The self-association, optical transition probabilities, and hetero-association with chlorogenic acid of nicotinamide were obtained from UV-Vis spectroscopy in aqueous solution at room temperature (293K). The dimerization constant of nicotinamide ( ) was obtained using dimer model by nonlinear curve fitting technique. The hetero-association constant in the system of molecules of nicotinamide with chlorogenic acid were obtained in aqueous solution using a Benesi-Hildebrand equation. In order to characterize the binding system of the dimerization reactions for the self and hetero-association of the compound, the thermodynamic parameters were investigated using Vant’s Hoff equation at the temperature range (293 to 299K). As a result, the change of enthalpy obtained for the self and hetero-association is and respectively. The values of change in the thermodynamic parameters indicated that the hydrophobic interaction and electrostatic forces subsequently plays the major role in the binding reaction between the molecules of nicotinamide and its complexes with chlorogenic acid, respectively. In addition, the optical transition probabilities of nicotinamide were also calculated in the wavenumber regions by using integrated absorption coefficient techniques. Finally, the results of this study are very important for understanding the binding reaction in biological system, nature and strength of the transition in molecular interaction, absorption spectral interpretation, and in providing stringent test of atomic and molecular structure calculations in theoretical work of the compounds.
Vitamins are organic chemical compounds, which are very important for an organism as a vital nutrient to sustain life since they play an important role in normal metabolism process, growth and vitality (Hassan, 2012). Nicotinamide (Vitamin B3) is an active, water soluble and amide form vitamin that is found in small amounts in natural foodstuff (Hassan, 2012). It can be obtained through synthesis in the body or as a dietary source and supplement of different food sources such as chicken, pork, beef, fish, legumes, nuts, grain products, mushrooms, yeast extracts, and coffee (Hassan, 2012; FSANZ, 2011; DiPalma and Thayer, 1991). It functions to the coenzymes nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADP), and is a precursor of essential enzymatic reactions in the body including adenosine triphosphate (ATP) production (Williams and Ramsden, 2005; Rolfe, 2014; Shalita and Smith, 1995; Jackson et al., 1995). Lack of nicotinamide leads to pellagra, fatigue, loss of appetite, pigmented rashes of the skin, oral ulcerations, dermatitis, dementia and death (Maiese et al., 2009; Hegyi et al., 2004).
Functional foods and bioactive components such as chlorogenic acid of different fruits and vegetables are beneficial for human health (Liu, 2003; Crowe, 2013) and because they present in popular drinks and foods (that is, in coffee, tea, cola beverages and chocolates). They are the most widely consumed of all behaviorally active drugs in the world (Clifford, 1979; Bolton and Null, 1981). Chlorogenic acid (CGA) is a main phenolic natural product that possesses many health benefits, including antioxidative (Wen et al., 2004; Richelle et al., 2001; Ayaz et al., 2008), antibiotic, anti-hypercholesterolemia, antihypertensive (Svilaas et al., 2004), as a selective inhibitor for the production of glucose in liver (Schwab, 2001), in plant metabolism or glucose absorption (Svilaas et al., 2004), used in disorders such as obesity, diabetes, and cancer (Wang et al., 2008; Clifford et al., 2010), chemopreventive, and other biological activities (Nazzaro et al., 2009).
With the increasing use of food supplements and the rapid development of new types of drugs, food/drug interactions are currently a great field of interest. Interaction of bioactive compounds with vitamins and aromatic drugs is one of the most common study areas, since the compounds can be found in various food sources and the interaction may affect the pharmacodynamics and pharmacokinetics of the compounds (Crowe, 2013). Thus, this study is important because knowledge of the association, optical transitional probability and thermodynamic parameters are very important for understanding the binding reaction in biological system, nature and strength of the molecular interaction in liquid solutions, to characterize the electron transition probabilities, interpreting the absorption spectra, and in providing stringent test of atomic and molecular structure calculation in theoretical works (Belay, 2013; Bayliss, 1950; Mac Rae, 1957; Milonni and Eberly, 1988; Ataklti et al., 2016). Chlorogenic acid was studied for self and hetero-association with ethidium bromide (EB) (Belay, 2013) and with five antibiotics drugs (amikacin, ampicillin, ciprofloxacin, erythromycin, and vancomycin) for the application against bacteria (Hemaiswarya and Doble, 2010). To the best of our knowledge the self and hetero-association, optical transitional probability and thermodynamic parameters of the nicotinamide to elucidate structures, optical transition and thermodynamic properties of the molecules are not yet investigated using UV-Vis spectroscopy. UV-Vis spectroscopy is the simplest techniques to study such kind of interactions, since the technique is highly sensitive, rapid and can be easily implemented (Belay, 2013; Niazi et al., 2006; Ataklti et al., 2016). Therefore, the objective of this work is to investigate the self-association, optical transition probability and hetero-association with chlorogenic acid of nicotinamide using UV-Vis spectroscopy.
Nicotinamide (NIC, Figure 1a) and chlorogenic acid (CGA, Figure 1b) from Sigma-Aldrich were used for measurements without any further purification. All solutions were made using doubly distilled water. The solutions were stored in the dark to avoid photo degradation of the compounds. For the electronic absorption measurements of the solutions, Perkin-Elmer Lambda 19 UV-Vis Spectrophotometry with double monocromator using 1 cm fused quartz cuvette in a spectrum range 200 to 500 nm was used at room temperature (293K). The analyzed spectra were obtained by subtracting the spectrum of pure solvent (water) from that of the solution containing of the compounds. A digital balance with accuracy of 0.0001 g, measuring cylinders, pipettes, and volumetric flasks, magnetic stirrer with hot plate and beakers where also used.
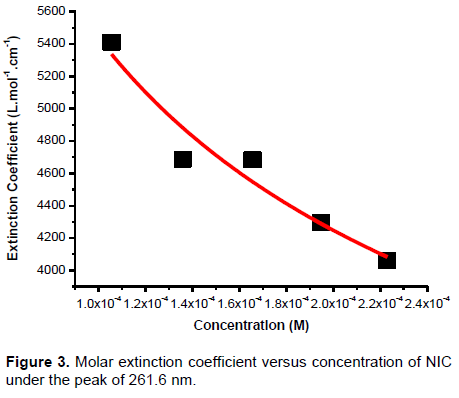
The self-association of NIC was studied over the concentration range of . The absorbance as a function of concentration has been measured at absorption maxima 261.6 nm to obtain the greatest accuracy of detection. For numerical analysis of the self-association, dimer model equation was used by fitting to the experimental data using non-linear curve fitting based on Levenberg-Marquardt algorithm using Origin 8 software (Ataklti et al., 2016; Belay, 2010). The molar extinction coefficients and equilibrium constants were used as searching parameters, in order to achieve minimum discrepancy between the experimental data and equations.
Moreover, the complexation of NIC-CGA the constant NIC concentration was titrated by CGA solutions in a concentration range of . The numerical values of the association constant and molar extinction coefficient of the complexes between NIC-CGA at their maximum wavelength was analyzed using the Benesi-Hildebrand approach by a linear curve fitting for the experimental data with that of the theoretical values obtained from equation (16) using Origin 8 software.
Similarly, the thermodynamic parameters such as enthalpy, gibbs free energy and entropy of the self-association of NIC and its hetero-association with CGA were studied at the temperature range (293 to 299K), respectively. These parameters have been determined using the model of Vant’s Hoff’s equation by linear curve fitting for the experimental data with that of the theoretical values.
The integrated absorption technique which is more powerful in measuring the intensity of absorption light has been used to calculate the optical transition probabilities (transition dipole moment, oscillator strength and integrated absorption cross-section) (Ataklti et al., 2016; Belay, 2010; Belay, 2013). The pure nicotinamide was studied by integrating the absorption coefficient and molar decadic absorption coefficients in the wave number regions of 20000 to 40000 cm-1. Usually, the UV-Vis spectrophotometer measures the concentration in terms of absorbance versus wavelength; this was recalculated into absorption coefficient or molar decadic absorption coefficient versus wave number using Origin 8 software.
Self-association of nicotinamide
Figure 2 shows the concentration dependence of self-association spectra of NIC measured in dually distilled water at room temperature (293K) with one absorption peak observed at the wavelength 261.6 nm. The quantitative analysis for self-association of nicotinamide was carried out using the concentration dependent molar extinction coefficient of the molecules at its maximum wavelength.
Numerical analysis was carried out using dimer model and the equation derived according to the following molecular equilibrium in solutions (Belay, 2010; Belay, 2012; Ataklti et al., 2016).
where and are monomers and dimers of the compound, respectively and is the equilibrium dimerization constant. The overall concentration of the dissolved molecules in the solution, using the mass conservation law can be written as:
The contribution of the monomer and dimer to the molar extinction coefficient of the solution is commonly considered to be additive and
where are molar monomer extinction coefficients, molar dimer extinction coefficients, equilibrium mole fraction of the molecules in the monomer concentration and equilibrium mole fraction of the molecules in the dimer concentration, respectively.
The concentration can be derived from the mass conservation law given in equation (2) by substituting on the account of equation (3). Thus, the known dimer model is obtained as,
In Equation 6, there are three unknown parameters , and which can be obtained from fitting dimer model equation to the experimental data as shown in Figure 3. The obtained values of the dimerization constants , monomer extinction coefficient and dimer extinction coefficient of NIC at wavelength 261.6 nm are , and , respectively. The deviation of the Beer-Lambert’s law at high concentration and dependence on concentration, suggest the existence of self-association process of the molecule (Antonov et al., 1999; Belay, 2012). The existence of self-association of nicotinamide may modify the pharmacokinetic properties of the compound.
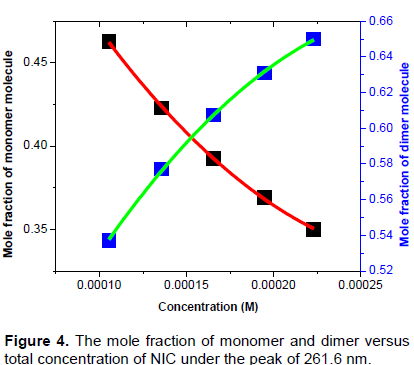
Figure 4 shows the mole fraction of monomer and dimer versus concentration of nicotinamide molecules under the peak of 261.6 nm. The graphs show increase and decrease in the mole fraction of dimer and monomer as the concentrations of the NIC is increasin gand it a linear curve fitting for the experimental data with that of the theoretical values obtained from equation (16) using Origin 8 software.
Similarly, the thermodynamic parameters such as enthalpy, gibbs free energy and entropy of the self-association of NIC and its hetero-association with CGA were studied at the temperature range (293 to 299K), respectively. These parameters have been determined using the model of Vant’s Hoff’s equation by linear curve fitting for the experimental data with that of the theoretical values.
The integrated absorption technique which is more powerful in measuring the intensity of absorption light has been used to calculate the optical transition probabilities (transition dipole moment, oscillator strength and integrated absorption cross-section) (Ataklti et al., 2016; Belay, 2010; Belay, 2013). The pure nicotinamide was studied by integrating the absorption coefficient and molar decadic absorption coefficients in the wave number regions of 20000 to 40000 cm-1. Usually, the UV-Vis spectrophotometer measures the concentration in terms of absorbance versus wavelength; this was recalculated into absorption coefficient or molar decadic absorption coefficient versus wave number using Origin 8 software.
Hetero-association of nicotinamide with chlorogenic acid
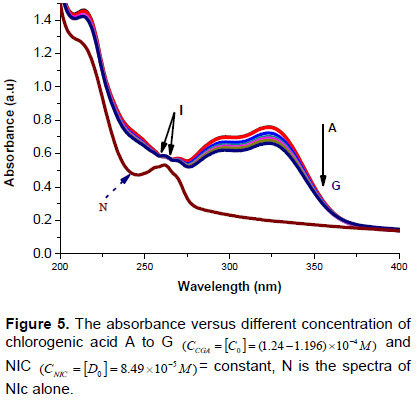
The mathematical approach used in physical chemistry for the determination of equilibrium constant called Benesi-Hildebrand approach were used for the quantitative analysis interaction of nicotinamide with chlorogenic acid (Benesi, 1949), under the condition . A constant of nicotnamide solution with different concentrations range of chlorogenic acid solutions were used to calculate the equilibrium constant and molar extinction coefficient of the interaction formation. When a chromophoric precursor is converted to product with different spectrums an isosbestic point is observed in overlaid spectra. In more complex reactions, the wavelength of isosbestic also changes if the molar absorptivity of the precursor changes and the fraction of the precursor converted to multiple product changes (Antonov et al., 1999). Figure 5 shows the effects of CGA concentration on the UV-Vis absorption spectra of NIC solutions. The addition of CGA to NIC solutions results in important spectral modification and red band shift were observed. As shown in Figure 5, the absorbance of NIC increases when CGA is added to the NIC solution. This indicates that, CGA promotes NIC for its pharmaceutical function. Moreover, the existences of isosbestic points were observed at wavelength of about 258 and 265 nm of the interaction which indicates the formation of complexes between CGA with NIC (Khopkar, 1998).
The equilibrium constant for the complex formation , is derived as:
From Equation 7, the equilibrium constant for the complex formation can be defined as:
where , , and are the equilibrium concentration of CGA, NIC, and association of NIC-CGA, respectively. The initial concentration of NIC and CGA is designated as:
The absorbance (A) for concentration [CD] according to Beers law is
The plot of vs gives a straight line with y-intercept and slope as shown in Figure 6. Thus, the equilibrium constant and molar extinction coefficient calculated by fitting Equation 16 to experimental data of Figure 6 are and respectively. The obtained results are in a good agreement with the results (Abraha et al., 2016) for the equilibrium constant for the complex formation.
Thermodynamic properties of self and hetero-association with CGA of nicotinamide
Heating the aqueous solution of NIC, NIC-CGA complex shows that the absorption spectra of the molecules are strongly dependent on the temperature in the range of 293 to 299K. The equilibrium constants of the molecules of the compounds at the aforementioned temperature were calculated at the peak of wavelengths of the self and hetero association using Equations 3 and 16, respectively. Figure 7a and b, shows the graph of versus of NIC and its hetero-association with CGA. The magnitude of the enthalpy was estimated from the slope of the approximating line according to Vant’s Hoff’s equation:
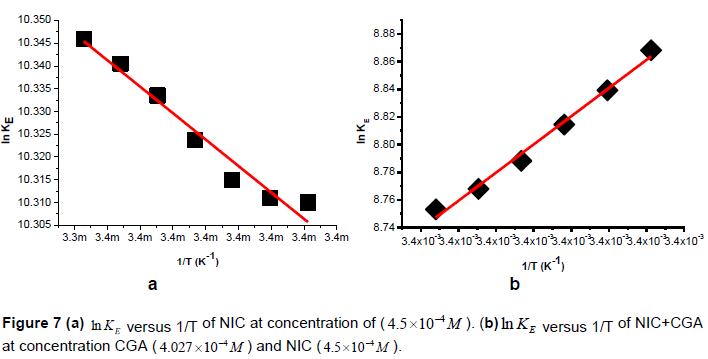
Where ∆H is the molar enthalpyhange,a linear curve fitting for the experimental data with that of the theoretical values obtained from equation (16) using Origin 8 software.
Similarly, the thermodynamic parameters such as enthalpy, gibbs free energy and entropy of the self-association of NIC and its hetero-association with CGA were studied at the temperature range (293 to 299K), respectively. These parameters have been determined using the model of Vant’s Hoff’s equation by linear curve fitting for the experimental data with that of the theoretical values.
The integrated absorption technique which is more powerful in measuring the intensity of absorption light has been used to calculate the optical transition probabilities (transition dipole moment, oscillator strength and integrated absorption cross-section) (Ataklti et al., 2016; Belay, 2010; Belay, 2013). The pure nicotinamide was studied by integrating the absorption coefficient and molar decadic absorption coefficients in the wave number regions of 20000 to 40000 cm-1. Usually, the UV-Vis spectrophotometer measures the concentration in terms of absorbance versus wavelength; this was recalculated into absorption coefficient or molar decadic absorption coefficient versus wave number using Origin 8 software.
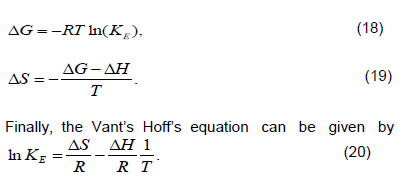
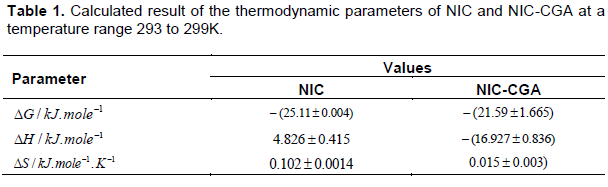
Plots of versus gives a straight line, whose slope and intercept can be used to determine ∆S and ∆H and Gibb’s free energy can be determined at a specific temperature using Equation 19. In order to characterize the force between NIC and NIC-CGA molecules, thermodynamic parameters on the given temperatures were analyzed using Vant’s Hoff’s equation.The thermo dynamic parameters ,Gibb’s free energy change(ΔG), enthalpy change (ΔH) and entropy change (ΔS) are important for confirming the binding mode. The calculated values for the Gibb’s free energy, enthalpy, and entropy of the molecules for the self and hetero association is obtained as Table 1. Since ΔH >0 and ΔS >0, hydrophobic interactions are dominant for the interaction of NIC molecules, but for the interaction between NIC-CGA (ΔH < 0 and ΔS >0), electrostatic forces plays the major role in the interaction. Moreover, the negative value for the Gibb’s free energy and enthalpy indicates that the absorption process of the compounds is continuous and exothermic reaction, respectively, and the positive value of enthalpy for the self-association shows that the process is endothermic reaction. Also, the positive value of entropy confirms the increasing randomness of the solution interface during the absorption process of the molecules of the compounds (Guo et al., 2014). The calculated result for change in entropy of the complexation is also similar with the previously reported thermodynamic results given by for Chlorogenic Acid in β-cyclodextrin Complex (Álvarez-Parrilla et al., 2010).
Optical transition properties of nicotinamide
Figure 8 represents the absorbance versus wavenumber obtained from the UV-Vis absorption spectra in water solvent at a concentration of . The optical transition probabilities of nicotinamide were obtained from the absorption spectra to characterize the strength of the electron transition and to interpret the absorption spectra. The molar decadic absorption coefficient which represents the ability of a molecule to absorb light in a given solvent at a given wavelength were calculated using Beer-Lambert’s law (Belay, 2013; Liptay, 1969) and integrated absorption coefficient is the sum of absorption coefficient for all frequencies. It is independent of line function which may be varying due to pressure, temperature, interaction of solute and solvent (Milonni and Eberly, 1988; Michale, 1999). The integrated absorption coefficient ( ) in the frequency (ν+dv) regions can be expressed by,
The integrated absorption cross-section ( ) is given by Milonni and Eberly (1988):
where is absorption coefficient and N is number density of the molecules.
Oscillator strength which represents the average number of electrons per atom that can be excited by the incident radiation which is an important parameter for providing the relative strength of electron transition and can be related as follows with the molar decadic absorption coefficient ( ) as a function of frequency (Georgakopoulous et al., 2004):
The transition dipole moment ( ) is a vector that depends on both ground state and excited state and couple the transition to the electric field of light and it is related with molar decadic absorption coefficient as (Liptay ,1969; Michale, 1999):
where . Using the Equations (21, 22, 23 and 24), the optical transition properties of nicotinamide calculated in bi-distilled water are presented in Table 2. The peak in the visible region of the compounds is due to electronic transitions of chromophore groups (Bakhkshiev, 1961; Firth et al., 1983).
The result of this investigation indicates that the molecule of nicotinamide aggregates with itself and with chlorogenic acid molecules in the solution. The calculated parameters for the self and hetero-association are important implication for interpreting the study of binding and kinetic chemical reaction system of the compounds. These parameters are very useful for understanding the nature and strength of its molecular interaction in liquid solutions, in order to characterize the electron transition probabilities and interpret the absorption spectra of the compound, for direct experimental application in the emission, absorption and dispersion and in providing stringent test of atomic and molecular structure calculation in theoretical works. In addition, knowledge of the mechanism of the association, thermodynamic properties and the optical transition probabilities of NIC are useful in order to design the advanced and controllable carriers of drugs and food components. Therefore, the investigated results have wider applications in optical characterization, pharmaceutical drug designing and food companies in terms of economic and scientific utility.