
Reduced crop contamination and improved safe food can be achieved through, reducing pollution at source, improved vegetable production and post harvest handling and using support for vegetable trading systems to improve food safety The increasing discharge of industrial wastes in this river basin is posing serious danger to the water resources and the health of people in the area.
The aim of this research work is to ascertain the level of tannery effluents pollution in the environment and advise on possible ways of reducing effluent pollution.
Description of study area
Nigeria is located approximately between latitude 4o and 14o North of the equator, and between longitude 2° 2’ and 15° east of the Greenwich meridian and Kano state which is ranked first in population with well over 9.0 million people, lies between latitude 12°00’ and 09.4°N and longitude 08°31’ and 07°29’E in Northern Nigeria. It is historically a commercial and agricultural State, and in fact the centre of commerce. The high population is brought about by the much economic and industrial activities taking place in the city. The major industries in the city include tanneries, textiles, chemicals and allied products. Kano City is located on the main watershed which separates the two main river basins in the metropolis. The main industrial areas of Kano - Bompai, Sharada, and Challawa - are located within the two river basins (Bichi and Anyata, 1999). The selected industrial sites were based on their appreciable size and location amongst other criteria.
Collection/treatment of effluent samples
Samples were collected from five tanneries sites of S1, S2 (Sharada) S3, S4 and S5 (Challawa) for three times between March and September each in plastic containers previously washed with detergents and 10% HNO3 acid and thoroughly rinsed with de-ionized water. Suitable volume of sample was taken, and then acidified with concentrated HNO3 to bring down the pH down to 2.0 this is to maintain the stability of the oxidation state of the various elements in solution and prevent precipitation. All the samples were taken to the laboratory and stored at 4ºC temperatures in a refrigerator till the analyses were completed. 100 cm3 of sample was then taken and added 5 cm3 concentrated HNO3, and then digested. The digested samples were analyzed for chromium, iron, copper, and lead concentrations by atomic absorption spectrophotometer (Buck Scientific210 Model). Meters were used to determine the pH and conductivity while gravimetric method was used for TDS and TSS (APHA, 1998).
The atomic absorption spectrophotometric analysis of heavy metal in tannery samples is summarized in Table 1.
In the present study, the range of mean for Cr, 3.33 - 5.79 mg/L has been reported in Table 1, which was higher than the maximum permissible limits of both the FEPA and WHO. In a similar study in the region 1.02 - 1.56 mg/L (Akan, 2007) and 2.49 mg/L (Dan’Azumi and Bichi, 2010), were reported. While in India 7.21 mg/L was reported (Deepali, 2010), and value as high as 9.00 mg/L has also been reported in Indian (Dikshit and Shukla, 1989). Cr is the major chemical used in tanning process and hence its discharge in the effluent was found to be higher than maximum permissible limits in all reported cases.
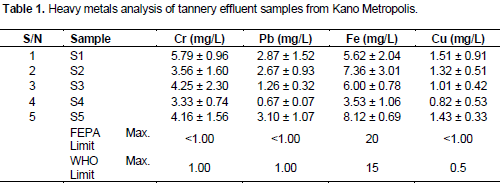
Continuous discharge of Cr in low concentration has been reported to be toxic to aquatic life and has been shown to disrupt the aquatic food chain. It dominated the tannery effluent with the highest deviation from the allowable concentration and has been reported by most literatures available. Chronic exposure to chromium may be associated with allergic dermatitis in humans, permanent eye injury high blood pressure and DNA damage (Scragg, 2006)
The observed concentration range of 0.67 - 3.10 mg/L for Pb which was higher than the acceptable limit but for site S4 was reported. This findings agreed with the values reported by most researchers from both Nigeria (Sangadoyin, 1995; Dan’Azumi and Bichi, 2010) and India (Begum et al., 2009). High concentration of Pb may result in metallic poisoning that manifests in possible human carcinogenic (Bakare-Odunola, 2005). High Pb concentration may result in birth defects, mental retardation, autism, psychosis, allergies, dyslexia, hyperactivity, weight loss, shaky hands, muscular weakness, abdominal pain, head ache, irritability, joint pain, fatigue, anemia and paralysis (beginning in the forearms). It is carcinogenic and toxic, affecting, the central nervous system, the kidneys or liver, skin, bones and teeth (Hogan, 2010). In comparison with a study where a concentration of 0.1 mg/L had resulted in development of neurological problems in fetuses and children, the result obtained in this study definitely requires urgent attention by all and sundry (Fatoki et al., 2005).
The analytical results revealed 3.53 - 8.12 mg/L., as the range for Fe, which was within allowable limit. Earlier studies in the region reported 1.23 - 1.16 (Fatoki et al., 2005) and 2.14 mg/L (Rehman and Anjum, 2010; Yusuff and Sonibare, 2004) but, value as high as 4.41 - 14.556 mg/L has also been reported (Dan’Azumi and Bichi, 2010). On the contrary much lower values of 0.351 mg/L (Tariq, et al., 2006) and 0.75 mg/L (Deepali, 2010) were reported in effluent released from tanneries in India. Fe is a necessary element found in nearly all living organisms considered at the border between macro and micro elements. Iron-containing enzymes and proteins often contain heme prosthetic groups that participate in many biological oxidations and in transport (Chandra et al., 2009).
For Cu a concentration ranges of 0.82 - 1.51 mg/L was reported for Cu which was higher than the permissible value. Similarly high concentrations were earlier reported in Kano State (Dan’Azumi and Bichi, 2010) and Lagos (Sangadoyin, 1995). On the other hand Deepali (2010) reported Cu content of 0.022 mg/L in Indian. Cu is an integral part of numerous enzymes, normal copper homeostasis is essential for human growth and development, as well as for disease control in livestock and poultry, a cofactor in enzymes including ferro-oxidase (ceruloplasmin), cytochrome – c –oxidase, superoxide dismutase and others. It plays a role in iron metabolism, melanin synthesis and central nervous system function. (Fisher, 2001), but at high concentration it affects the liver and causes kidney damage and gastrointestinal distress, bone diseases, renal failure, dermatitis, pulmonary cancer, have been reported (Hogan, 2010).
From Table 2, it can be seen that the pH values for sites S4 (3.96) and S5 (10.60) were outside the allowable limit but others were within. Akan et al. (2007) in Kano and Assefa and Ayalew (2014) from Ethiopia reported values that fell within the permissible limit. pH outside permissible limit adversely affects the availability of plant nutrients, heavy metals concentrations, growth of algae and micro organism (Akan et al., 2007).
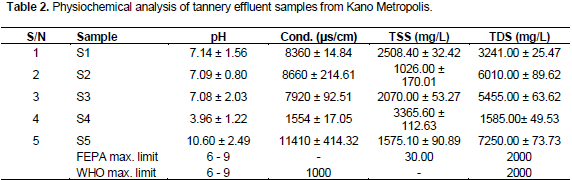
The range of conductivity obtained in this study was 1554 – 11410 μs/cm which was higher than the maximum permissible limits of both the FEPA and WHO. Other values reported were 3668- 4370 μs/cm (Assefa and Ayalew, 2014) and 6020 μs/cm (Ram, 2002). High values of conductivity indicates the presence of higher concentration of ions (Deepali et al., 2009).
TSS range of 1026.00 - 3365.60 mg/L was reported, but significantly low and high values have previously been reported 204 - 525 mg/L (Assefa and Ayalew, 2014) and 3491.9 - 9485.33 mg/L (Deepali et al., 2009).
The mean range of TDS was 1585.00 - 7250 mg/L, this value was more than the FEPA and WHO standard but for location S4 (1585.00 mg/L) with permissible value. Akan et al. (2007) report lower value of 661.4 - 1281.1 mg/L. On the contrary extremely high value of 42716.33 mg/L was reported in Indian (Raskin and Ensley, 2000;
Deepali et al., 2009). High TDS value increases the salinity of water and thus may render it unhealthy for drinking and irrigation purposes. This makes the discharge of wastewater into surface water harmful. Consumption of water with high concentrations of TDS has been reported to cause disorders of alimentary canal, respiratory system, nervous system, coronary system besides causing miscarriage and cancer (Reddy and Subba, 2001)..Earlier analysis by Ogabiela et al. (2007) substantiated this study by reporting values for conductivity, TSS, and TDS that were higher than permissible limit in Kano.
In this study it was observed that location S4 had low pH and the concentration of heavy metals were relatively lower and it concurred with the discovery that for pH above 6 heavy metals tend to remain insoluble and more inert (Raskin and Ensley, 2000). But this finding does not clear S4 Tannery as an industry complying with standard because the acidic state of the effluent is a serious breach of acceptable practice.
The results of this study show that the concentration of Cr, Pb and Cu were above the limits set by FEPA and WHO. The values for pH values for some sites were within allowable limits, but others were not. Conductivity and TSS values were above the allowable limit same with most TDS values. These findings rendered the effluents harmful to the surroundings and to keep the environment healthy, discharge of untreated tannery effluent into water bodies and lands should be strongly discouraged and regular monitoring of soil, plant and water quality to ascertain the safety level should be encouraged. In addition strict legislation and stringent standard practices must be enforced to prevent the indiscriminate disposal of untreated effluent into the environment.
The authors have not declared any conflict of interest.
REFERENCES
Akan JC, Moses EA, Ogugbuaja VO, Abah J (2007). Assessment of tannery industrial effluents from Kano metropolis, Kano State, Nigeria. J. Appl. Sci. 7:2788-2793.
Crossref |
|
|
|
Aklilu A, Mengistu S, Fisseha I (2013). Determining the Extent of Contamination of Vegetables Affected by Tannery Effluent in Ejersa Area of East Shoa, Ethiopia. Int. J. Sci. Res. Pub. 3:5. |
|
|
|
Americal Public Health Association (APHA) (1998). Standard Methods for the examination of water and waste water, 19th edition. American Water works Association water pollution control federation publication. Washington, D.C. |
|
|
Assefa W, Ayalew W (2014). Bahir Dar tannery effluent characterization and its impact on the head of Blue Nile River. Afr. J. Environ. Sci. Technol. 8(6):312–318.
Crossref |
|
|
|
Bakare-Odunola MT (2005). Determination of some metallic impurities present in soft drinks marketed in Nigeria. The Nig. J. Pharm. 4(1):51-55. |
|
|
Begum A, Ramaiah M, Harikrishna I, Khan I, Veena K (2009). Heavy Metals Pollution and Chemical Profile of Cauvery River Water. E-J. Chem. 6(1): 47-52.
Crossref |
|
|
Bichi MH, Anyata BU (1999). Industrial Waste Pollution in the Kano River Basin. Environ. Manage. Health 10(2):112-116.
Crossref |
|
|
Chandra R, Bharagava RN, Yadav V, Mohan D (2009). Accumulation and distribution of toxic metals in wheat (Triticum aestivum L.) and Indian mustard (Brassica campestris L.) irrigated with distillery and tannery effluents. J. Hazard. Mater. 162:1514.
Crossref |
|
|
|
Dan'Azumi S, Bichi MH (2010). Industrial Pollution and Heavy Metals Profile of Challawa River in Kano, Nig. J. Appl. Sci. Environ. Sanitation 5(1):23-29. |
|
|
|
Deepali KK (2010). Metals Concentration in Textile and Tannery Effluents, Associated Soils and Ground Water. New York Sci. J. 3(4):82-89. |
|
|
|
Dikshit VP, Shukla NP (1989). Waste recycling and pollution control in Indian tanneries. Indian J. Environ. Prot. 9(3):182-86. |
|
|
|
Fatoki OS, Lujiza N, Ogunfowokun OA (2005). Trace metal pollution in Umtata river. Water, S. A. 28(2):183. |
|
|
|
Fisher DC (2001). Copper. In: Sullivan JB Jr. Krieger GR. Clinical Environmental Health and Toxic Exposures. 2nd Edition, Lippincott Williams & Wilkins. |
|
|
|
Hogan CM (2010). Heavy metal. Encyclopedia of Earth. National Council for Science and the Environment. Eds Monosson E, Cleveland C. Washington DC. |
|
|
Malarkodi M, Krishnasamy R, Kumaraperumal R, Chitdeshwari T (2007). Characterization of heavy metal contaminated soils of Coimbatore district in Tamil Nadu. J. Agronomy. 6(1):147-151.
Crossref |
|
|
Mustafa S, Ahmad T, Naum A, Shah KH, Wassum M (2010). Kinetics of chromium ion removal from tannery wastes using Amberliti IRA 400c and its hybrids. Water Air Soil Pollution 210(1-4):43-50.
Crossref |
|
|
|
Ogabiela EE, Agunwa UB, Lawal FA, Awoeye LD (2007). Analysis of tannery effluent from the confluence of discharge point at sharada industrial estate in Kano, Nigeria. J. Chem. Soc. Nig. 32(2):17–19. |
|
|
|
Ram CS (2002). Compliance monitoring of industrial effluents standards in Nepal, State of the environment, Nepal. 6:15-16. |
|
|
|
Raskin B, Ensley D (2000). Phytoremediation of toxic metals: Using plants to clean up the environment, John Wiley & Sons, New York, NY, USA. |
|
|
|
Reddy PM, Subba RN (2001). Effects of industrial effluents on the ground water regime in Vishakapatnam. Pollution Res. 20(3):383-386. |
|
|
Rehman A, Anjum MS (2010). Cadmium uptake by yeast, Candida tropicalis, isolated from industrial effluents and its potential use in wastewater clean–up operations. Water, Air Soil Pollution 205:149-159.
Crossref |
|
|
Sangadoyin AY (1995). Characteristics and control of industrial effluent generated. Pollution Environ. Manage. Health 6(4):15-18.
Crossref |
|
|
|
Scragg A (2006). Environmental Biotechnology, Oxford University Press, Oxford, UK, 2nd edition. |
|
|
Tariq SR, Shah MH, Shaheen N, Khalique A, Manzoor S, Jaffar M (2006). Multivariate analysis of trace metal levels in tannery effluents in relation to soil and water- A case study from Peshawar, Pakistan. J. Environ. Manage. 79:20-29.
Crossref |
|
|
|
US Environmental Protection Agency (USEPA) (2000). EPA's Terms of Environment. U.S. Environmental Protection Agency. |
|
|
|
Yusuff RO, Sonibare JA (2004). Characterization of textile industries' effluents in Kaduna, Nigeria and pollution implications. Global Nest: Int. J. 6(3):211-20. |

