ABSTRACT
Investigations were conducted to compare gas produced from co-digestion of corncob, rice chaff, goat dung and dog dung with commercial gas. The study was carried out anaerobically at a temperature of 29.5 to 33°C (mesophilic condition) in a mini laboratory digester (bioreactor) fabricated using guage 16 metal sheets with 80-L capacity for a 90-day retention time. The shredded corn cob and rice chaff were mixed with water at ratio 4:1 (waste to water) and 3:1 (waste to water) respectively and mixed with goat and dog dungs at ratio 2:1 (waste to water). The control sample collected from a domestic cooking gas cylinder contained 56.12% CH2, 0.14% NH3, 0.22% CO, 0.23% H2S and 43.20% CO2. The result obtained for the biogas analysis shows that Sample E (75:25) contains 63.54% CH4, 0.93% NH3, 0.84% CO, 0.54% H2S, 34.12% CO2. The value recorded for sample E represented the highest value obtained among the samples. Also the gas composition shows a good substitute for commercial gas.
Key words: Corn cob, rice chaff, goat dung, dog dung, biogas, commercial gas, bioreactor and digestates.
Utilization of non-renewable energy in excess can cause problems of energy crisis. The use of energy sources such as fuel derived from fossil raw materials is a fuel that is not easily recyclable and requires a long process to produce the fuel. Therefore, there are the needs for new alternative energy sources that are renewable. One of the energy technologies that comply with these requirements is biogas technology (Wahyuni, 2011). Production of biogas from waste and organic residue with various types of anaerobic digesters method has been widely studied, both experimental and theoretical, for decades. Biogas can be obtained from wide range of organic wastes e.g. animal waste, industrial waste water and municipal solid wastes (Tuesorn et al., 2013). Biogas production has been carried out by several researchers and can be produced by
anaerobic digestion with
anaerobic organisms, which digest material inside a closed system, or
fermentation of biodegradable materials. Biogas is primarily
methane (CH
4) and
carbon dioxide (CO
2) and may have small amounts of hydrogen sulfide, moisture and
siloxanes. The gases
methane,
hydrogen, and
carbon monoxide (CO) can be combusted or oxidized with oxygen. This energy release allows biogas to be used as a fuel; it can be used for any heating purpose, such as cooking, as well as in a gas engine to convert the energy in the gas into electricity and heat. Biogas can be compressed, the same way
natural gas is compressed to
CNG, and used to power
motor vehicles. Biogas can be cleaned and upgraded to natural gas standards, when it becomes bio methane (Huertas et al., 2011). In view of search for the solutions of anaerobic digestion of animal and plant wastes as well as exploring the potentials of all decomposable wastes, the ability to generate biogas with the blend of goat, dog and plant waste (that is, corn cob) is investigated. Though the waste from goat and dog may be small as compared to cow and other livestock, they could be useful in enhancing the viability of other major wastes (particularly the plant waste – corn cob). This study aims at investigating the viability of the blend of goat, dog dung and corn cob as waste to produce combustible biogas when used as major feedstock or enhance the quality of others as a blend.
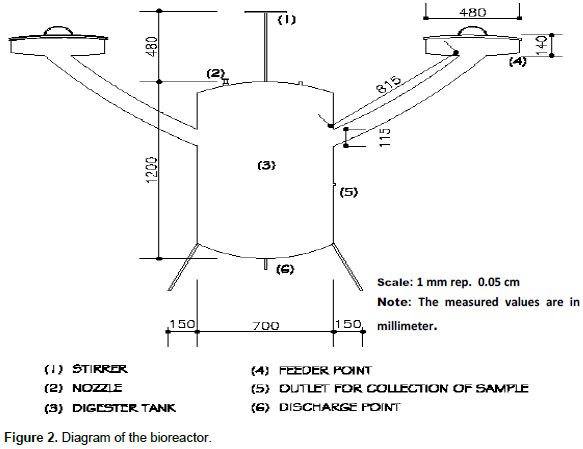
The study was conducted using 80-L metallic digester. The digester was designed and constructed with guage 16 metal sheets as described by Eze and Ojike (2012). Corn cobs were procured from local roasted corn sellers in Ondo and Lagos States while rice chaff was obtained from a local rice milling industry in Ekiti State. The corn cobs were milled using the dry attrition mill (made by Addis Nigeria, Asiko A11 double grinding mill) so as to reduce the sizes and increase the surface area of the materials for faster degradation. The rice chaff was boiled for 15 min to reduce the lignin content which tend to prevent enzymatic breakdown of the chaff. Goat dung was collected freshly from a local abattoir (Odo-Eran) in Cele area of Lagos, while the dog dung was collected at a veterinary outlet in Surulere Lagos. Corn cob (0.2 kg) and rice chaff (0.1 kg), with goat dung (20 kg) and dog dung (10 kg) was mixed with 60 L of water before digestion. Six bioreactors were used for five different sample ratios of goat and dog dungs blended with the feedstock with the negative control as follow; Sample A: corn cob, rice chaff, goat dungs (25%), dog dung (25%); Sample B: corn cob, rice chaff, goat dungs (50%), dog dung (50%); Sample C: corn cob, rice chaff, goat dungs (75%), dog dung (75%); Sample D: corn cob, rice chaff, goat dungs (25%), dog dung (75%); Sample E: corn cob, rice chaff, goat dungs (75%), dog dung (25%) and Sample F: corn cob, rice chaff, negative control while cooking gas served as positive control. The chromatography system is composed of the gas chromatography equipment and a recorder for plotting chromatographs. The equipment model is Hp6890 with HP ChemStation and Rev. A09.01 (1206) software. The carrier gas was helium at 20 ml/min flow rate with the inlet temperature of 145°C while the inflow of the carrier gas was 26 ml/min in the column with dimensions and type of 30 m 8 mm 0.85 mm 30 m 1 mm and Heysep DB 100/120; Deerfield, Illinois respectively. The oven temperature was programmed at 140°C in 6 min, ramped at 50°C/min and maintained at 175°C.
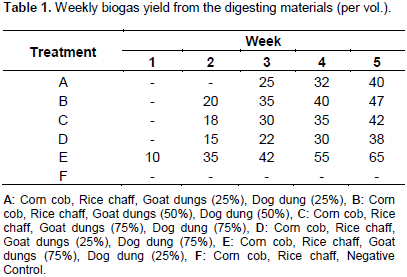
Table 1 revealed the gas production level during the five weeks of biodegradation of the test materials within the six bioreactors subjected to various treatments. The weekly biogas yield for all sample ratios for five weeks from the six bioreactors indicated that gas production started in the first week with E treatment with gas yield of 10 per volume. Same tends was observed by Vivekanandan and Kamaraj (2011) in a study using cow dung as co-substrate with rice chaff at different substrate ratio and the first yield was noticed on the 3rd day of digestion. The E treatment also has the highest gas yield of 65 per volume on the fifth week which can be related to the findings of Vivekanandan and Kamaraj (2011) on rice chaff and cow dung as co-substrate at two different ratios and the report showed that the digester case with the highest dung ratio produces the highest yield. Also, Okoroigwe (2005) generated biogas by combining cow and dog dung and linked the high yield to the increased nutrient provided by the combined manure which makes for catabolism and metabolism of the methanogenic bacteria. Previous findings by Eze et al. (2007) and Iyagba et al. (2009) re-affirm that blending animal wastes and crop residues improves the blend digestibility and gas production arising from additional nutrients and gas improved carbon to nitrogen ration.
Figures 1 and 2 shows, both the picture and the scaled drawing of the bioreactor used in this work. Figures 3 to 8 shows the variation of peaks of component hydrocarbons and other gases processed with the waste for the various treatments A to F and Table 2 presents the comparative percentage of the biogas with the commercial gas. The raw gas contains several impurities, like water, dust, H2S, CO2, siloxanes, hydrocarbons, NH3, oxygen and several other elements that must be removed in order to reach certain standards of quality. Adegun and Yaru (2013) reportedly removed these impurities from the biogas by first passing it through a solution of sodium hydroxide for the absorption of carbon dioxide and hydrogen sulphide components of the biogas and through a filter dryer to (dehydrate) absorb the moisture that may have accompanied it before passing to the spooter and then compressing it into the gas cylinder. The biogas obtained from the samples as presented in Table 1 comprises methane (CH4), Ammonia gas (NH3), Hydrogen sulphide gas (H2S), Carbon dioxide (CO2), and Carbon II oxide. The percentage of methane obtained for all samples tested are higher than the other gas produced. These results are similar to those obtained by Adegun and Yaru (2013) who worked on cattle dung biogas as a renewable energy for rural laboratories and Swan et al. (2012) who worked on low pressure separation techniques of biogas into methane and carbon dioxide employing polydimethylsiloxane PDMS membrane. The quantity of methane produced shows how effective the bioreactor was.

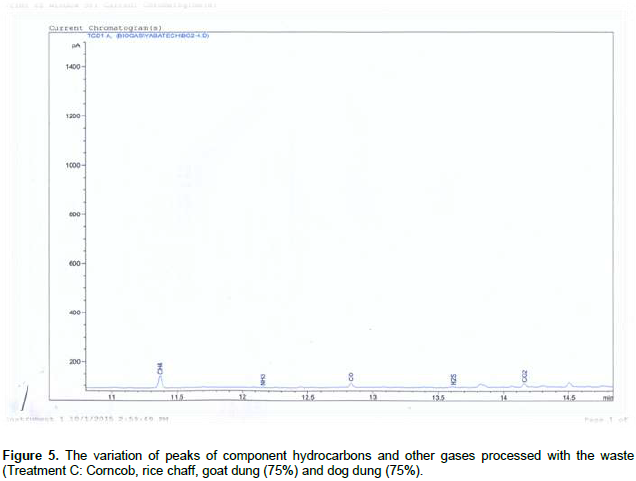
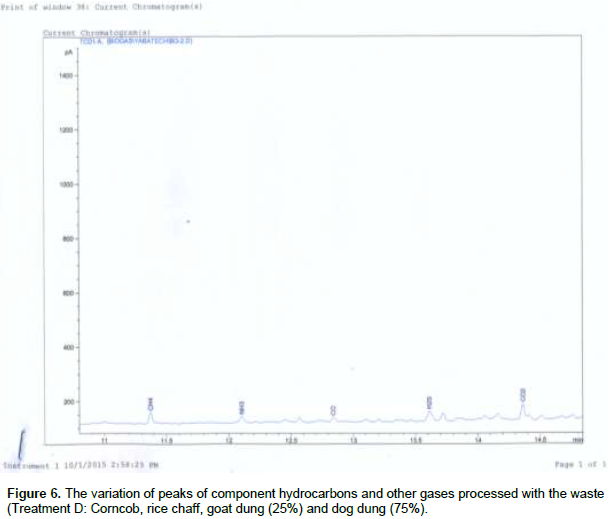
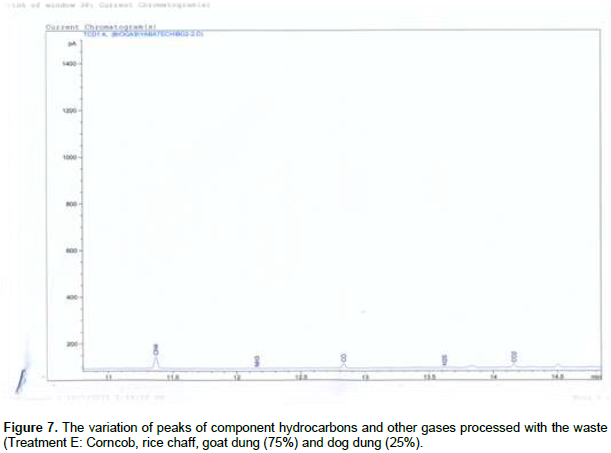
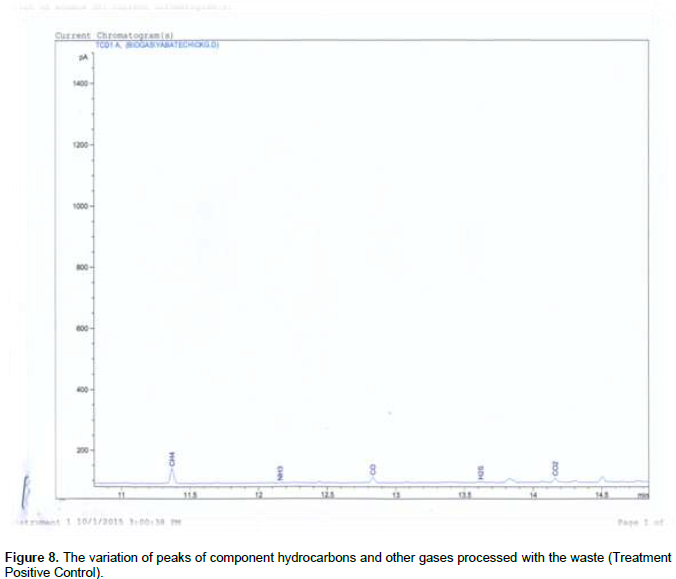
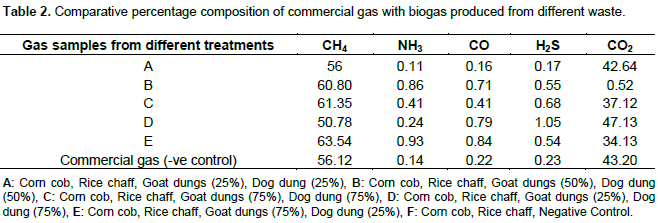
Also the highest methane content was recorded by sample E containing the highest goat to dog dung ratio which corresponds to the high volume produced by the sample. The value is also higher than that produced by the control sample which suggests that at the right bioreactor condition the biogas yield can be high enough to be compressed and used for domestic cooking. The result obtained also sees samples B and C producing higher methane content than that obtained in the control sample. Ammonia (NH3) is often removed from gas by a washing process with diluted nitric or sulfuric acid especially in industrial large scale cleaning processes. The use of these acids demands installations made of stainless steel that can be expensive for small scale applications like biogas cleaning. Ammonia (NH3) can also be removed with units filled with activated carbon and is also eliminated in some of the CO2-removing units, like adsorption processes and absorption processes with water (Hagen et al., 2001). Hydrogen sulfide gas is a colorless, very poisonous, flammable gas with the characteristic foul odor of rotten eggs, and results mostly from the bacterial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers.
This process is commonly known as anaerobic digestion and is the main process in biogas formation (Anon, 2012). Due to its corrosive nature, H2S have to be removed in an early state of the biogas upgrading process. The removal of carbon dioxide (CO2) is based on the principle of separation of CO2 and CH4 by using an absorbent as solvent. The mostly used method is the use of water as physical absorbent typically at a pressure of 1000-2000 kPa (Tynell, 2005). Water solvent is also effective on H2S absorption (Wellinger and Lindberg, 2005; Krich et al., 2005; Tynell, 2005). After scrubbing, the water can be regenerated by using a stripping low pressure column, where it is brought into contact with air or steam and inert gas in case of high concentration of H2S. This induces the CO2 to move into the gas phase according to the chemical equilibrium. The separation efficiency of this technique is 95%. This technology is simple and relatively inexpensive; moreover, the loss of CH4 is relatively small (less than 2%) because of the large difference in solubility of CO2 and CH4 (Schomaker et al., 2000; Krich et al., 2005).
The yield (biogas) produced in this study shows that the feed stock used in the work has high biogas generating potential which shows that anaerobic digestion technique is a variable option for generating energy at low cost while also combating environmental and health hazards that could result from indiscriminate disposal of the waste which serves as the material for the generation of utilizable and renewable energy.
The authors whose names are listed in this journal certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
REFERENCES
|
Adegun IK, Yaru SS (2013). Cattle dung biogas as a renewable energy source for rural laboratories, Centre for Research and Development (CERAD) The Federal University of Technology, Akure, Nigeria.
|
|
|
|
Anon ND (2012). Bio KW.Biogas system for co-digestion of cattle manure, renewable resources, food residues, grease separator sludge and slaughter house waste, Bio Kraftwerk GmbH & Co. KEG.
|
|
|
|
|
Eze IS, Anyanwu CN, Oparaku OU, Okoye COB (2007). Animal Manure: A Resource or a Waste? 37th Annual Conference of Nigerian Society of Chemical Engineers.
|
|
|
|
|
Eze JI, Ojike O (2012). Anaerobic production of biogas from maize wastes. Int. J. Phys. Sci. 7(6):982-987.
|
|
|
|
|
Hagen M, Polman E, Jencen J, Myken A, Jonsson O, Dahl A (2001). Adding gas from biomass to the gas grid, Malmo, Sweden: Swedish Gas Center: Report SCG P 118.
|
|
|
|
|
Huertas JI, Giraldo N, Izquierdo I (2011). Removal of H2S and CO2 from Biogas by Amine Absorption in Mass Transfer in Chemical Engineering (ed. J. Markos), ISBN 978-953-307-619-5, Intech.
View
|
|
|
|
|
Iyagba ET, Mangibo IA, Mohammed YS (2009). The study of cow dung as a co-substrates with rice husk on biogas production. Sci. J. Res. essays 4(9):861-866.
|
|
|
|
|
Krich K, Augenstein A, Barmale J, Benemann J, Rutledge B, Salour D (2005). Upgrading Dairy Biogas to Biomethane and Other Fuels. In: Andrews K., Editor. Biomethane from Dairy Waste - A Sourcebook for the Production and Use of Renewable Natural Gas in California.s.l.:California: Clear Concepts, pp. 47-69.
|
|
|
|
|
Okoroigwe EC (2005). Adaptation of plastic technology in the production of biogas digester.An M. Eng. Project Report.Department of Mechanical Engineering, University of Nigeria, Nsukka.
|
|
|
|
|
Schomaker AHHM, Boerboom AAM, Visser A, Pfeifer AE (2000). Anaerobic digestion of agro-industrial wastes: information networks e technical summary on gas treatment, Nijmegen, Nederland: AD-NETT; Report No. FAIR-CT.
|
|
|
|
|
Swan KA, Sapkala RS, Sapkal VS (2012). Low Pressure Separation Technique Of Biogas Into CH4And CO2 Employing PDMS Membrane. Int. J. Adv. Eng. Technol. III(I):311-315.
|
|
|
|
|
Tuesorn S, Wong WS, Champreda V (2013). Enhancement of biogas production from swine manure by a lignocellulolytic microbial consortium. Bioresour. Technol. 144:579-586.
Crossref
|
|
|
|
|
Tynell A (2005). Microbial growth on pall-rings e A problem when upgrading biogas with the technique absorption with water wash, Stockholm, Sweden: SvenskaBiogasforeningen and Swedish Gas Center: 53 p. Report No.: 610408 ISSN1651-5501.
|
|
|
|
|
Vivekanandan S, Kamaraj G (2011). The study of biogas production from rice chaff (karukka) as Co-substrate with cow dung. Indian J. Sci. Technol. 4(6).
|
|
|
|
|
Wahyuni S (2011). KongresIlmuPengetahuanNasional (KIPNAS)ke 10.
|
|
|
|
|
Wellinger A, Lindberg A (2005). Biogas Upgrading and Utilisation. IEA Bioenergy Task 24: Energy from Biological Conversion of Organic Waste. Available at:
View
|
|