Full Length Research Paper
ABSTRACT
The borderline oxacillin-resistant Staphylococcus aureus (BORSA) strains have a low-level resistance to penicillinase-resistant penicillins. The aim of this study was to study the prevalence and the antimicrobial susceptibility of BORSA strains in Bamako. A retrospective study was conducted at the University Teaching Hospital of the Point G in Bamako. The S. aureus strains were isolated on Columbia agar supplemented with 5% sheep blood, nalidixic acid and colistin. Antimicrobial susceptibility testing was performed using the disc diffusion method on Mueller-Hinton agar. β-lactamase production was determined by nitrocephin disc. Among 735 nonrepetitive strains of S. aureus, 41 (5.6%) were BORSA and 335 (45.6%) methicillin-resistant S. aureus (MRSA). The prevalence of BORSA strains was 25 (4.9%) and 16 (7.2%) in hospital and out-patients areas, respectively. Of the 41 BORSA strains, five were not β-lactamase producers. Cefoxitin (100%), cephalothin (97.6%), gentamycin (90.2%), amikacin (90.2%), netilmicin (87.8%), pristinamycin (87.8%), tobramycin (82.9%), fusidic acid (83%), lincomycin (78%), amoxicillin combined with clavulanic acid (73.2%), sulfonamides (73.2%), kanamycin (73.2%) fosfomycin (73%) and chloramphenicol (70.7%) were the most active against the BORSA strains. The prevalence of BORSA strains was not high in this study, and the strains were susceptible to a great range of antibiotics.
Key words: Antimicrobial susceptibility, borderline oxacillin-resistant Staphylococcus aureus, prevalence, Bamako (Mali).
INTRODUCTION
Staphylococcus aureus belongs to the Staphylococcaceae family (Foster and Geoghegan, 2015). The S. aureus infections appear under various clinical aspects such as urinary tract infections, abscesses, septicemia, vaginitis, and/or pleuropulmonary infections (Avril et al., 2000). The borderline oxacillin-resistant Staphylococcus aureus (BORSA) has a low level of resistance to penicillin resistant to penicillinases which are oxacillin, cloxacillin and methicillin, that is, antibiotics which are not hydrolyzed by staphylococcal β-lactamases (Hryniewicz and Garbacz, 2017). β-lactams are the antibiotics of choice in the treatment of staphylococcal infections (Hryniecz and Garbacz, 2017).
The resistance of the BORSA strains is typically linked to hyperproduction of β-lactamases or in some cases to other mechanisms which are the production of an inducible methicillinase with plasmid mediation, the modification of genes of proteins binding penicillin (PBP) (Cros et al., 2010; Hryniecz and Garbacz, 2017; Maalej et al., 2012). The activity of oxacillin on the BORSA strains is restored by β-lactamase inhibitors such as clavulanic acid or sulbactam (Hryniecz and Garbacz, 2017; Maalej et al., 2012). β-lactamase inhibitors do not restore oxacillin activity when altered proteins binding penicillin occurs (Hryniecz and Garbacz, 2017).
The resistance to methicillin, on the contrary, is due to the acquisition of a new PBP, the PBP2a or PBP2' encoded by chromosomal genes mecA or mecC (mecALGA251) (Hryniecz and Garbacz, 2017; Maïga et al., 2017). In addition, the BORSA strains are not classified as either methicillin-resistant S. aureus strains or methicillin-susceptible S. aureus strains (Hryniecz and Garbacz, 2017). The methicillin resistance is crossed between penicillins and cephalosporins (Fleurette, 1989). The BORSA strains, also called methicillin-resistant borderline S. aureus, do not have PBP2a or PBP2' (Montanari et al., 1990). The BORSA has been described in Tunisia (Maalej et al., 2012).
In Mali, to the best of our knowledge, no study has been focused on the borderline-oxacillin resistant Staphylococcus aureus stains. Thus, the aim of our study was to determine the prevalence and susceptibility to antibiotics of the BORSA strains in Bamako both for epidemiological and clinical purposes.
MATERIALS AND METHODS
Study design and settings
A retrospective study was conducted between January 2014 and December 2020 at the medical biology and hospital hygiene laboratory of the University Teaching Hospital (UTH) of the Point G, Bamako Mali. The UTH of the Point G is the third-pyramidal reference in Mali, and has 522 beds divided between the surgical, intensive care and medical departments.
Bacterial strains
Patients admitted to the different departments of the UTH of the Point G, were hospitalized patients (in-patients), those who were not hospitalized and coming directly to the laboratory for different reasons were called out-patients.
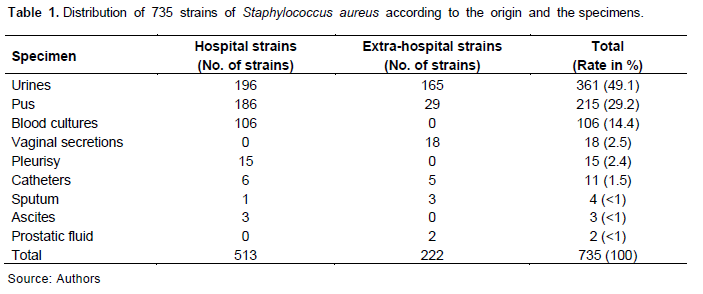
The specimens collected were mainly respiratory origin, urine, pus, blood cultures, and/or vaginal secretions (Table 1).
The isolation of S. aureus strains was carried out on Columbia agar supplemented with nalidixic acid (15 mg/l), colistin (10 mg/l) and sheep blood (5%) at 37°C at the oven.
Given the retrospective study design, we included in this study, the S. aureus strains with complete drug susceptibility results with additional details of sampling source and date. On the other hand, the S. aureus strains without information of the sampling source and/or origin were not included. In this study hospital strains refer to strains isolated from hospitalized patients at the UTH of the Point G, and extra-hospital strains those from out-patients (non-hospitalized patients).
While the molecular methods for identification are rapid and could save more time and labor, they are quite expensive and not implemented in our laboratory yet. Thus, the identification of the strains of S. aureus was made by the combination of Gram staining (bioMérieux, France), the presence of a catalase (bioMérieux, France), coagulase (Bio-Rad, France), the Pastorex reagent Staph Plus (Bio-Rad, France) and the API STAPH gallery (bioMérieux, France).
Susceptibility to antibiotics test
The antimicrobial susceptibility testing was carried out on Mueller-Hinton agar (Bio-Rad, France) by the disc method (Agar diffusion method) (Kahlmeter and Turnidge, 2012). The strains of S. aureus were classified as "susceptible", "intermediate" or "resistant" according to the recommendations of the Antibiotic Committee of the French Society of Microbiology/European Committee on Antimicrobial Susceptibility Testing in 2015 (CA-SFM/EUCAST) (Amara et al., 2022; Bonnet et al., 2010). For the purpose of analysis, the strains of S. aureus classified as <<intermediate>> to the antibiotics tested were considered resistant.
Laboratory procedure
The antimicrobial susceptibility testing was performed on Mueller-Hinton agar poured in a Petri dish. Five to eight colonies identical and isolated from an 18 to 24 h culture of S. aureus were suspended in a 5 ml of sterile saline solution which was calibrated to 0.5 McFarland. One milliliter of this suspension was then added to 9 ml of sterile distilled water (dilution 1:10). This second suspension is poured over the entire surface of the Mueller-Hinton agar poured into a Petri dish. The excess is poured into bleach. The seeded agar is left to dry for 15 min at 37°C inside the incubator. Please kindly note that the incubator is not used to dry seeded agar plate. Instead, the plates are allowed to stand on the bench for a while, before the antibiotics dics are introduced. After the seeded agar was dried, the blotting paper discs impregnated with the antibiotic discs to be tested are placed on the surface of the agar using a disc dispenser according to manufacturer’s instructions (Bio-Rad, France).
After a first diffusion of the antibiotics at 30 min at room temperature, the Petri dish is incubated at 37°C for 18 to 24 h, in the inverted position (cover down). The paper disc impregnated with oxacillin is placed on the surface of another seeded agar in Petri dish (diameter 90 mm) and placed in the incubator at 30°C for 24 to 48 h in the inverted position also. The reading is performed in measuring the diameter of inhibition of each antibiotic disc using a caliper in contact of growth.
Antibacterial agents tested
The antibiotics tested are penicillin G (6 µg), cephalothin (30 µg), oxacillin (5 µg), amoxicillin combinated with clavulanic acid (20 µg + 10 µg), cefoxitin (30 µg), gentamycin (10 UI), kanamycin (30 UI), tobramycin (30 µg), netilmicin (30 µg), amikacin (30 µg), streptomycin (10 µg), erythromycin (15 µg), lincomycin (15 µg), pristinamycin (15 µg), chloramphenicol (30 µg), tetracycline (30 µg), sulfonamides (200 µg), trimethoprim(5 µg), norfloxacin (5 µg), fosfomycin (50 µg) and fusidic acid (10 µg) (Bio-Rad, France).
β-lactams resistance phenotypes
While the S. aureus strains susceptible to cefoxitin and oxacillin were considered methicillin-susceptible S. aureus (MSSA), those resistant to cefoxitin and oxacillin have been considered methicillin-resistant Staphylococcus aureus (MRSA) (Cattoir and Leclercq, 2012; Mougeot et al., 2001). In addition, the S. aureus strains resistant or intermediate to oxacillin and susceptible to cefoxitin were considered as borderline oxacillin-resistant Staphylococcus aureus (BORSA) (Hryniewicz and Garbacz, 2017). Moreover, the BORSA strains resistant or intermediate to penicillin G produced β-lactamase. The production of β-lactamase was confirmed using the nitrocephine (cefinase) disc (bioMérieux, France). The penicillin G-susceptible BORSA strains with a fuzzy border were not β-lactamase producers. We compared the susceptibility of the BORSA strains to that of randomly selected MSSA strains.
Ethical consideration
The use of the results of the susceptibility to antibiotics test of isolated S. aureus strains was done after patients signed an informed consent. The results were de-identify with anonymous numbers, and the publication of the results was made with the permission of the leadership of the Point G’s UTH.
Statistical analysis
The results of the susceptibility to antibiotics were used to monitor the bacterial resistance’s profile at the Point-G’s UTH and Bamako.
Data entry and computer processing were done using Epi Info version 7.1 software. The χ² test was used to compare our proportions with a significance level of p ≤ 0.05.
RESULTS
Global characteristics of patients and isolates
In total, 735 non-repetitive strains of S. aureus were isolated from 734 persons between January 2014 and December 2020 and included in the study. The mean age of patients was 47.61 ± 19.17 years with extremes of three and 98 years. The sex ratio (male/female) of patients was 0.94. They were in 513 (69.8%) of hospital strains and 222 (30.2%) out-patients’ strains.
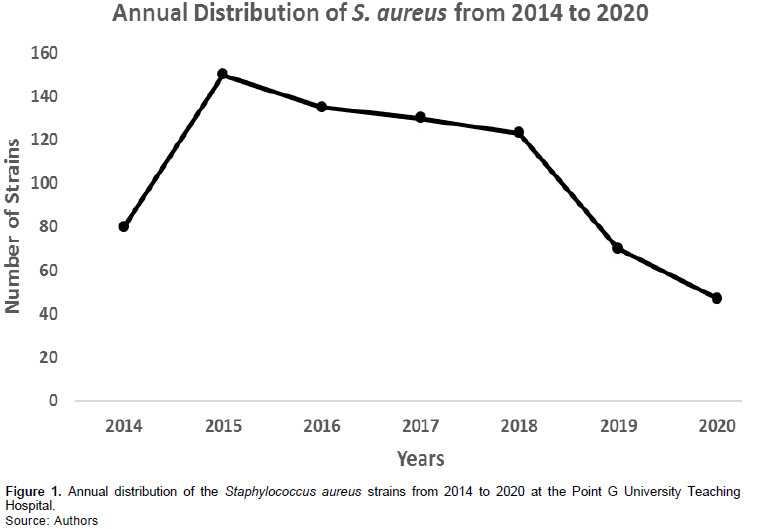
The annual frequency of the S. aureus strains during the study period is presented in Figure 1.
Frequency of BORSA within S. aureus strains
Of the 513 hospital strains of S. aureus, 25 (4.9%) were BORSA, 243 (47.4%) MRSA and 245 (47.8%) MSSA. At the same time, of 222 out-patients’ strains, 16 (7.2%) were BORSA, 92 (41.4%) MRSA and 114 (51.4%) MSSA. Thus, the BORSA strains prevalence was independent of origin (p= 0.18).
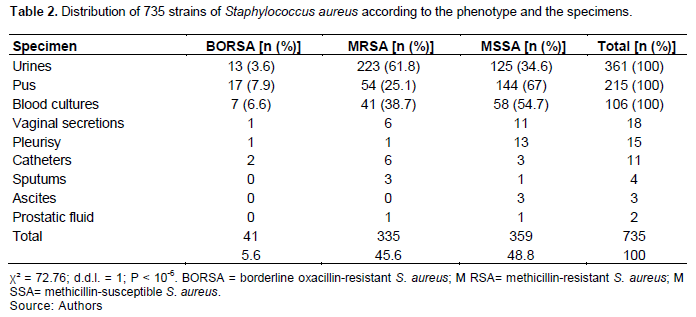
The distribution of the 735 strains according to the phenotype of resistance to β-lactams and the sample is reported in Table 2. The prevalence of the BORSA strains was independent of the sampling sites. Moreover, the prevalence of the MRSA strains was higher in the urine than in the other samples (p ? 10-6).
The bacteriological characteristics of the BORSA hospital and extra-hospital strains are reported in Table 3. Two out-patients’ BORSA strains were isolated from urine in the same patient in April 2017: one of them had diminished susceptibility to norfloxacin, chloramphenicol and streptomycin.
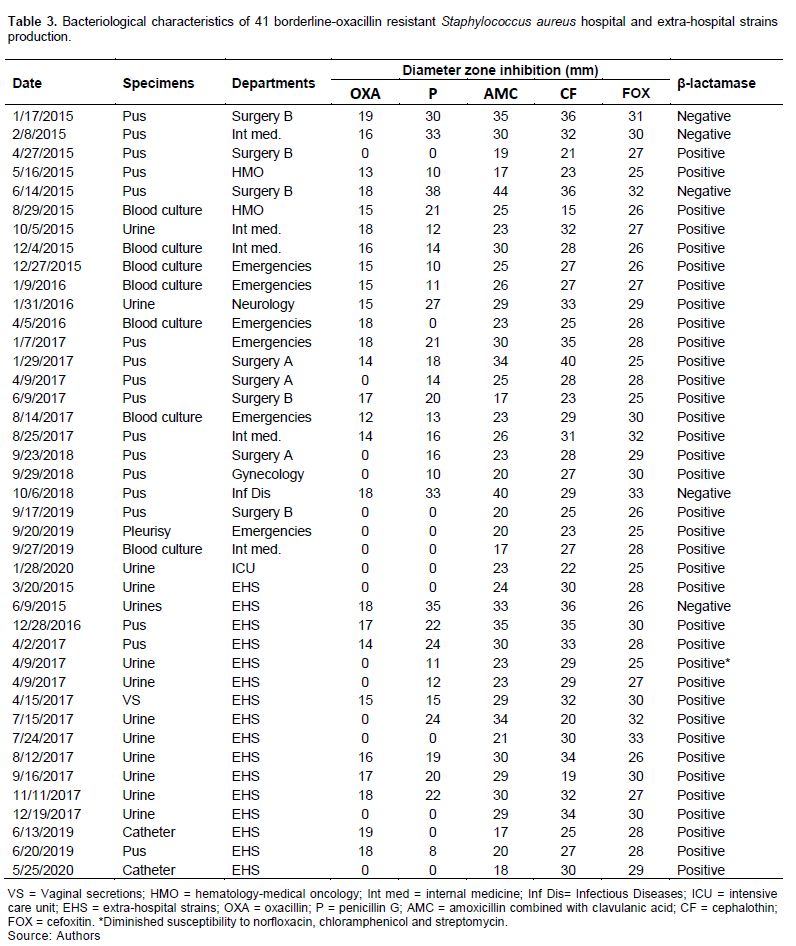
Moreover, of the 25 BORSA hospital strains, four did not produce β-lactamase, and of 16 BORSA extra-hospital strains, one did not produce β-lactamase.
Susceptibility profile of S. aureus strains
All our BORSA strains were resistant to oxacillin, but susceptible to cefoxitin. In addition, 40 (97.6%) were susceptible to cephalothin, 30 (73.2%) to the combination of amoxicillin + clavulanic acid, and five (12.2%) to the penicillin G. Among the 36 BORSA strains producing β-lactamase, 25 were susceptible to the combination amoxicillin + clavulanic acid and 11 were resistant.
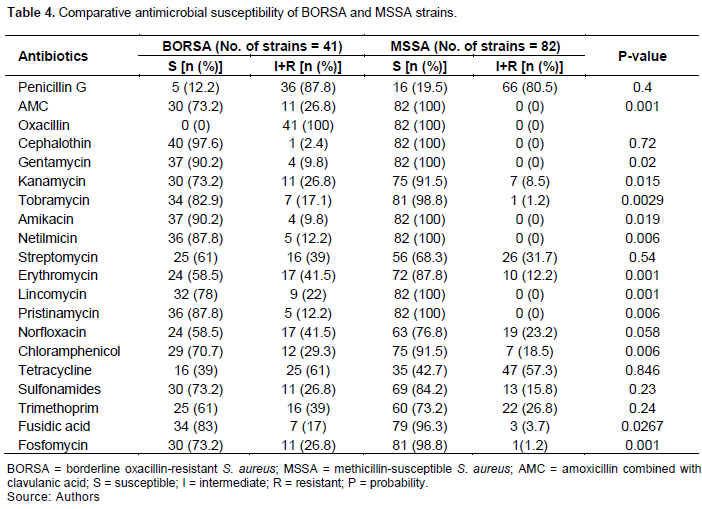
The comparative antibiotic susceptibility of the BORSA and MSSA strains is reported in Table 4. The most active antibiotics on the BORSA strains were cefoxitin, cephalothin, gentamycin, amikacin, netilmicin, amoxicillin + clavulanic acid association, tobramycin, pristinamycin, fusidic acid, lincomycin, chloramphenicol, sulfonamides, kanamycin, and fosfomycin. Moreover, MSSA strains were more susceptible to amoxicillin + clavulanic acid, kanamycin, tobramycin, erythromycin, lincomycin, pristinamycin, chloramphenicol, fusidic acid and fosfomycin the BORSA strains: the differences are statistically significant.
DISCUSSION
In this study, we determine the prevalence and susceptibility to antibiotics of the BORSA strains between 2014 and 2020, using the disk method (agar diffusion technique): an international reference technique (Kahlmeter and Turnidge, 2012). To the best of our knowledge, this study is the first study of its kind conducted in Bamako, Mali making difficult comparison of previous data from Mali. The identification of our strains was based on their morphological, physiological and biochemical characteristics (Avril et al., 2000; Fleurette, 1989). The identification of S. aureus was characterized by its susceptibility to lysostaphin, nitrofurantoin and its resistance to the vibriostatic compound O/129 and to bacitracin (Fleurette, 1989).
The results of the antibiogram were interpreted in accordance with the recommendations of the Antibiogram Committee of the French Society of Microbiology and the European Committee on Antimicrobial Susceptibility Testing (Amara et al., 2022; Bonnet et al., 2010).
Other higher sensitivity and specificity methods were used for the detection of resistance to methicillin such as the oxacillin disc placed either on a Petri-dish of Mueller-Hinton agar incubated between 25 and 30°C and observed after 24 and 48 h or on a Petri-dish of hypersalted Mueller-Hinton agar incubated at 37°C, the use of probes recognizing mec genes, and the agglutination reactions of latex particles sensitized with anti-PBP2a antibodies (April et al., 2000). But the agglutination reactions of latex particles sensitized with anti-PBP2a antibodies is not routinely used (Maalej et al., 2012).
Currently, the identification of the MRSA and BORSA strains are based on the use of oxacillin and cefoxitin discs on Mueller-Hinton agar incubated at 37°C and examined 24 h later. For oxacillin, the interpretation must be done within 48 h if there is a low amount of culture (Bonnet et al., 2010; Cattoir and Leclercq, 2012). The use of the cefoxitin disc alone could make it possible to wrongly classify the BORSA strains among those with MSSA. The use of cefoxitin (30 µg) and moxalactam (30 µg) discs make it possible to identify MRSA at the Saint-Louis Hospital in France with excellent sensitivity (Felten et al., 2002).
In this study, the BORSA strains prevalence was 5.6% and independent of the sampling site and the origin of the strains. The MRSA strains prevalence was 46.2% and was higher in the urine than in the other samples (Table 2). As observed in Myanmar (Soe et al., 2021), this high prevalence in urine samples, may have occurred because of urinary catheterization practice and the colonization by MRSA of indwelling urinary catheters. In Tunisia, of the 1,895 strains of S. aureus isolated between 2006 and 2011 at the University Hospital of Sfax, 451 (21.9%) were MRSA and 23 (1.2%) BORSA (Maalej et al., 2012). These rates are lower than ours. In a previous study conducted in 2007 in our department, the frequency of MRSA strains was 50.3 and 50.8% among hospital and out-patients’ settings, respectively (Tchougoune, 2007). Those rates are closed to these current findings. Usually, the prevalence of the BORSA varies from 1.4 to 12.5% (Hrynievicz and Garbacz, 2017), and our rate is within the intermediate values (Table 2).
In this study, we isolated the BORSA strains from different collection sites, and we did not find a correlation between the strains and the site of collection (Table 2). This was the same in Sfax (Tunisia), and in Toronto (Canada) (Maalej et al., 2012; Leahy et al., 2011). Moreover, the hospital BORSA strains were coming from different units, and it was the same in Tunisia and Canada. In addition, we did not observe an outbreak of the BORSA strains during the study period, and this was the same in Tunisa as well (Maalej et al., 2012). Moreover, when analysis the 16 BORSA strains isolated from children with cystic fibrosis by pulsed-field restricted DNA electrophoresis in Toronto: no link was observed between the strains (Leahy et al., 2011). No link between the 23 strains was observed in Tunisa (Maalej et al., 2012).
Of the 41 BORSA strains, five did not produce β-lactamase (Table 3), and this was the same in Canada, where out of 16, four did not produce β-lactamase (Leahy et al., 2011). At the same time in Tunisia, all the BORSA strains produced β-lactamase (Maalej et al., 2012). This could probably be explained by the sample size which was different in the three countries.
In contrast to Tunisia and Canada (Leahy et al., 2011; Maalej et al., 2012), the combination of amoxicillin + clavulanic acid was not active on 11 BORSA strains out of 41 (Tables 3 and 4). This is probably explained by other mechanisms (Croes et al., 2010; Hryniewicz and Garbacz, 2017; Jupeau-Vessières and Scavizzi, 1994; Maalej et al., 2012). Moreover, both BORSA strains in Mali and Tunisia were susceptible to cefoxitin (Table 3) (Maalej et al., 2012).
In addition, penicillin G, cephalothin, streptomycin, norfloxacin, tetracycline, sulfonamides and trimethoprim were not more active on MSSA strains than on BORSA strains (Table 4). Gentamycin (90.2%), amikacin (90.2%), tobramycin (82.9%), netilmicin (87.8%) and kanamycin (73.2%) were the most active aminoglycosides on our BORSA strains (Table 4). Among the BORSA strains isolated in Sfax, 13 out of 23 strains were susceptible to kanamycin, 20 to gentamycin and 19 to tobramycin. Fusidic acid (83%), fosfomycin (73%) and chloramphenicol (70.7%) had good activity on our BORSA strains (Table 4). All the BORSA strains isolated in Sfax were susceptible to fosfomycin, chloramphenicol and glycopeptides (Maalej et al., 2012). Among our BORSA strains, one out of two strains was susceptible to erythromycin (Table 4). Eleven out of 23 BORSA strains were susceptible to erythromycin in Sfax, Tunisia (Maalej et al., 2012). Thirteen BORSA strains out of 16 were susceptible to erythromycin in Toronto, Canada (Leahy et al., 2011).
Thus, the search of BORSA and MRSA strains is a priority at the UTH of the Point G in Bamako.
Our study has some limitations including the retrospective approach which may have precluded to firmed conclusion because of the loss of sensitive information. In addition, we have more patients who were not included due to missing data either on sample types and origins or culture results and have used patients who were diagnosed in urban Bamako district only who may have different results from rural areas of Mali.
Despite these limitations, we studied a relatively high sample size to determine the prevalence and the antimicrobial susceptibility of the borderline oxacillin-resistant Staphylococcus aureus strains in Bamako in sample types. In addition, given that almost all the regions in Mali are getting a bacterial culture system, thus, a larger sample size and sampling from all the different regions of Mali would be essential for obtaining a true country profile of BORSA and MRSA phenotypes.
CONCLUSION
The BORSA strains prevalence is not high in Bamako and was not associated with the origin and sampling site. The BORSA strains were susceptible to many molecules: cefoxitin, cephalothin, gentamycin, amikacin, netilmicin, tobramycin, the combination amoxicillin + clavulanic acid, pristinamycin, acid fusidic, lincomycin, chloramphenicol, sulfonamides, kanamycin, and fosfomycin. In contrast, antibiotics such as erythromycin, norfloxacin, tetracycline, trimethoprim, and streptomycin should not be used in the treatment of infections caused by strains of the BORSA strains in Bamako
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Amara M, Aubin G, Cattoir V, Dortet L, Goutelle S, Jeannot K, Lepeule R, Lina G, Marchandin H, Mérens A, Ploy MC, Schramm F, Varon E (2022). Comité de l'Antibiogramme de la Société Française de Microbiologie/European Committee on Antimicrobial Susceptibility Testing. Recommandations 2022. Avalable on: |
|
|
Avril JL, Dabernat H, Denis F, Monteil H (2000). Bactériologie clinique, 3rd édition. Paris: Ellipses. 602 p. |
|
|
Bonnet R, Cavallo JD, Chardon H, Chidiac C, Courvalin P, Dabernat H, Drugeon H, Dubreuil L, Guery B, Jarlier V, Jehl F, Lambert T, Leclerq R, Nicolas-Chanoine M-H, Plesiat P, Quentin C, Soussy CJ, Varon E, Weber P (2010). Comité de l'Antibiogramme de la Société Française de Microbiologie/European Committee on Antimicrobial Susceptibility Testing. Recommandations 2010. Avaliable on: |
|
|
Cattoir V, Leclercq R (2012). β-lactamines et staphylocoques. In. Courvalin P, Leclercq R (eds). Antibiogramme, 3rd édition. Paris: Eska pp.137-145. |
|
|
Croes S, Beisser SP, Terporten PH, Neef V, Deurenberg RH, Toberingh EE (2010). Diminished in vitro antibacterial activity of oxacillin against clinical isolates of borderline oxacillin-resistant Staphylococcus aureus. Clinical Microbiology Infections 16:PMID: 20880412 |
|
|
Felten A, Grandry B, Lagrange PH, Casin I (2002). Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): a disk diffusion method with cefoxitin and moxalactam, the vitek2 system and the MRSA- screen latex agglutination test. Journal of Clinical Microbiology 40: PMID: 12149327, PMCID: PMC120619. |
|
|
Fleurette J (1989). Staphylocoques et microcoques. In. Le Minor L, Véron V (eds).Bactériologie médicale, 2nd édition. Paris: Flammarion pp. 773-794. |
|
|
Foster TJ, Geoghegan JA (2015). Staphylococcus aureus. In. Tang YW, Sussman M, Liu D, Poxton I and Schwartzman J (eds). Molecular medical microbiology, Volume II. 2nd edition. New York, NY: Elsevier pp. 655-674. |
|
|
Hryniewicz MM, Garbacz K (2017). Borderline oxacillin-resistant Staphylococcus aureus (BORSA) - a more common problem than expected? Journal of Medical Microbiology. |
|
|
Jupeau-Vessières AM, Scavizzi MR (1994). Evolution de la résistance bactérienne aux antibiotiques. Encyclopédie médico-chirurgicale Maladies Infectieuses 16 p. |
|
|
Kahlmeter G, Turnidge J (2012). Techniques phénotypiques. In. Courvalin P, Leclercq R (eds). Antibiogramme, 3rd édition. Paris: Eska pp. 59-69. |
|
|
Leahy TR, Yau YCW, Atenafu E, Corey M, Ratjen F, Waters V (2011). Epidemiology of borderline oxacillin-resistant Staphylococcus aureus in pediatric cystic fibrosis. Pediatric Pulmonology 46. PMID: 21337531. |
|
|
Maalej SM, Rhimi FM, Fines M, Mnif B, Leclercq R, Hammami A (2012). Analysis of borderline oxacillin-resistant Staphylococcus aureus (BORSA) strains in Tunisia. Journal of Clinical Microbiology 50. PMID: 22814459 PMCID: PMC3457433. |
|
|
Maïga A, Dicko OA, Tchougoune LM, Fofana DB, Coulibaly DM, Maïga II (2017). Haute prévalence des souches de Staphylococcus aureus résistantes à la méticilline au centre hospitalier universitaire du Point G à Bamako (Mali). Mali Médical 32(3):1-8. |
|
|
Montanari MP, Tonin E, Biavasco F, Varaldo PE (1990). Further characterization of borderline methicillin-resistant Staphylococcus aureus and analysis of penicillin-binding proteins. Antimicrobial Agents and Chemotherapy 34. PMID: 2360829 PMCID: PMC171719. |
|
|
Mougeot C, Guillaumat-Taillet J, Libert JM (2001). Staphylococcus aureus: nouvelle détection de la résistance intrinsèque par la méthode de diffusion. Pathologie Biologie 49. PMID: 11367553. |
|
|
Soe PE, Han WW, Sagili KD, Satyanarayana S, Shrestha P, Htoon TT, Tin HH (2021). High Prevalence of Methicillin-Resistant Staphylococcus aureus among Healthcare Facilities and Its Related Factors in Myanmar (2018-2019). Tropical Medicine and Infectious Disease 6:70. |
|
|
Tchougoune LM (2007). Prévalence des souches de Staphylococcus aureus résistantes à la méticilline au CHU du Point G [thèse]. Bamako: Université des Sciences, des Techniques et des Technologies de Bamako 74 p. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0