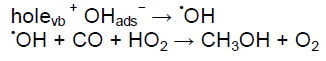ABSTRACT
The aim of this study is to simulate co-catalysts of titanium nitride nanotubes (TiN-NTs) with Cu nano-particles for converting carbon monoxide (CO) and water into methanol. This method may provide a new way to reduce carbon-monoxide levels in the atmosphere rising due to our planet's heavy use of fossil fuels as well as to produce alternative fuels. Using TiN-NTs has shown the efficiency to remove CO from the atmosphere. There are four positions for CO and TiN-NTs (4, 4) that we have investigated, passing of CO endohedrally and between the nanotubes. We study the structural, total energy, thermodynamic and conductive properties of converted CO on Cu nano-particles in TiN-NTs to methanol. The electronic and geometric structures and thermodynamic properties are quantum mechanically calculated for these situations by DFT methods at the semi-empirical ZINDO/1 level, B3LYP/6-31G basis sets. The thermodynamic properties show which interactions are endothermic. The heat of exhaust can be used for CO conversion to CH3OH or other products.
Key words: Carbon monoxide (CO), Methanol, Titanium nitride nanotubes (TiN-NTs), ZINDO/1, environment.
Carbon monoxide (CO) is a colorless, odorless, and tasteless gas that is somewhat less dense than air. It is dangerous to humans and animals when withstood in larger levels, though it is also produced in standard pet metabolism in minimal amounts, and is considered to have some standard organic functions. In the air, it is spatially variable and temporary, having a role in the formation of ground-level ozone (Riduan et al., 2009).
Solid state gas sensors are most important in semiconductor processing, medical diagnosis, environmental sensing, and personal safety etc. (Kolmakov et al., 2003). The high surface to volume ratio of nano-forms makes them natural contenders as new sensors and suitable surface to remove and converted pollutants.
TiN-nanotubes (TiN-NTs) have attracted great interest due to their unique electronic properties and nanometer size. Because of these unique properties, they are great potential candidates in many important applications such as nanoscale electronic devices, chemical sensors and field emitters. The effect of gas adsorption on the electrical resistance of a TiN-NT has received great attraction because of fast response, good sensitivity of chemical environment gases and low operating temperature (Paulose et al., 2006).
The products of the carbon monoxide (CO) and water (H2O) reaction depend on metal oxide catalysis, temperature and pressures [Li et al., 2007]. The most important challenge is that of the catalyst. The current commercial catalysts are co-catalysts of titanium nitride nanotubes with Cu- nanoparticles that help converts carbon monoxide and water into methanol using sunlight as the power source for sun gas conversion (Figure 1). There are some methods such as options for carbon monoxide and carbon dioxide removal from the atmosphere include afforestation and chemical approaches like direct air capture of CO from the atmosphere or reactions of CO with minerals to form carbonates [Varghese et al., 2009; Farha et al., 2010; Yang et al., 2012; Aresta and Dibenedetto, 2004; Hohenberg and Kohn, 1964]. Methane and methanol are major products of the chemical industry and also a feedstock for many chemicals. However CO conversion to methanol and other product is challenging.
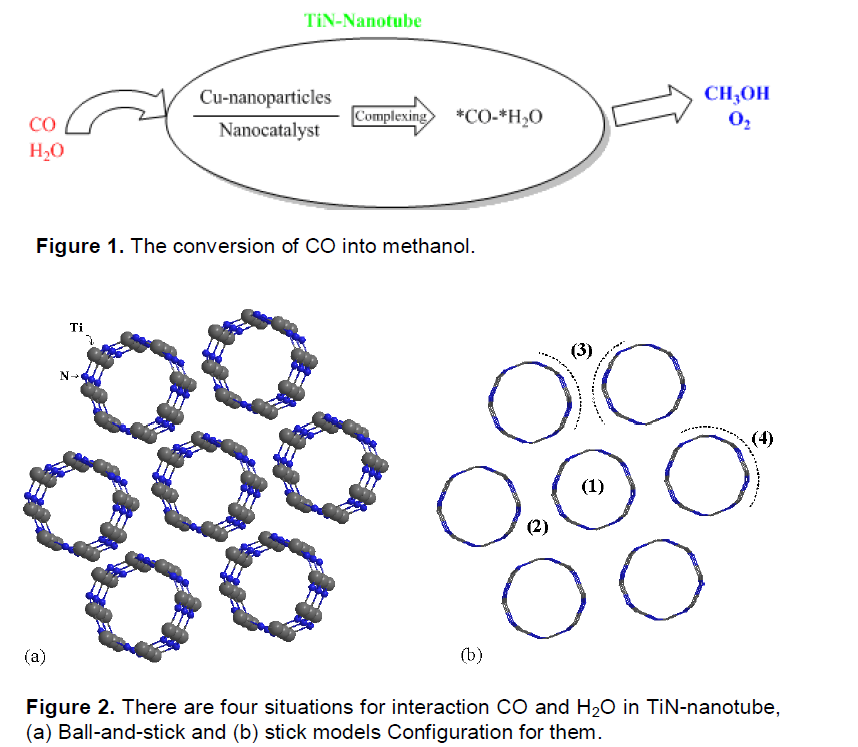
As shown in Figure 2, there are four situations in which CO and H2O can pass between TiNNTs-Cu. In this work, the 1st and 2nd situations are investigated for them. In Figures 3 and 4, TiN- nanotubes with Cu-nanoparticle simulated by ball-and-stick models, CO and H2O converted to CH3OH and O2.
Interaction between CO and H2O on Cu nanoparticle in TiN-nanotubes is investigated using ZINDO/1 method by semi empirical. We study the structural, total energy, thermodynamic properties of them in room temperature. All the geometry optimization structures were carried out using Gaussian program package. Density Functional Theory (DFT) optimized their intermediates and transient states. The results show a sensitivity enhancement in resistance and capacitance when CO and H2O are converted to CH3OH and O2.
THE COMPUTATIONAL METHODS
The computational approach consists of three stages: First, all the geometry optimization structures in present work were performed by employing DFT, using Becke’s three parameter hybrid with the Lee-Yang-Parr correlation functional, B3LYP (Becke, 1993; Yang and Parr, 1988) method with the 6-31G basis sets. Then the thermodynamic properties of them were calculated for different distances. We can use the information obtained from semi-empirical calculations to investigate many thermodynamic aspects of chemical processes. The heat of formation is calculated by subtracting atomic heats of formation from the binding energy. ZINDO/1 has been used widely to calculate heats of formation, molecular geometries, dipole moments, ionization energies, electron affinities, and other properties (
Ridley and
Zerner, 1973; Zerner, 1991). At the end, the reaction pathway is analyzed in order to confirm the structure of the transition state obtained.
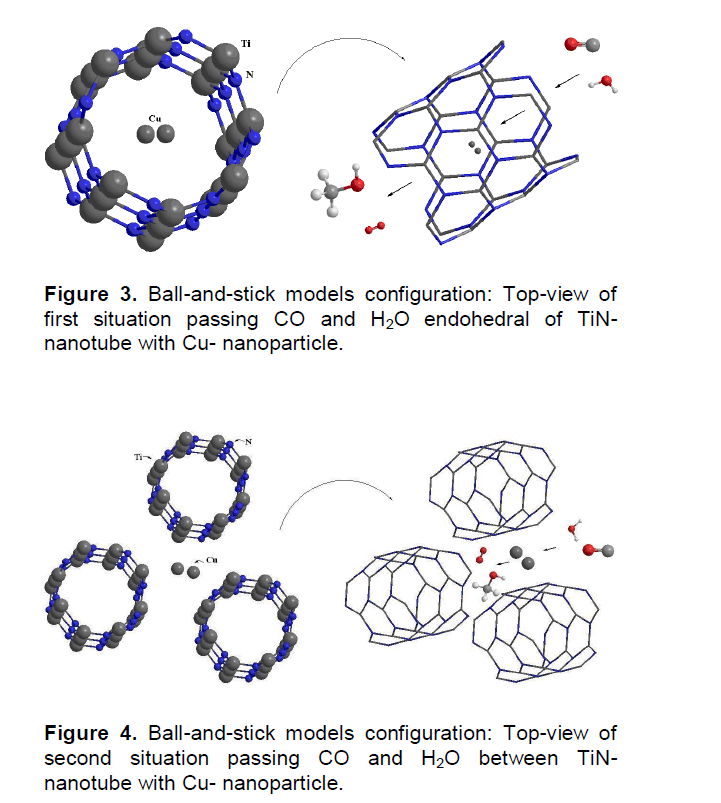
In this study, entry carbon monoxide and water on Cu nanoparticles between titanium nitride nanotubes have been simulated in three steps (Figures 3 and 4), and then in steps 4 and 5 are transition state conversion of them to CH3OH and O2. The conversion was completed in 6th step, methanol and oxygen are produced, and then it is excretion from TiN-NTs in 7th or 8th step. The electronic structure and the thermodynamic properties are calculated for all steps by ZINDO/1.
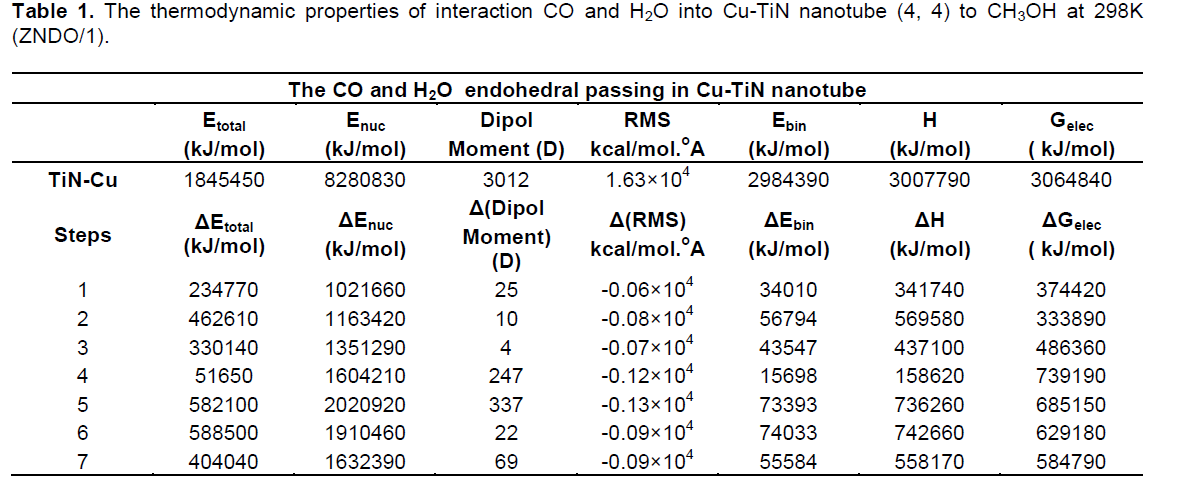
The electronic structures and properties of CO converted to CH3OH have been investigated using hybrid density functional theories with the basis set B3LYP/6–31G use correct logical order. In this work, we use TiN-NTs from kind of armchair TiN nanotubes (4, 4) that show in Figures 3 and 4. When CO gas from the exhaust exits to the filter of TiN-NTs with Cu nanoparticles (into or between of TiN-NTs), electron exchange between them occurs and CO converts to CH3OH or other products. Table 1 show thermodynamice parameters of monoxide carbon and water endohedral passing in TiN-NT by seven steps and their passing between TiN-nanotubes (second state) shown in Table 2. The thermodynamic properties for all steps are without the effect of the catalyst in the reaction for example:
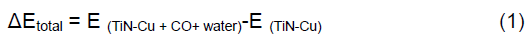
The study includes conformational searches (and further refinement by DFT) and semi- empirical by ZINDO/1 methods. The most significant property is the ZINDO/1, which is, finding a good correlation between the ZINDO/1 and the substitution pattern on this conversation. ZINDO/1 has been used widely to calculate heats of formation, molecular geometries, dipole moments, ionization energies, electron affinities, and other properties (Reslan et al., 2012; Dondela et al., 2005).
In Tables 1 and 2, the dipole moment (D) measurement gives an idea about the degree of polarity for approached of CO and H2O to Cu nanoparticles into and between TiN-NT. When CO passing in nanotube, dipole moment (D) decrease, so in 4th and 5th steps increase polar because in these steps occur exchange electron between them and is formed CH3OH and O2 for two situations. The root mean square (RMS) gradient (kcal/mol. Å) is different for this interaction at room temperature.
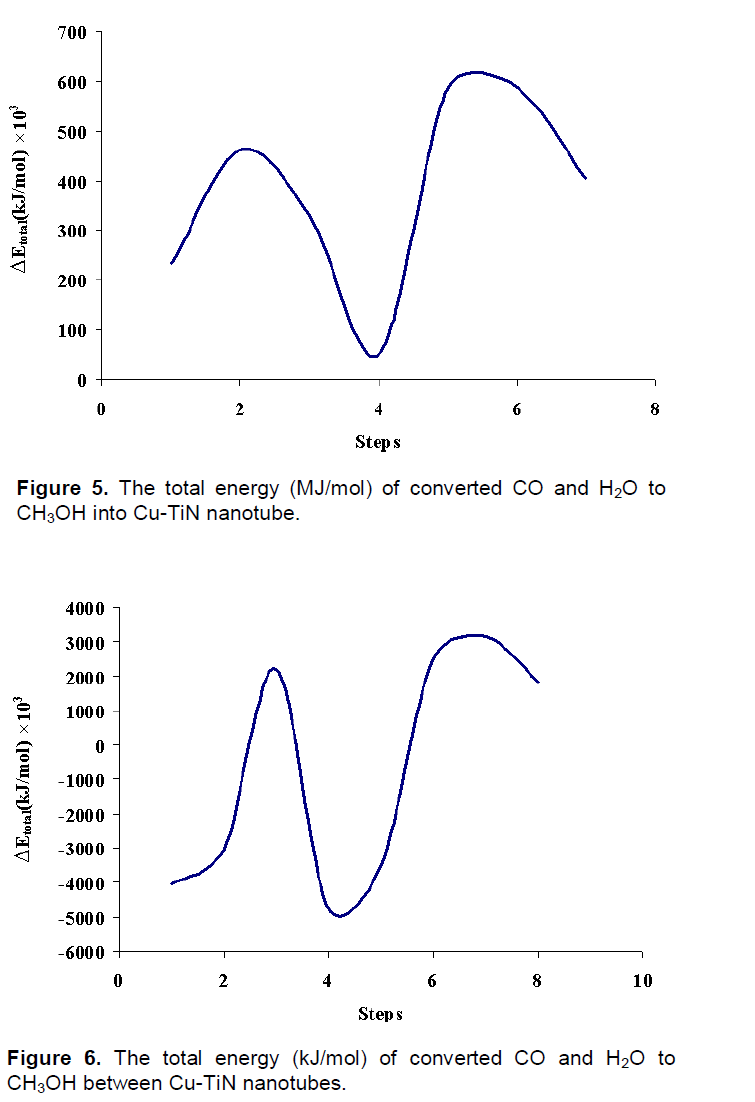
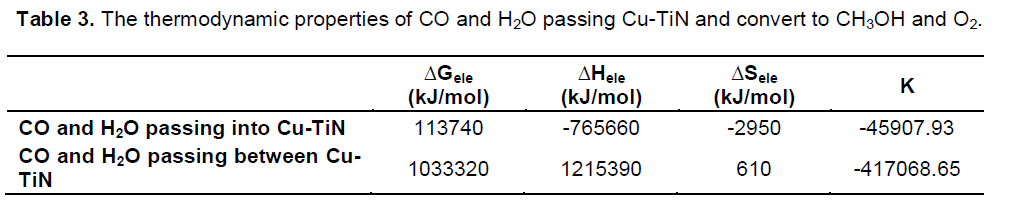
The total energy (Etotal) is minimum amount for 4th step, is 51650 (endohedral) and -4737300 kJ/mol (between tube), after its conversion, Etotal increase to 582100 kJ/mol in 5th step for endohedral passing and -3521920 kJ/mol for between passing (Figures 5 and 6).
The ΔE
nuc, ΔE
bin, ΔH and ΔG
elec increase in 5
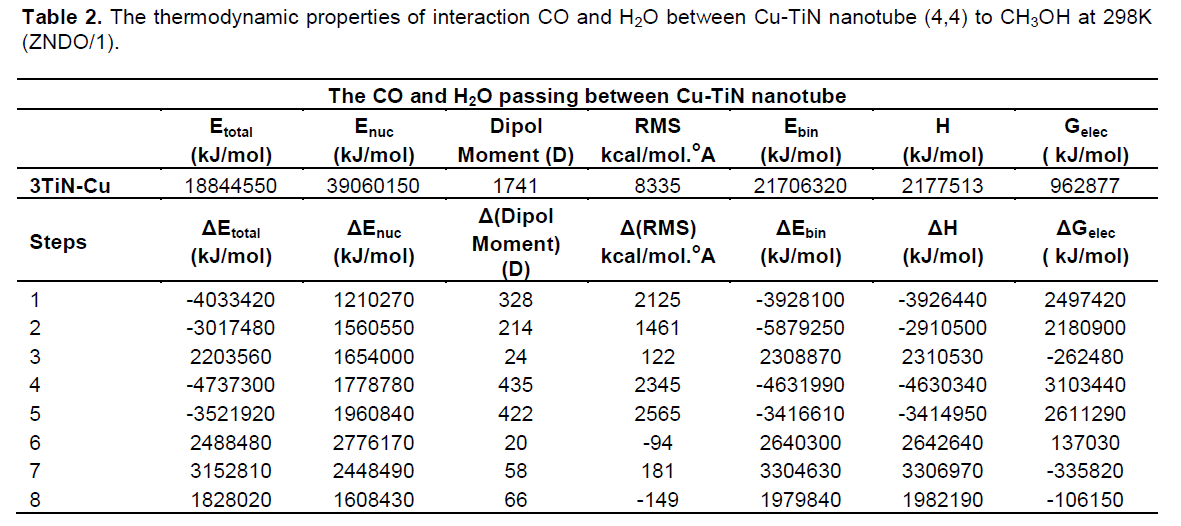
th step that methanol molecule is formed in this interaction for tow situations. Thermodynamic equilibrium constants,
K, for these interactions were calculated by the related standard Gibbs free energy difference
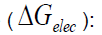
Where,
T is the transition temperature and
R is gas constant. The entropy difference (
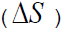
) at the phase transitions are given by:
Where, ΔH is the electronic enthalpy difference. In Table 3 is shown the other thermodynamic properties for these interactions.
Thermodynamic parameters (ΔGele, ΔHele and ΔSele) for CO and H2O converted to CH3OH and O2 were calculated and the results suggest that the nature of adsorption is endothermic.
This method needs energy that can be provided from heat of exhaust gas or solar energy. The calculation of thermodynamics is evaluated due to the comparison with experimental values. The calculated data are in good agreement with the experimental spectra (Varghese et al., 2009).
Conversion of CO by TiN-nanotube is caused changing in the electric structure of nanotube, because CO and
H2O are adsorbed on Cu in TiN-NT by weak bond (Figures 3 and 4). The electric resistance for them is following as:
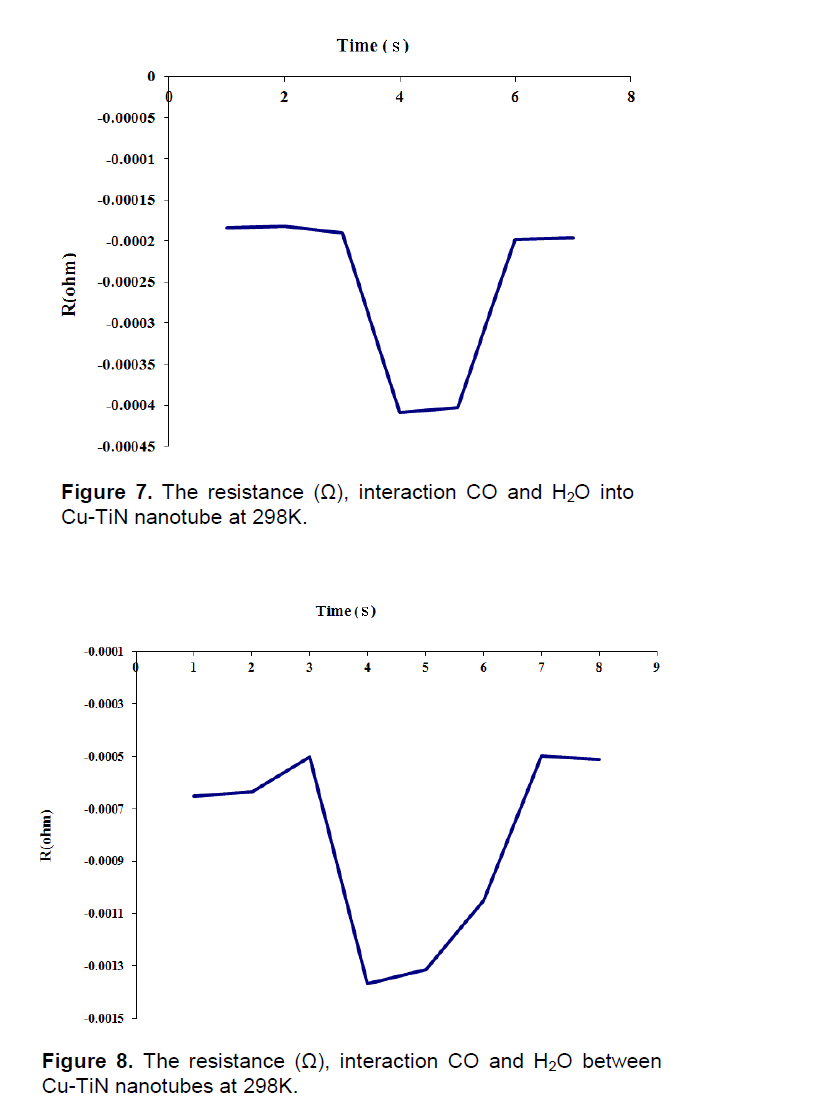
Where, n, F and t are electron number of conversion, faraday constant and time (h) respectively (Lee, 2005). After adsorption of CO and H2O on surface of Cu nanoparticles in TiN-NT and transition electron between them, the electric resistance to time (s) decreased that showed in Figures 7 and 8, when methanol is formed, the electric resistance is increasing.
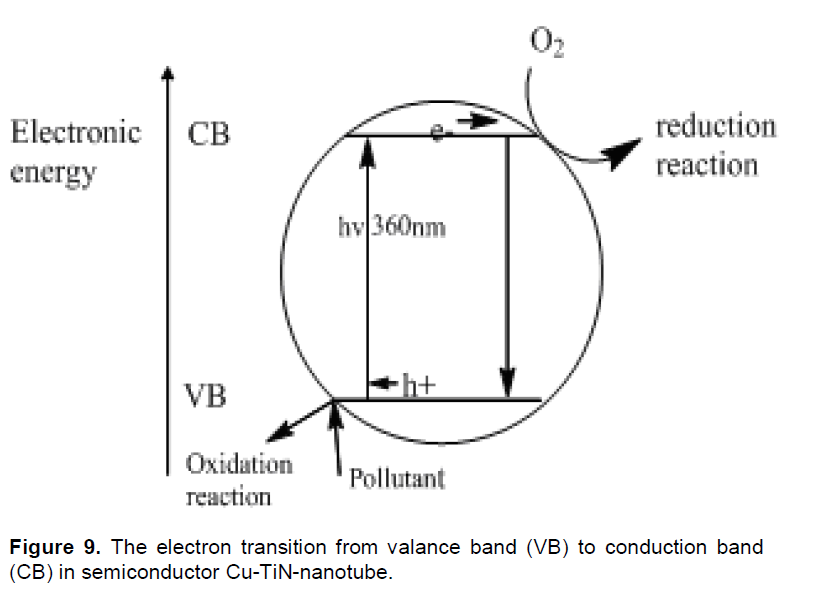
TiN-nanotube is used as a photocatalyst in photocatalysis process, because is a semiconductor (Varghese et al., 2009). The absorption of a photon (hν) with ultra-band energy from UV irradiation source is caused TiN activation (Figure 9). The transmission of an electron (e−) from the valence band to the conduction band is caused highly reactive positive holes (h+) in the valence band, therefore this transfer is been to adsorption and conversion to pollutants on the Cu nanoparticles into low-risk products in environment:
Oxidative reaction:
After adsorption of CO onto the nanosurface and transference of electrons between them, the electric resistance decreased. The TiN-NTs are semiconductors, photo-active catalysts, able to utilize near-UV light, biologically, chemically inert, photo-stable and inexpensive, therefore TiN-NT is appropriate for this conversion in environment.
The co-catalysts of Cu and TiN with a nano structure significantly enhance the photocatalytic reduction of CO with H2O to CH3OH and O2. In this manuscript this conversion to CH3OH is calculated, the local minimum geometries of them were determined at B3LYP/6-31G level. The ZINDO/1 method is used for calculation of thermodynamic parameters these interactions. The calculation which shows heat reaction formation (?H) is endothermic for these reactions. These reactions need sun, photo active or other energy in presence of visible light. We propose, TiN nanotubes with Cu nanoparticles to form filter to be installed in exhaust of automobile and heat of exit gases provides energy to the converter. These techniques have been highly considered by researchers and industrial men because of their low cost, high adsorption efficiency, selective operation, easy and hazardless application.
The authors have not declared any conflict of interest.
REFERENCES
Aresta M, Dibenedetto A (2004). The contribution of the utilization option to reducing the CO2 atmospheric loading: research needed to overcome existing barriers for a full exploitation of the potential of the CO2 use. Catal. Today. 98:455–462.
Crossref
|
|
|
Becke D (1993). Densityfunctional thermochemistry. III. The role of exact exchange. J.Chem.Phys. 98:5648-5652.
Crossref
|
|
|
|
Dondela B, Peszke J, Åšliwa W (2005). Semiempirical ZINDO/S, AM1 and ab initio HF/STO-3G and HF/6-31G study of quaternary salts of diazaphenanthrenes with ethyl bromide and 1,3-dibromopropane. J. Mol. Structure. 753(1–3):154–164.
Crossref
|
|
|
|
Farha OK, Yazaydin O, Eryazici I, Malliakas C, Hauser B, Kanatzidis MG, Nguyen ST, Snurr RQ, Hupp JT (2010). De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2:944–948.
Crossref |
|
|
|
Hohenberg P, Kohn W (1964). Inhomogeneous electron gas. Phys. Rev.136:B864-B871.
Crossref
|
|
|
|
Kolmakov A, Zhang Y, Cheng G, Moskovits M (2003). Detection of CO and O2 using Tin Oxide Nanowire Sensors. Adv. Mater. 15(12):997-1000.
Crossref
|
|
|
|
|
Lee S (2005). Encyclopedia of chemical processing and design. Taylor & Francis, 2005. ISBN: 0824755634, 9780824755638.
|
|
|
|
Li J, Zhao Z, Kazakov A, Chaos M, Dryer FL, Jr JJS (2007). A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. Int. J. Chemical Kinetics. 39(3):109–136.
Crossref
|
|
|
|
Paulose M, Shankar K, Yoriya S, Prakasam HE, Varghese OK, Mor GK, Latempa TA, Fitzgerald A, Grimes CA (2006). Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 μm in Length. J. Phys. Chem. B 110(33):16179-16184.
Crossref |
|
|
|
Reslan R, Lopata K, Arntsen C, Govind N, Neuhauser D (2012). Electron transfer beyond the static picture: A TDDFT/TD-ZINDO study of a pentacene dimer. HE. J. Chem. Phys. 137:22A502-6.
Crossref |
|
|
|
Ridley J, Zerner M (1973). An intermediate neglect of differential overlap technique for spectroscopy: Pyrrole and the azines. Theoretica chimica acta. 32(2):111-134.
Crossref
|
|
|
|
Riduan SN, Zhang Y, Ying JY (2009). Conversion of carbon dioxide into methanol with silanes over N-Heterocyclic Carbene Catalysts. Angew. Chem. 121:3372–3375.
Crossref
|
|
|
|
Varghese OK, Paulose M, LaTempa TJ, Grimes AG (2009). High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano. Lett. 9(2):731-737.
Crossref |
|
|
|
Yang DA, Cho HY, Kim J, Yang ST, Ahn WS (2012). CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 5:6465–6473.
Crossref
|
|
|
|
Yang LW, Parr RG (1988). Development of the colle-salvetti correlation-energy formula into a functional of the electron density Phys. Rev. B: Condens. Matter. 37:785-789.
Crossref |
|
|
|
Zerner M (1991). Reviews in computational chemistry, Volume 2, Eds. K. B. Lipkowitz and D. B. Boyd, VCH, New York, P. 313.
Crossref
|
|


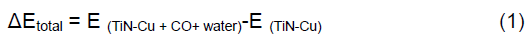
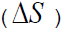 ) at the phase transitions are given by:
) at the phase transitions are given by: