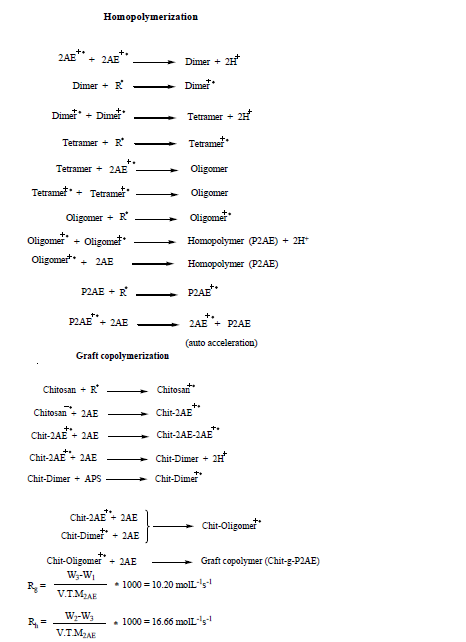
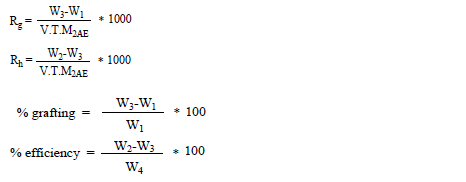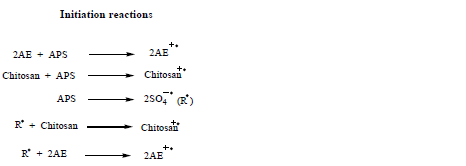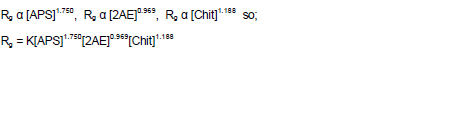Many graft copolymers of chitosan and vinyl monomers have been synthesized and evaluated as flocculants, paper strengthener and drug-releaser (Singh et al., 2006). It has potential applications ranged from biomedicine and pharmacy to water treatment (Yu et al., 2007). Chitosan has both reactive amino and hydroxyl groups that can be used to chemically alter its properties under mild reaction conditions (Hosseini et al., 2010). Kinetics of graft copolymerization investigated from different methods (Li et al., 2002; Mahdavinia et al., 2004).
In addition, among various methods, graft copolymerization is most attractive because it is a useful technique for modifying the chemical and physical properties of natural polymers (Hosseini and Entezami, 2005; 2003; Hosseini and Gohari, 2013; Hosseini, 2006; 2013; Armes and Miller, 1988). Polyaniline are commonly synthesized by chemical or electrochemical oxidation of aniline in acidic aqueous solution (Abdolahi et al., 2012; Hosseini et al., 2010, 2013a). However, other polymerization techniques have now been developed such as enzymatic (Silva et al., 2005), ultrasonic irradiation (Liu et al., 2002), polymerization using electron acceptors (Su and Kuramoto, 2001) and under electric and magnetic fields (Hosseini et al., 2009, 2013b).
Although, the method of preparation is easy, the process ability is found to be very difficult. In order to diversify these difficulties, the electrically conducting polymers are made blend with conventional polymers.
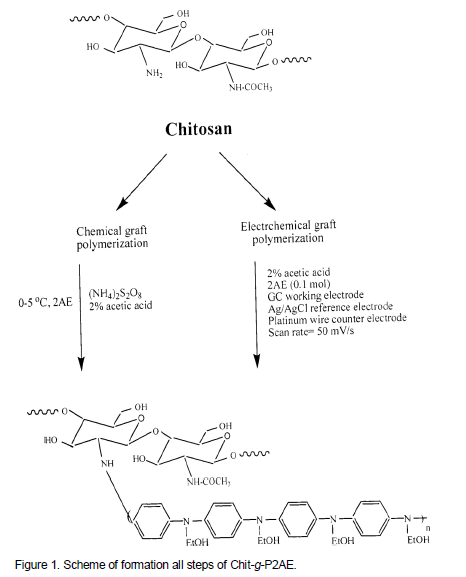
In the preceding works, the authors have reported chemical and electrochemical synthesis of conducting graft copolymer of vinyl acetate with pyrrole and studied of its gas and vapor sensing (Hosseini et al., 2013a). In continuation, we have synthesized PANI grafted onto polyvinylpropionate (Hosseini and Entezami, 2005). Then investigation of sensing effects of polyaniline grafted on polystyrene for cyanide compounds (Hosseini, 2006) and graft copolymer of polypyrrole grafted on polystyrene for some of toxic gases (Hosseini and Entezami, 2005) were reported too. In this work, conducting polymer was employed to initiate the graft copolymerization of 2AE onto chitosan. So, the grafting P2AE onto chitosan was carried out by chemical polymerization. Therefore, effects of concentration of ammonium peroxydisulfate (APS), 2AE, reaction time and temperature on graft copolymerization were studied by determining the grafting percentage, grafting efficiency and percentage add-on. In continuation, efficiency and grafting percentages and electrical conductivities of graft copolymer was measured. Figure 1 showed all steps of formation of Chit-g-P2AE.
Grafting procedure
A typical graft copolymerization study was carried out as follow. Chitosan (W1 g) was immersed in definite concentration of acetic acid (2%) in a glass tube and thermo stated at 45°C for 30 min. The solution was deaerated by passing pure nitrogen gas for 30 min. Required amount of monomer, 2AE was added and de-aerated for another 15 min. Graft copolymerization was initiated by the addition of calculated volumes of APS (using standard solutions). The time of adding the oxidizing agent, APS was taken as the starting time for the reaction. The polymerization conditions were selected in such a way that no polymerization occurred in the absence of added oxidant. This was ascertained by a separate experiment. At the end of the reaction time, the reaction was arrested by blowing air into the glass tube to freeze further reactions. The grafted chitosan fiber along with the homopolymer poly 2-anilinoethanol (P2AE) were filtered from the reaction mixture using a G4 sintered crucible and washed well with 1M HCl for several times, dried (at 80°C for 4 h) and weighed till to get constant weight. The weight of chitosan to be (W1, g) and the total weight of the grafted polymer along with the homopolymer was called W2, g.
The mixture of the grafted chitosan/P2AE and the homopolymer, P2AE was soxhlet extracted with N-methyl pyrrolidone (NMP) for several hours to separate the homopolymer. The extraction process was repeated till the separation of the homopolymer from the grafted sample was completed. This was ascertained by drying the polymer grafted in vacuum till to get constant weight (W3, g). The difference in W3-W1 gives the weight of the grafted P2AE. The difference in W2-W3 gives the weight of the homopolymer, P2AE, formed and W4 is the weight of monomer used.
Electrochemical synthesis of Chit-g-P2AE
Electrochemical polymerization carried out by coating chitosan on surface GC disk working electrode, then growth P2AE onto chitosan in acidic solution. Chit-g-P2AE was prepared by applying intended potential to the electrode using potentiostate. In this electrolysis, a standard three-electrode cell, without any cell partition using a GC working electrode and Ag/AgCl as a reference electrode were employed. The electrolyte solution consisted of 0.1 M 2-anilinoethanol in 20 mL of 2% acetic acid in water. The potential range for electrochemical polymerization and the scan rate were -0.5 to 2 V (versus Ag/AgCl) and 50 mVs-1, respectively.
Graft copolymerization of synthetic polymers onto chitosan can introduce desired properties and enlarge the field of the potential applications of them by choosing various types of side chains.
IR Spectroscopy
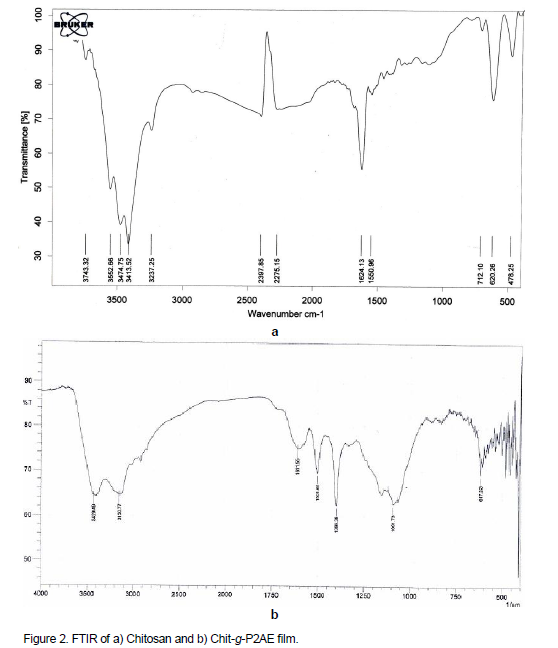
Structural changes of Chit-g-P2AE were confirmed by FTIR spectroscopy (Figure 2a and b). The spectrums of chitosan (Figure 2a) observe the characteristic absorption bands around 3413 and 1624 cm-1. The strong peak around 3413 cm-1 due to the stretching vibration of O–H, the extension vibration of N–H, and inter hydrogen bonds of the chitosan. In graft copolymer, the peak at 3100-3420 cm-1 is of quite reduced intensity and broad, (due to overlapping of O–H and -NH2 stretching groups of chitosan) (Figure 2b). There is a sharp peaks in 1510 and 1389 cm-1, which is related to aniline ring and 1101 cm-1 related to C-N stretching vibration bond. Reduced intensity of this peak with respect to chitosan shows that appreciable N–H at chitosan has been grafted with P2AE chain. From the IR data, it is clear that the grafted copolymer Chit-g- P2AE had characteristic peaks of P2AE of chitosan, which could be strong evidence of grafting.
Scanning electron microscopy
Figure 3(a-c) shows the SEM micrographs of chitosan and Chit-g-P2AE after purification. Figure 3a shows chitosan surface is monotonous. Figure 3b provides direct evidence that polymer films are monotonous and unruffled. This sample has distinct one-phase morphology; so, it can confirm grafting of polymer without homopolymer. The photograph in Figure 3c shows that the cast film of Chit-g-P2AE is homogeneous and continuous. It is well known that chitosan has the good ability of degradation. Therefore, P2AE grafted with chitosan can improve its biodegradability. Chit-g-P2AE can play an important role in enhancing the compatibility physical properties of chitosan and conducting polymers.
Conductivity measurements
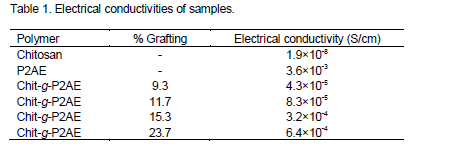
The conductivities of both grafted and ungrafted chitosan were measured. The Chit-g-P2AE showed a good conductivity value better than pure chitosan. It was found that the conductivity values increased with increase in percentage grafting (Table 1). This confirms the chemical grafting of P2AE onto chitosan matrix.
Study of cyclic voltammetry
The cyclic voltammograms for Chit-g-P2AE films grown and blank at different scan rates in acidic solution, GC disk as working electrode and Ag/AgCl reference electrode, with a reduced anodic potential limit are presented of Figure 4(a-c). Two pairs of well resolved oxidation peaks A and B which progressively developed at 0.9 and 1.3 V can be seen in Figure 4(a). First curve shows oxidation of chitosan moieties in the precursor, start to be oxidized for polymerization and red-ox behavior as well and it is electroactive completely.
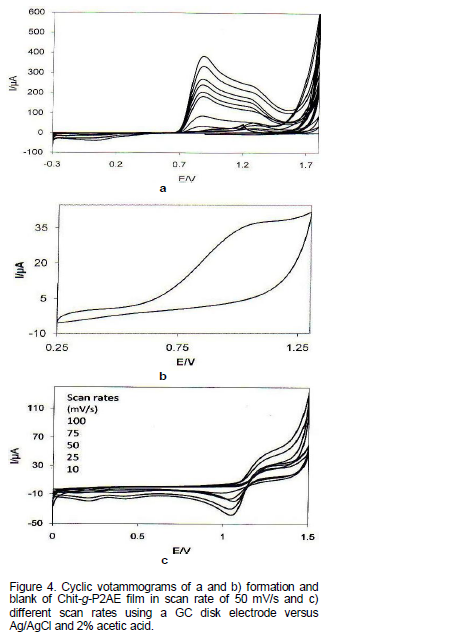
This strongly resembles the behavior of P2AE. Only a peak, the so called “middle peaks” B, which belong to the degradation products (Hosseini, 2006) is poorly recognized. The potentials of the first peaks A in Chi-g- P2AE are slightly less positive than the corresponding values in P2AE. With the same electrochemical conditions, the curve shapes of Chit-g-P2AE almost replicate the electrochemical behaviour of P2AE, confirming once more that the mechanism of the oxidation processes in Chit-g-P2AE are the same as in the case of P2AE. Figure 4(b, c) showed that cyclic voltammograms of Chit-g-P2AE in blank and different scan rates, respectively. Herein, leucoemeraldine is converted into emeraldine in form of oxidation doping in peak and in continuation of the fall in peak, emeraldine is oxidized to pernigraniline and then, in the emeraldine is reduced to leucoemeraldine.
In both reduction systems, during transferring electron from polymer chain, to neutralize the load, the anions in the electrolyte exits to the polymer structure. In Figure 4a, reduction peaks in 50 mV/s scan rate was not seen, but reduction peak increased by decreasing scan rates. So, we can see redox peak in 10 mV/s, as well.
Differential scanning calorimetry (DSC) and thermo-gravimetric analysis (TGA) investigation
Figures 5 and 6 showed TGA and DSC of Chit-g-P2AE. Figure 5 shows that, polymer started to become soften from 30°C up to 205°C, first. It approximately loses 23% of its weight, which is due to humidity and existing solvent in polymer chains. Secondly, at temperature about 205 to 244°C approximately loses 8% of its weight, which is due to degradation of chitosan and side chain of P2AE. Third, at temperature of about 244 to 350°C, it starts to experience structural degradation and approximately loses 20% of its weight, which is due to degradation of polymer backbone. Chit-g-P2AE is thermally stable at temperatures below 205°C and in temperatures above 205°C; the polymer starts degradation and completely decomposed at above 350°C.
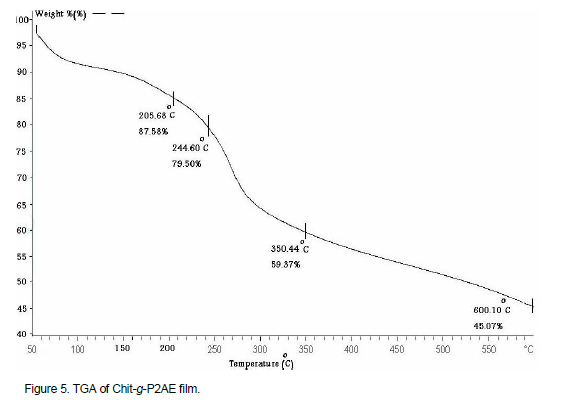
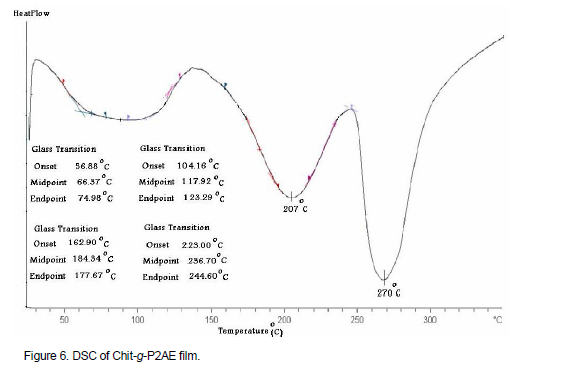
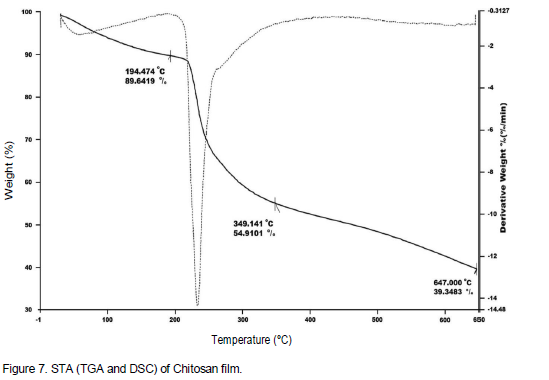
As shown in Figure 6 for Chit-g-P2AE, DSC curve, the endothermic peaks appear in 56 to 123°C (80°C), 162 to 230°C (207°C) and 245 to 300°C (270°C) regions. The endothermic peaks are related to TGA degradation steps. The TGA and DSC curves show that Chit-g-P2AE has more thermal resistance than chitosan and P2AE. This curve has a nearly softening temperature in 200°C and destruction initiation in above than 200°C and in both polymers chitosan and P2AE is less resistance. Figure 7 showed STA (DSC and TGA) thermmograms of chitosan for comparison. The STA curves show that Chit-g-2AE has a little more thermal resistance than chitosan and lower than P2AE. The TGA curve for chitosan has a softening temperature in 194°C and destruction initiation in less than 200°C and for Chit-g-P2AE degradation initiation over than 205°C.
Rate measurements
The rate of grafting (Rg), rate of homopolymerization (Rh), grafting and efficiency percentages were calculated as follows:
where t = reaction time, M = molecular weight of the monomer and V = total volume of the reaction mixture.
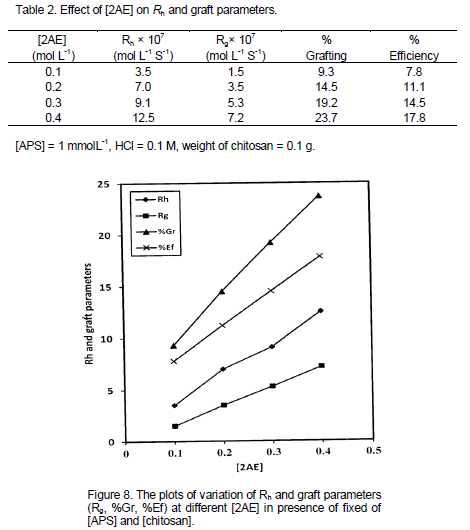
Effect of monomer concentration on Rh and graft parameters
Experimental results obtained by changing the [2AE] in the range from 0.1 to 0.4 mol L-1 using APS as an initiator is given in Table 2 while keeping other experimental conditions as constant. The effect of varying the [2AE] on Rh and graft parameters such as Rg, % grafting (%Gr) and % efficiency (%Ef) are represented in Table 2 as well. The Rh and graft parameters value increased with increase in [2AE]. In an attempt to have further confirmation about the dependence of Rh and graft parameters (Rg, %Gr, %Ef) on [2AE] a different set of experimental conditions were made as represented in Figure 8. The plots of Rh and graft parameters vs. [2AE] were drawn. The plot indicates the first order dependence of Rh and Rg on [2AE] and intercept of the plots Rh and Rg vs. [2AE] were noted.
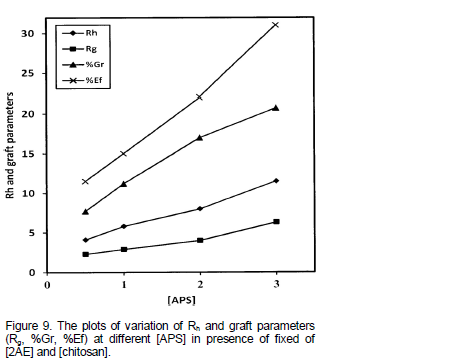
Effect of initiator concentration on Rh and graft parameters
The effects of varying the [APS] on Rh and graft parameters are presented in Table 3. The [APS] was varied from 0.5 to 3 mmol L-1 while keeping other experimental conditions as constant. Here again, the Rh and Rg value showed increasing trend with [APS]. In a separate set of experimental conditions different from the above mentioned conditions, the effect of [APS] on Rh and graft parameters were studied (Figure 9). The plots of Rh, Rg, %Gr and %Ef vs. [APS] were drawn. Then direct plots were found to be linear and passing through the origin. Figure 8 indicated the first order dependence of Rh and Rg on [APS].
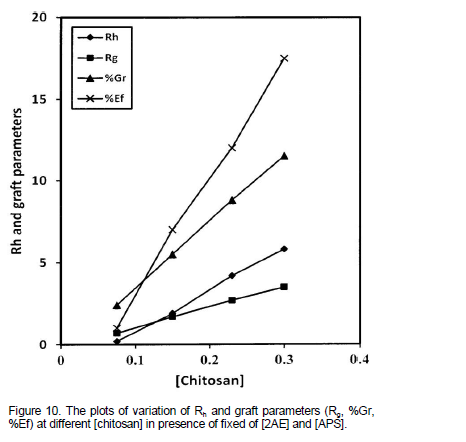
Effect of amount of chitosan on Rh and graft parameters
The effect of amount of chitosan on Rh and graft parameters were studied under the conditions mentioned in Table 4. The chitosan weight was varied between 0.075 to 0.30 g while keeping other experimental conditions as constant. Rh and Rg increased with increase in amount of chitosan. In an attempt to quantify the order dependences, the effect of the amount of chitosan on Rh and graft parameters were studied under a set of different experimental conditions as specified in Figure 10. The plots of Rh and graft parameters were drawn. The slope values of the plots were found to be close to one indicating first order dependence of Rh and Rg on weight of chitosan. These plots were found to be linear and passing through the origin. The linear plots support the clear first order dependence of Rh and Rg on backbone amount.
Mechanism of graft copolymerization
A probable mechanism is proposed here to explain the experimental results obtained. The mechanism suggested for graft copolymerization of P2AE onto chitosan in this paper is based on the mechanism proposed by few research groups. Shim et al. (1990), Bhadani et al. (1996) and Anbarasan et al. (2000) explained the formation of homopolymer via radical cation and mechanism for the graft copolymerization of PANI onto various natural polymers by chemical and electrochemical methods. Taking the above mechanisms as basis, a probable mechanism is suggested here to explain the modification of chitosan through chemical grafting. Probable mechanism for APS initiated graft copolymerization of 2AE onto chitosan.
In a grafting system of 2AE, APS and chitosan, the relation of the rate of grafting (Rg) with the monomer, chitosan and initiator concentrations after calculations of slop of lines from Tables 2 to 4 can be written as:
Graft copolymerization was employed as an important technique to obtain a chemically and electrochemically modified chitosan. The 2AE was successfully grafted onto the chitosan backbone in an aqueous acidic medium condition. There are higher graft percentage and lower homopolymer formation. The grafting process was confirmed by IR analysis. Based on the TGA and DSC results, it was found that the grafted chitosan was more thermally stable than ungrafted one due to the incorporation of 2AE, which may broaden the range of chitosan application. In addition, the SEM micrographs indicate that the graft copolymer is efficient to improve the compatibility of binary blend of chitosan and P2AE.