ABSTRACT
Barite is barium sulfate with the chemical formula of BaSO4. Unlike barite witherite (BaCO3) it is not chemically inert, it can dissolve in water and, when dissolved, can cause an environmental hazard. Barite forms in many geologic environments with both metallic and nonmetallic minerals. Minerals associated with the ore are a main concern when mining and processing barite. In many barite deposits, silica is present,if the level of SiO2 is high enough with prolong exposure to particle size of less 10 µm pose a health hazard, and the ore must be handled so as to reduce the respirable silica level. If unoxidized minerals such as pyrite are present with the barite, acidicrunoff could result from ground-water or storm water content. The runoff may also leach additional minerals from the ore, resulting in high minerals concentration. Both the runoff of this water as well as the accumulation of this water in mining pits is of possible environmental concern. These issues must be addressed within the context of the environmental requirements in place in the jurisdiction within which the mining occurs.This paper is aimed at characterizing Barite from Bukkuyum local government area of Zamfara state of Nigeria, using Empyrean diffractometer DY 674 (2010) for XRD phase analysis of the powdered sample. The peaks generated match those of a BaSO4 in ICDD PDF 2 (2010) database. Minipal 4 energy dispersive X-ray Fluorescence (XRF) provided the elemental analysis in their oxides, with SiO2 of 4.55% content and 19.5% of SO3 that could be the source of silicosis and acidic runoff, respectively, that are of health and environmental concern. The SEM provided the photomicrograph picture with the fiber histogram giving the statistics of particle size of 2.19, 8.73 and 20.70µm, allwith the objective of giving adequate information of the environmental hazards associated with its exploration base on the findings of these characteristics.
Key words: Characteristics, barite, exploration, environmental, health, hazard.
Abbreviation:
Abbreviations: OSHA,Occupational safety and health administration; PDF,powder diffraction file; XRF, X-ray fluorescence; SEM, scanning electron microscope.
Barite is barium sulfate with the chemical formula of BaSO4, unlike barite the carbonate of barium mineral is witherite (BaCO3) itis not chemically inert. Witherite can dissolve in water and, when dissolved, can cause an environmental hazard. Barite by far the predominant barium mineral used by industry because of it highdensity and chemical inertness, it is ideal for use in the petroleum, automotive, and paint industries, and in other applications (Paul, 2006; Brobst, 1994).Barite forms in many geologic environments and can be found with both metallic and nonmetallic minerals. The barite can either be the major component of the mineral or minor fraction. In most cases, the barite most be a major component of the mineral and must be substantial thickness for the ore to be economic. Mineral associated with the ore are a main concern when mining and processing barite. In many barite deposits, quartz is present with the barite. If the level of quartz is high enough, the ore must be handled so as to reduce the respirable silica level (Gianella,1940; Ketner,1963; Papke, 1984). Exposure to silica can be deadly, and limiting that exposure is essential. For the particle size the Occupational Safety and Health Administration (OSHA) proposes a new permissible exposure limit, calculated as an 8htime-weighted average, of 50 micrograms of respirable crystalline silica per cubic meter of air (50µg/m3).
OSHA also proposes other ancillary provisions for employee protection such as preferred methods for controlling exposure, respiratory protection, medical surveillance, hazard communication, and recordkeeping (Harbenand Bates, 1990; DuhamandHanor, 1967; Suresh et al., 2007). If unoxidized minerals such as pyrite are present with the barite, acid mine drainage or low-pH runoff could result from ground-water or storm water content with the pyrite. The acidic runoff may also leach additional minerals from the ore, resulting in runoff with a low pH and high minerals concentration. Both the runoff of this water as well as the accumulation of this water in mining pits is of possible environmental concern. These issues must be addressed within the context of the environmental requirements in place in the jurisdiction within which the mining occurs (Siegel, 2002; Nordstrom, 2008; Oden, 2012).
This paper is aimed at characterizing Barite from Bukkuyum local government area of Zamfara state of Nigeria, with the objective of giving adequate information of the environmental and health hazards associated with it exploration base on the findings of these characteristics which is a function of its geological origin. The characterization includes chemical analysis, mineralogical examination, and particle-size distribution of the crushed ore, depending on the nature of the deposit. Chemical analysis and mineralogical data allow identification of the minerals present. The size distribution gives an idea to grind ability and examining the size fractions will indicate liberation size.
Experimental procedure
The sample of Barite was collected from Bukkuyum local government area, Zamfara state of Nigeria (Figure 1). Using, Empyrean diffractometer DY 674 (2010) for (XRD) phase composition analysis, Minipal 4 energy dispersive X-ray Fluorescence (XRF) for elemental analysis and Scanning Electron microscope (SEM) formicrograph morphological picture of the kaolin sample.
XRD Analysis of the sample
The sample of Barite was analyzed using the reflection-transmission spinner stage using the Theta-Theta settings. Two-Theta starting position is 0.00483 and ends at 75,000 with a two-theta step of 0.026 at 3.57 s per step. Tube current was 40mA and the tension was 45VA. Fixed Divergent Slit size of 1° is used and the goniometer radius is 240mm. The intensity of diffracted X-rays is continuously recorded as the sample and detector rotate through their respective angles. A peak in intensity occurs when the mineral contains lattice planes with d-spacings appropriate to diffract X-rays at that value of θ, although each peak consists of two separate reflections (Kα1and Kα2), at small values of 2θ the peak locations overlap with Kα2 appearing as a hump on the side of Kα1, Greater separation occurs at higher values of θ. Typically these combined peaks are treated as one. The 2λposition of the diffraction peak is typically measure as the center of the peak at 80% peak height.
Results are presented as peak positions at 2θ and X-ray counts (intensity) in the form of a table, and x-y plot. Intensity (I) is reported as peak height intensity. That is intensity above background, or as integrated intensity, the area under the peak. The relative intensity is recorded as the ratio of the peak intensity to that of the most intense peak (relative intensity = I/I1 x 100).The d-spacing of each peak is then obtained by solution of the Bragg equation for the appropriate value of λ. Once all d-spacing have been determined, automated search/match routines compared the unknown to those of known materials. Because each mineral has a unique set of d-spacing, matching these d-spacings provides an identification of the unknown sample. A systematic procedure was used by ordering the d-spacings in terms of their intensity beginning with the most intense peak. Files of d-spacings for hundreds of thousands of inorganic compounds are liable from the International Centre for Diffraction Data as the Powder Diffraction File (PDF).The peaks obtained from the analyses were matched with the minerals from ICDD database which is attached to the software of the machine.
XRF Analysis of the sample
Solid pellet sample of l0g was placed in the special "dekat “plastic cup sample holders. The sample was then loaded on the sample changer and the position label was noted.Beads used for the major oxide analysis were SiO2, AI2O3, Fe2O3, TiO, MgO, CaO, K2O and P2O5 expressed in weight percent were prepared.10g of pulverized sample was weighed and mixed exactly with 5times of X-Ray flux (66.0% lithium Tetraborate, 34.0% Lithium Metaborate).This weighed mixture was mixed properly in a Platinum crucible and ignited in apre-set furnace at 1150°C for 10 min,immediately, the weighed mixture inthe crucible was placed on the crucible holder, and the preparation process commenced as the start button was pressed on. It was heated, mixed and automatically cast into a dish (mold). During this process a releasing agent was added (ammonium iodide tablet). Upon completion of cooling the cold glass beadwas unload ready for analysis. The glass bead was labeled and slotted into the Minipal 4 energy dispersive XRF for major oxide analysis.For XRF Trace elemental Analysis,the pellets were prepared by weighing l0g of oven dried pulverized sample and 5.0g flux(cellulose powder) added as a binder. This is mixed thoroughly with glass rod in a bowl. The well mixed was then compressed by applying a pressure of 1500kgmusing manual compressor. The steps carried out for measurements were; Loading the sample, and opening the measure window for the required application.
SEM of the sample
The Solid Barite sample pellet was prepared for scanning electron microscopy by crushing and grinding. The SEM has an electron optical column in which a finely focused electron beam can be scanned over a specified area of the specimen, small particles mounted on a stub. Resolution in the SEM ranges from about 50 to 25? (5 to 2.5 nm).In the electron column of the SEM, a high intensity electron beam is scanned across the specimens. The impact of an electron beam on the surface of a solid sample causes various types of radiation signals that were recorded by detector above the specimen.
XRD Phase analysis
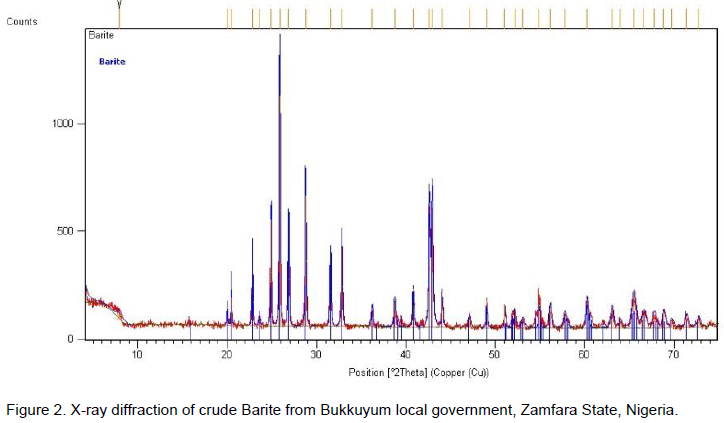
From the Figure 2, it show the XRD peaks of the crude Barite powder, which indicates peaks corresponding to Barite with chemical formula BaSO4 on ICPDS card number 00-024-0020.
XRF Elemental analysis
From the Table 1, it has substantiate the XRD results by providing the elemental composition in their oxides, oxides of particular interest are BaO of 71.46%, Al2O3 of 14.98%, Fe2O3 of 0.58%, SO3 of 19.5%, CaO of 0.71%, MgO of 0.03%, and LOI of 0.04%. The SiO2 of 4,55% can cause silicosis especially with prolong exposure to the mineral mining and processing with particle size less than 10µm.The high percentages ofSO3 and Fe2O3indicates possible acid run-off from ground water with pyrite content.
SEM Morphological analysis
From the Figure 3 and 4, it shows the fracture surface and the grind ability of the mineral with particle size distribution of2.19, 8.73, 20.70µm of the crude barite.From the fiber histogram statistics, about 62 to 65% are less than 10µm. Depending on application the specification of particle size are provided.
Based on the experimental results analysis, the following conclusions have been presented.
1. XRD analysis confirms the Barite BaSO4 phase.
2. The XRF presented the elements in their oxides SiO2 of 4.55%, Al2O3 of 1.31%,K2O of 0.06%, Na2O of 0.07%, SO3 of 19.5%, CaO of 0.09%, MgO of 0.03%, TiO2 of 1.6%, Fe2O3 of 0.27%, MnO of 0.01%, BaO of 71.46% and LOI of 1.05%.
3. The SEM shows the morphology along with statistics of particle size of 2.19, 8.73 and 20.70µm.
4. Finally, it can be concluded that barite from Bukkuyum local government area of Zamfara state hasSiO2of 4.55% content as such the primary health risk is the inhalation of "respirable" particles smaller than 10µm. Generally, the smaller the particles are the greater hazard and potential injury to the lungs. Dust particles larger than these are not capable of penetrating the defense mechanisms of the lung. Prolonged exposure may cause delayed chronic silicosis. Chronic silicosis may take many years of exposure to develop, but with acute exposure rapid development can occur. In latter stages of silicosis, known as complicated or conglomerate silicosis, lung function may be reduced, resulting in symptoms of shortness of breath.The content of SO3 of 19.5% can possibly lead to acidic low-pH runoff from ground-waterthat may also leach additional minerals from the ore, resulting in high minerals concentration of environmental concern(NIOSH, 1992; NLMC, 1991).
The autor (s) did not declare any conflict of interest.
Authors would like to thank (NGRL), Kaduna for their kind help of XRD and XRF analysis, and also Department of Chemical Engineering, ABU, Zaria for the SEM morphology analysis.
OSHA, Occupational safety and health administration; PDF, powder diffraction file; XRF, X-ray fluorescence; SEM, scanning electron microscope.
REFERENCES
|
Brobst DA (1994). Barium minerals. Pages 125-134 in Industrial Minerals and Rocks.6thedition.Edited by D.D. Carr, Littleton, CO: SME.
|
|
|
|
Duham AC, Hanor JS (1967). Controls of barite mineralization in the western United States. Econ. Geol. 62:82-94.
Crossref
|
|
|
|
|
Gianella VP (1940). Barite deposits in northern Nevada. AIME Trans. 144:294-299.
|
|
|
|
|
Harben PW, Bates RL (1990). Barite. In: Industrial Minerals: Geology and world Deposits. London: Metals Bulletin Plc. pp. 10-18.
|
|
|
|
|
Ketner KB (1963). Bedded barite deposits of the Shoshone range, Nevada. Professional Paper 475-B. U.S. Geological Survey (USGS).
|
|
|
|
|
NIOSH (1992). NIOSH recommendations for occupational safety and health: compendium of policy documents and statement Cincinnati, OH: US. Department of health and human services public health service, centers for disease control, National Institute of occupational safety and health, DHHS (NIOSH) publication No 92-100.
|
|
|
|
|
NLMC (1991). The hazardous Substances data bank: Barium Sulfate. Bethesda MD: National Library of Medicine.
|
|
|
|
|
Nordstrom DK (2008). The groundwater quality before mining in a mineralized region, Lessons from the Questa Project. Geosciences J. 12(2):139-149.
Crossref
|
|
|
|
|
Oden MI (2012). Barite veins in the Benue Trough: field characteristics, the quality issue and some tectonic implications. Environ. Nat. Res. 2(2):21-32.
Crossref
|
|
|
|
|
Papke KG (1984).Barite in Nevada, Bulletin 98; Nevada Bureau of Mines and Geology.
|
|
|
|
|
Paul M (2006). Barium minerals. In: Industrial Minerals and Rocks.7th edition. Edited by D.D. Carr, Littleton, CO: SME. pp. 219-225.
|
|
|
|
|
Siegel FR (2002). Environmental Geochemistry of Potential Toxic Metals Springer, Heidelberg.
Crossref
|
|
|
|
|
Suresh S, Dinakar N, Prasad TNKV, Nagajyothi PC, Damodharam T, Nagaraju A (2007). Effects of barite mine on groundwater quality in Andhra Pradesh, India. Mine Water Environ. 26:119-123.
Crossref
|
|