ABSTRACT
The aim of this study was to evaluate the bacteriological load in Moringa oleifera Lam. leaves consumed in Guinea Savannah vegetation zones of Nigeria, via: Abuja (Gwagwalada market), Southern Guinea Savannah; Katsina (Daura market), Northern Guinea Savannah and Sokoto (Central market), Sudan Guinea Savannah. Three fresh and dried M. oleifera Lam. leafy samples each of 50 g were randomly collected per market location for analysis of total viable cells (cfu/mL) using standard procedures of analyses. The bacterial load in each sample was determined in triplicates and analyzed with SPSS Version 16. Bacterial isolates were classified on the basis of cultural morphology, Gram reaction and biochemical tests. Results showed bacterial growth on Nutrient, Mannitol and MacConkey media. Sabouraud dextrose, Brilliant green and Salmonella-Shigella media recorded no growth in all the leave extracts analyzed. This could be ascribed to the selective nature of the Sabouraud dextrose, Brilliant green and Salmonella-Shigella media, and suggested that fungi/yeast, Salmonella spp. and Salmonella-Shigella species were not among the bacterial contaminants or that the active ingredient component-Pterygospermin, in M. oleifera leaves extract inhibited the growth of micro-organisms in the leaves extract. The study recorded two pathogenic bacteria from all the locations, with S. aureus being more dominating, followed by Escherichia coli and these organisms suggest health hazards. Consumers and vegetable vendors should be educated on proper hygienic handling, transportation and storage of vegetables to avoid bacteriological food spoilage and other related health issues.
Key words: Moringa oleifera leaves, nutritional quality, bacteriological load, vegetables.
Vegetables are good source of food because of their riches in nutritional quality which include beta- carotene,
ascorbic acid, minerals, fibers and essential oils which play significant physiological role in human body as an antioxidant, stimulating enzymes, destroying bacteria and reducing diseases such as heart and cancer. The phytochemical compounds in green leafy vegetables possess antimicrobial properties and include alkaloids, anthraquiones, flavonoids, phenols, tannins, terpenoids and saponins (Paulsamy and Jeeshua, 2011). Internal system of antioxidants exists in human body to get rid of excessive free radicals from metabolism, but exogenous/ natural antioxidant which green leafy vegetables can provide is needed (Yanishlieva et al., 2006).
The vegetables, including Moringa leaves, either fresh or dried, are available, accessible, and affordable at the least costs to every household, including the rich and the poor (Osuagwu et al., 2014; Monica et al., 2015). Moringa oleifera Lam. leaf is consumed worldwide because of its nutritional quality including macro and micro nutrients, for medicinal purposes and industrial uses in water effluent treatments (Joshi and Mehta, 2010; Moyo et al., 2011; Xiaompin et al., 2011). Different parts of M. oleifera Lam. plant are sources of proteins, vitamins, minerals and phytochemical compounds which exhibit pharmacological and biotechnological potentials. On the other hand, the leaves, flowers, pods and seeds of the plant are considered essential food source of high nutritional quality in developing countries such as Nigeria. M. oleifera Lam. leaves can be eaten cooked or fresh and can be stored dried for long period unrefrigerated without loss in nutrient quality. Osuagwu et al. (2014), documented that room/shade drying is the best processing method that preserves the nutritional quality of M. oleifera Lam. leaves.
M. oleifera plant (Moringa or drumstick) is native to sub-Himalaya region of Northwest India. It is widely distributed throughout Africa, Southeast Asia, the Caribbean Islands and South America (Miracle Tree, 2014). Health workers now treat malnutrition in small children and pregnant and nursing women with M. oleifera leave powder because of its nutrients. The iron content of the leave is very high and the powder is prescribed for the treatment of anemia in the Philippines (Dhaka et al., 2011; Joshi and Mehta, 2010; Moyo et al., 2011; Xiaompin et al., 2011; Osuagwu et al., 2014; Monica et al., 2015). M. oleifera leaves contain phenolics and flavonoids compounds which exhibits various biological activity including antioxidants, anticancinogenic, immunomodulatory, antidiabetes and the regulation of thyroid status (Jung, 2014). Also, M. oleifera leaves is often the only source of protein, vitamins and minerals to the less privileged in the society and the leaves are used in the control of hypertension because the Na/K ratio content of the leaves is low (Fahey, 2005; Kasolo et al., 2010).
Sun drying is a traditional method of preservation of agricultural produce including vegetables such as Moringa leaves, grains, seeds and fruits in Africa (Wilhelm et al., 2004). Although, this practice is carried out under poor unhygienic conditions, it confers on agricultural produce storage stability, reduces losses, makes food available for consumption during scarcity, inhibits the growth of food spoilage microorganisms including bacteria, viruses, fungi, and parasites (Osuagwu et al., 2014; Karam et al., 2016). The process is slow and takes much time to achieve the required food safety limit of food contaminants by World Health Organization (Food Safety Programme, 2002). Besides, the process is carried out in an opened poor unhygienic condition which enhances the increase of microbial contamination from the environment, human and animal activities (Vivas et al., 2010; Beuchat et al., 2013). Among the drying methods: room, sun, solar, oven, lyophilization, commercial food dehydrator, Osuagwu et al. (2014), reported that room/shade drying is the best processing method that preserves the nutritional quality of M. oleifera Lam. leaves. Thus, Witthuhn et al. (2005) and Barkari-Golan and Paster (2011), reported that the microbial cells count and pathogens isolated from commercially and conventionally produced fresh and dried fruits and vegetables are higher than the international accepted limits (103 CFUg-1 for fungi and 101 CFUg-1 for bacteria). Similarly, Beuchat et al. (2013) and Finn et al. (2013) have isolated pathogenic strains of Salmonella and E. coli from home dried food products.
Green leafy vegetables, either fresh or dried, are examples of few original processed food that carry high risk of contamination with pathogenic bacteria such as members of Gram negative including Escherichia coli, Salmonella species, Pseudomonas aeruginosa and members of Gra positive such as Staphylococcus aureus and Bacillus cereus from the soil, human and animal excreta, water, harvest and processing procedures (Braga et al., 2005; Pandy and Singh, 2011; Sapkota et al., 2012). Thus, microbial loads in food stuffs are a measure of the degree of food contamination by microorganisms and related contaminants and this has been demonstrated by many researchers including Bhila et al. (2010), reported mean log total bacteria count of 18.5; yeast and mould of 12.9 in wet cabbage; Khazaei et al. (2011), documented microbial critical points of Saffron from farm in Iran, using two methods of sampling: hands and forceps picking. Recorded microbial mean of 2×102±1.1×103 for samples picked by forceps and 4.66×102±5×103 for samples picked by bear hands; Kumar et al. (2013), assessed microbial quality of 36 fresh vegetables from several regions of Ropar, Punjab, India. The major contaminants recorded include yeast and mould, and E. coli, in cauliflower, pea, cabbage and potato. They further reported that the microbial loads found in low economic area was significantly higher than the one recorded in high economic area; Pinky and Nishant (2015) investigated the bacteriological load of 5 fresh vegetables: potato, tomato, cauliflower, cucumber and spinach, from Mandi at Dehradun, in India. They reported total viable cells count found as follows: cucumber: 5.8 × 108 CFU/ml; potato: 5.0 × 108 CFU/ml; tomato: 4.2 × 108 CFU/ml; cauliflower: 4.0 × 108 CFU/ml; and spinach: 3.8 × 108 CFU/ml. Organisms identified on the basis of morphology, Gram stains and negative stains, were Entrobacter aerogenes, Serretia entomophila, B. cereus, Listeria monocytogene, Proteus vulgaris and Micrococcus; Singla and Kamboj (2017), enumerated microbial load in vegetables irrigated with sewage water in village Banur, in Patiala district, Punjab, India. They documented microbial load of mean values (MPN/100 g) ranging from 353 × 102 in tomato (of organism Lycopersicon esculentum var. esculentum) to 605 × 102 in Radish (of organism Raphanus sativus).
M. oleifera plant is abundant all over Nigeria, and the products serve multipurpose values to meet recent human challenges which include malnutrition, diseases, hunger, portable water, and employment (Osuagwu et al., 2014; Monica et al., 2015). Traditional home drying of fruits and vegetables is practiced in Guinea Savannah vegetation zone of Nigeria, where drying processing of vegetables is carried out under poor hygienic and sanitary practices due to lack of awareness, education, food safety and legislation. This lack of knowledge of good hygiene and sanitary processing of vegetables, creates high potential risk of microbial contamination and enhances easy transmission of pathogenic microorganisms to humans. However, the leaves of M. oleifera Lam are widely consumed in Nigeria, but the bacteriological load in M. oleifera Lam. leaves consumed in Guinea Savannah zones of Nigeria, via: Abuja (Gwagwalada market), in Southern Guinea Savannah, Katsina (Daura market), in Northern Guinea Savannah and Sokoto (Central market), in Sudan Guinea Savannah, has hardly been documented, and that is why this study was undertaken.
Sampling
Locally processed fresh and powdered leaves of M. oleifera were randomly collected from three locations in Guinea Savanna vegetation zones via: Abuja (Gwagwalada market) in Southern Guinea Savanna; Katsina (Daura market) in Northern Guinea Savanna, and Sokoto (Central market) in Sudan Guinea Savanna of Nigeria. Three fresh and powdered leaf samples of approximately 50 g per market location (East, West and North) were collected aseptically into a sterile polythene zip bags for analysis.
Analytical methods
APHA (2001), Witthuhn et al. (2005); Gupta et al. (2010) and Ntuli et al. (2013) methods were adopted for the microbial load analysis of the samples. Each parameter was determined in triplicates and their mean values recorded.
Microbial load analysis
Procedures of APHA (2001), Witthuhn et al. (2005), Gupta et al. (2010) and Ntuli et al. (2013), were adopted for the enumeration, isolation and characterization of bacteria and fungi. Analysis of each analyte was done in triplicates and their means recorded.
Preparation of media
Mannitol agar (selective for isolation of S. aureus); Nutrient agar (general purpose, used for total bacteria count); MacConkey agar (Differential and selective, used for total coli form count); Sabouraud Dextrose agar (Selective, used for total fungi count); Brilliant green agar (selective for isolation of Salmonella species) and Salmonella-Shigella agar (Salmonella-Shigella species). All the media were prepared according to the manufacturer’s instruction and were autoclaved (XY-280A Model) at 121°C for 15 min under 1.6 kg.cm2 pressure.
Pre-treatment of samples
Fresh leaves were the only pre-treated samples, since the other samples were in powdery form. The healthy fresh leaves were thoroughly washed for 5 times with sterile distilled water (autoclaved) in a sterile stainless 60 cm basin (surface sterilized with 70% alcohol), in order to remove extraneous substances on the leaves. Thereafter, the leaves were collected in a sterile plastic sieve (surface sterilized with 70% alcohol) to drain the water, then ready for analysis.
Sample preparation
Each leave sample (fresh and powdered) was analyzed in triplicates. Twenty grams sample was weighed using aeAdam analytical balance, model N17250, and suspended in 80 mL of sterile 0.1% (w/v) peptone water (Oxoid, Cambridge, UK) in a 500 mL sterile plastic beaker and homogenized for 2 min on a vortex mixer. A four tenfold serial dilution was carried out on the supernatant of each sample in triplicates. A 10 mL of water sample was mixed with 90 mL of peptone water using vortex mixer. A serial dilution of 10-1 to 10-5 of each sample was pour-plated in triplicates on each specific medium.
Bacterial count
One milliliter of the small volumes of the most diluted (10-3 and 10-4) of each dilution of each sample, was pipetted separately into different sterile Petri dishes containing 20 mL of sterile molten medium of various specific media used for pour plating. The setup was mixed together by swirling and allowed to solidify. Thereafter, inoculated plates were incubated in an incubator (UK, Gallenkamp 340 model) at 37°C for 24 h for total aerobic count, total coliform, Salmonella spp., Salmonella-Shigella spp. and at 25°C for 5 days for fungi/yeast. Plates with colonies between 20 and 300 were counted with the colony counter. Mannitol agar was used for the count of S. aureus; Nutrient agar was used for total bacteria count; MacConkey agar was used for total coli form count; Sabouraud Dextrose agar was used for total fungi count; Brilliant green agar was used for Salmonella spp. count and Salmonella-Shigella agar was used for the count of Salmonella-Shigella spp. (Witthuhn et al., 2005; Ntuli et al., 2013).
Microbial load analysis
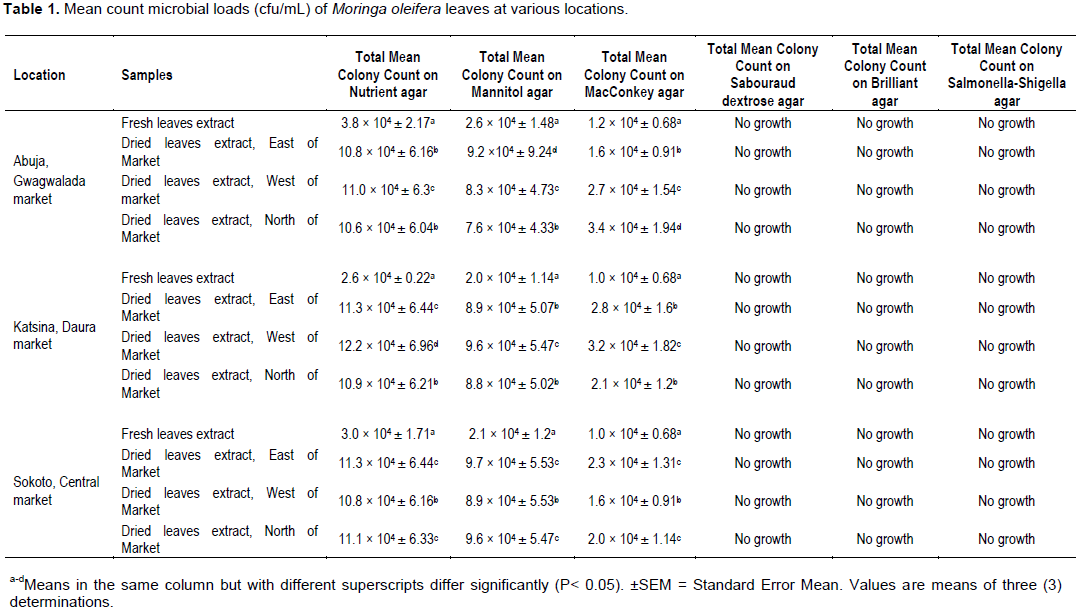
Microbial load of food measures the degree of food contamination by micro-organisms and related contaminants. The mean values of microbial load analysis of M. oleifera Lam. leaves from three different locations in Guinea Savannah of Nigeria, is presented in Table 1. Bacterial growths were recorded in all the nutrient, mannitol and MacConkey agar media of leave extracts analyzed (Table 1). Nutrient media is a general- purpose media that allows the growth of many bacteria. Mannitol media is a selective media that allows the growth of Staphylococcus species and MacConkey media is a differential and selective media that allows the growth of coli form species. Sabouraud dextrose, Brilliant green and Salmonella-Shigella media recorded no growth in all the leave extracts analyzed (Table 1). This could be ascribed to the selective nature of the Sabouraud dextrose, Brilliant green and Salmonella-Shigella media, and suggested that fungi/yeast, Salmonella spp. and Salmonella-Shigella spp. were not among the bacterial contaminants or that the active ingredient component-Pterygospermin, in M. oleifera leaves extract inhibited the growth of micro-organisms in the leaves extract.
However, numerous bacterial growths were observed in all the nutrient, mannitol and MacConkey media of leave extracts (Table 1). Mean bacterial count in nutrient agar varied between 2.6 ×104±0.22 to 3.8×104±2.17 cfu/mL fresh leave extracts and 10.6×104±6.04 to 12.2×104±6.95 cfu/mL dried leave extracts (Table 1). Similarly, bacterial mean count in mannitol agar ranged from 2.0 × 104±1.14 to 2.6 × 104±1.48 cfu/mL fresh leave extracts and 7.6 × 104±4.33 to 9.7 × 104±5.53 cfu/mL dried leave extracts (Table 1). Also, mean bacterial count recorded in MacConkey agar ranged between 1.0 × 104±0.68 and 1.2 × 104±0.68 cfu/mL fresh leave extract and 1.6 × 104±0.91 to 3.4 × 104±1.94 cfu/mL dried leave extract (Table 1). In all the incubated samples, the highest microbial load (12.2 × 104±6.95 cfu/mL) was recorded in Katsina dried leave, west of the market; while the lowest microbial load (1.0 × 104±0.68 cfu/mL) was reported by Katsina and Sokoto fresh leave extracts (Table 1). The study recorded two pathogenic bacteria from all the locations, with Staphylococcus spp. being more dominating followed by coli form species (Table 1). These are indicator organisms for poor hygienic conditions. However, the bacterial counts in nutrients, Mannitol and MacConkey media of the study are higher than the international stipulated limits of 101 cfu/mL in fresh and dried leafy vegetables (Food Safety Programme, 2002; FAO/WHO, 2014). This is a suggestive of poor hygiene and sanitary conditions during processing. However, there is distinct statistical significance (P<0.05) among the recorded mean values of microbial loads of the analyzed leave samples when compared with the microbial load of fresh leave samples.
It is observed that the results of the study agreed with the works of Bhila et al. (2010), who reported mean log total bacteria count of 18.5, in wet cabbage; Khazei et al. (2011), documented microbial mean of 2 × 102±1.1×103 for samples picked by forceps and 4.66 × 102±5×103 for samples picked by bear hands from the analysis of microbial critical points of saffron from farm in Iran, using two methods of sampling; Kumar et al. (2013) and Pinky and Nishant (2015), recorded bacteriological load in 5 fresh vegetables from Dehradun, India; Cucumber: 5.8 × 108 cfu/mL; Tomato: 4.2 × 108 cfu/mL; Spinach: 3.8 × 108 cfu/mL: Cauliflower: 4.0 × 108 cfu/mL.
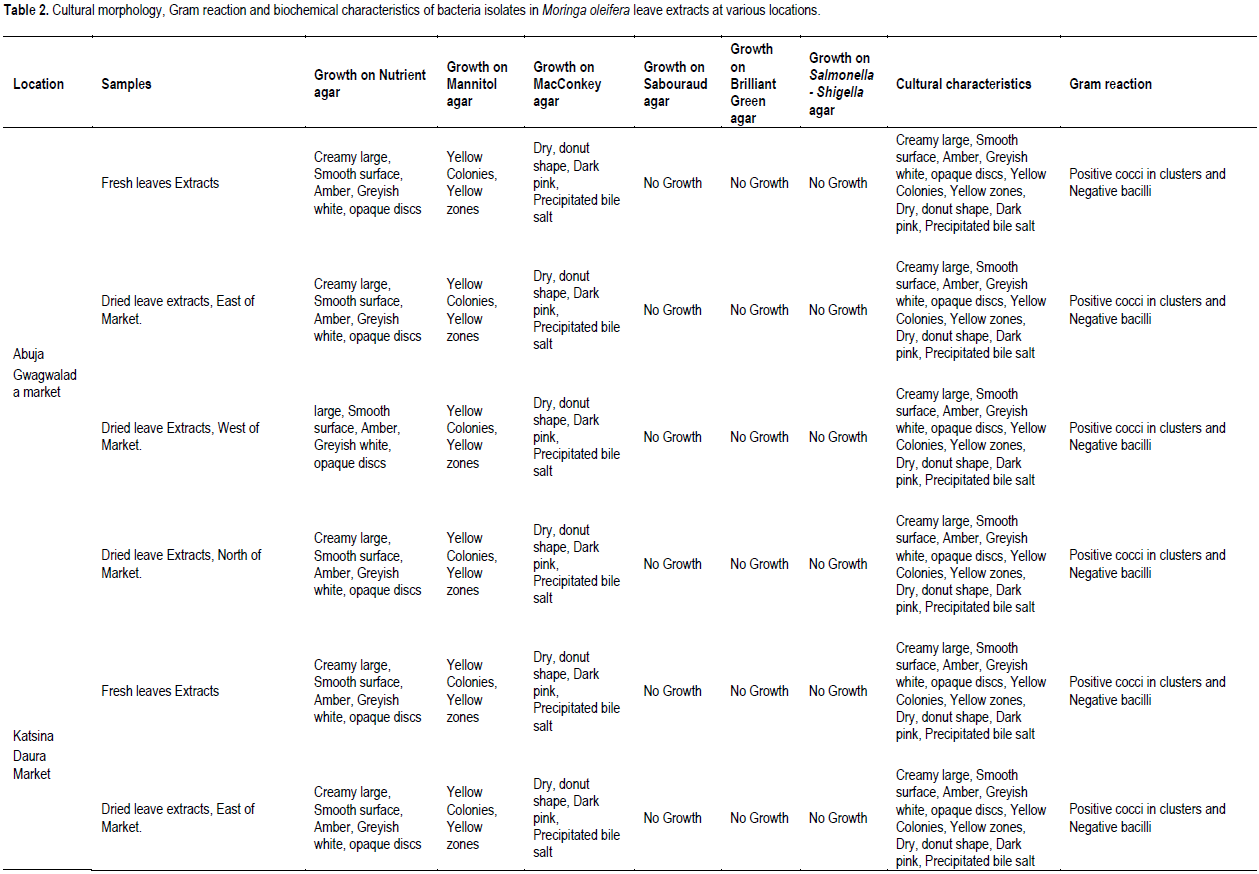
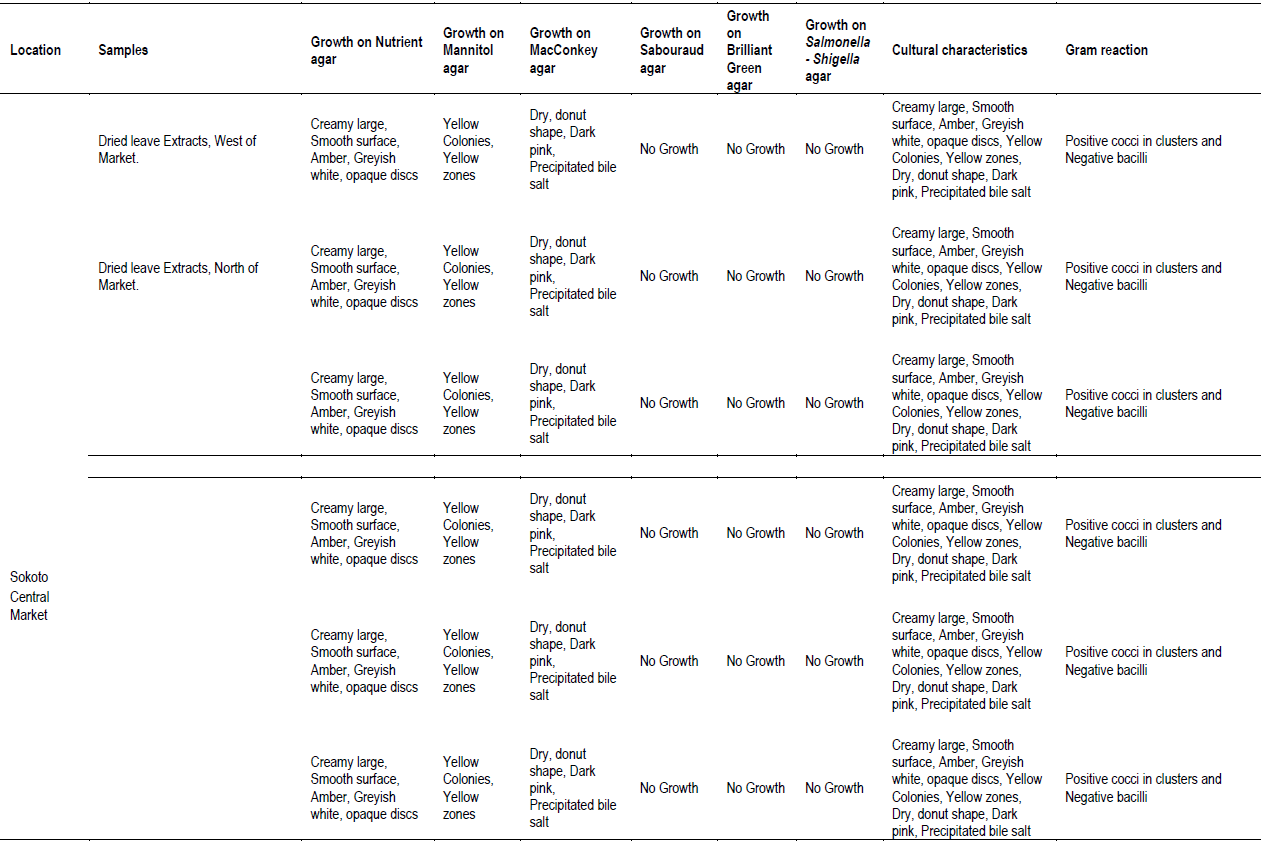
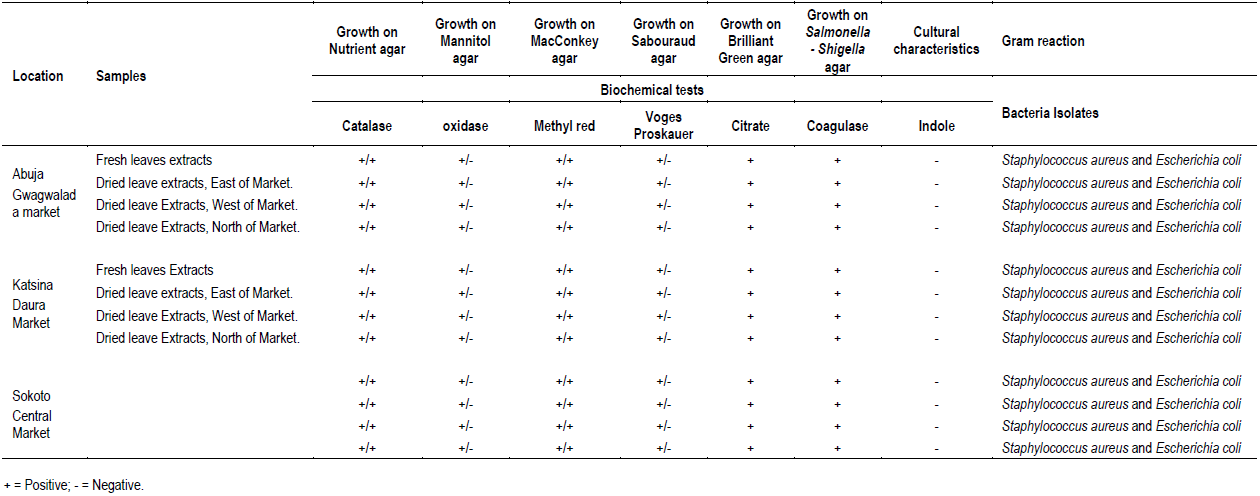
The cultural morphology, Gram reaction and biochemical characteristics of bacteria isolates in M. oleifera leave extracts at various locations investigated are presented in Table 2. Nutrient, Mannitol and MacConkey agars recorded bacterial colony in all leave extracts (Table 1). Sabouraud dextrose, Brilliant green and Salmonella-Shigella media recorded no growth in all the leave extracts analyzed (Table 1). All the bacteria colony growth in nutrient agar showed mixed cultural morphology of creamy large, smooth surface, circle and pasty, slightly opalescent and amber in color, large colony, thick, greyish white, moist, and opaque/ translucent discs (Table 2). Bacteria colony growth in MacConkey agar showed cultural morphology of dry, donut shaped and dark pink, surrounded with dark pink area of precipitated bile salts (Table 2). Bacteria colony growth in Mannitol agar showed cultural morphology of yellow colonies with yellow zones (Table 2). Bacterial colony was Gram stained in each of the media that recorded colony growth. Bacteria colony growth in nutrient agar showed mixed results of dark purple, clustered cocci and pink color bacilli, under the microscope, indicating Gram positive cocci in clusters and Gram-negative bacilli (Table 2). The bacterial colony tested positive to catalase, Methyl red, citrate, and negative to Oxidase, Voges Proskauer, biochemical tests confirming bacteria colony in nutrient media to be Staphylococcus spp. and coli form species (Table 2). Bacteria colony growth in MacConkey agar was pink colored bacilli, under the microscope, indicating Gram negative bacilli (Table 2). The bacteria colony tested positive to catalase, Methyl red and negative to Oxidase and Voges Proskauer, biochemical tests confirming bacteria colony in MacConkey media E. coli (Table 2). Bacteria colony growth in Mannitol agar was dark purple, clustered cocci, under the microscope, indicating Gram positive cocci in clusters (Table 2). The bacteria colony in Mannitol agar tested positive to catalase, citrate, coagulase, methyl red, Voges Proskauer and negative to indole and oxidase, and biochemical tests confirming bacteria colony S. aureus (Table 2). In conclusion, the bacteriological load of M. oleifera Lam. leaves consumed in the studied areas are E. coli and S. aureus.
Total plate count of bacteria (CFU/mL)
Microbial load of each sample was determined as CFU/mL and was calculated from the expression:
CFU/ml = Number of colonies × Dilution factor / Volume of inoculums (Prescott et al., 2002).
Characterization and identification of isolates
The isolates were classified on the basis of cultural morphology (opaque/translucent discs, donut shape and dark pink, creamy large smooth surface, yellow colonies with yellow zones, thick greyish white, opalescent and amber in color), Gram reaction and Biochemical tests and matched against standard microbial cultures (Witthuhn et al., 2005; Cheesebrough, 2005; Gupta et al., 2010; Ntuli et al., 2013).
Statistical analysis
The research experimental design was a factorial experiment fitted into a complete randomized design. Four treatments, three locations and two factors of three replicates each were involved. The data were subjected to statistical analysis to evaluate the differences between microbial loads of the samples. The data were analyzed with the SPSS, version 16 for windows in a general linear model. The mean separation of data analyzed was done with the Duncan Multiple Range Test P < 0.05. The results were expressed as mean and standard error of mean. The difference was considered significant at P < 0.05.
The results of this investigation reveal that the bacteriological load of M. oleifera Lam. leaves consumed in the studied areas are E. coli and S. aureus. This poses potential public health hazard to consumers as the samples fell short of meeting international food safety standard. Vegetable consumers and vendors should be educated on proper hygienic handling, transportation and storage of vegetables to avoid bacteriological food spoilage and other related health issues. However, results from this study would be valuable for further risk assessment of the impact on human health of consuming agricultural produce, especially home dried seeds, fruits, grains and vegetables such as M. oleifera Lam. leaves. Among the bacteria pathogens isolated, S. aureus, was the dominant bacteria. Bacteria contamination may be due to improper handling, storage and poor hygienic conditions.
Incidences of food borne diseases and infections caused by contaminated fresh/dried vegetables by micro-organisms can be avoided by applying proper hygiene and sanitary practices. Traditional drying of fruits and vegetables including M. oleifera Lam. produce should be carried out under good hygienic conditions to avoid microbial contamination including enteric pathogens such as E. coli, Salmonella and Shigella spp. Vegetables such as carrots, tomatoes, radishes, cabbage, cucumber, and lettuce, which are frequently consumed raw without proper processing must be thoroughly washed 3 to 4 times with clean water to remove extraneous materials, thereafter, are soaked in 0.85% sodium chloride solution for 5 to 10 min to eliminate pathogenic microorganisms. Then, are rinsed thoroughly in clean water for 3 to 4 times before consumption. Government policies to embrace measures that are focused on monitoring and evaluating food safety, good hygiene and sanitary practices through education, training and re-training programmes for food vendors and consumers in relation to food processing from farm to table.
The authors have not declared any conflict of interest.
The authors thank the Department of Microbiology, Federal University of Lafia, Nasarawa State, Nigeria, for providing all the necessary laboratory facilities for the analysis of vegetable samples.
REFERENCES
|
American Public Health Association (APHA) (2001). Compendium of Methods for the Microbiological Examination of Foods, Washington, DC, American Public Health Association (APHA).
|
|
|
|
Barkari-Golan R, Paster N (2011). Mycotoxins in Fruits and Vegetables. Elsevier Science. 1st Edition, Academic press. pp. 45-74.
|
|
|
|
|
Beuchat L, Komitopoulou E, Beckers H, Betts R, Bourdichon F, Fanning S, Joosten H, Ter Kuile B (2013). Low- water activity food.s increased concern as vehicles of foodborne pathogens. Journal of Food Protection 76(1).
Crossref
|
|
|
|
|
Bhila TE, Ratsaka MM, Kanegoni A, Seibret F (2010). Effect of sun drying on Microbes in non-conventional agricultural by- product. South African Journal of Animals Science 40(5).
|
|
|
|
|
Braga LC, Shupp JW, Cummings C, Jett M, Takahashi JA, Carmo LS (2005). Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. Journal of Ethnopharmacology 96(1-2):335-339.
Crossref
|
|
|
|
|
Cheesebrough M (2005). District laboratory practice in tropical countries, Part 2. Cambridge University Press.
Crossref
|
|
|
|
|
Dhakar R, Pooniya B, Gupta M (2011). "Moringa the herbal gold to combat malnutrition". Chronicles of Young Scientists 2(3).
Crossref
|
|
|
|
|
Fahey JW (2005). Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Part 1. Tress for Life Journal 1(5):5-20.
|
|
|
|
|
FAO/WHO (2014). Ranking of Low Moisture Foods in support of Microbiological Risk Management: Preliminary report of FAO/WHO expert consultation on ranking of low moisture foods. Part 1- Main Report, Rome/Geneva. FAO/WHO.
|
|
|
|
|
Finn S, Condell O, McClure P, Amezequita A, Fanning S (2013). Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Frontiers in Microbiology 4:331.
Crossref
|
|
|
|
|
Food Safety Programme (2002). WHO global strategy for food safety: safer food for better health, Geneva, Switzerland.
|
|
|
|
|
Gupta RN, Kartik V, Manoj P, Singh PS, Aika G (2010). Antibacterial activities of ethanolic extracts of Plants used in folk medicine. International Journal of Research in Ayurveda Pharmacy 1(2):529-535.
|
|
|
|
|
Joshi P, Mehta D (2010). Effect of dehydration on the nutritive value of drumstick leaves. Journal of Metabolomics and Systems Biology 1(1):5-9.
|
|
|
|
|
Jung IL (2014). Soluble extract from Moringa oleifera leaves with a new anticancer activity". PloS one 9(4):e95492.
Crossref
|
|
|
|
|
Karam M, Petit J, Zimmer D, Baudelaire D, Scher J (2016). Effects of drying and grinding in production of fruit and vegetable powders: A review. Journal of Food Engineering 188:32-49.
Crossref
|
|
|
|
|
Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal- Okeng JW (2010). Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural Communities. Journal of Medicinal Plants Research 4:753-757.
|
|
|
|
|
Khazaie N, Jouki M, Kalbasi A, Travacol PH, Rajabifar S, Motamedi FS, Jouk A (2011). A study of microbial critical points of saffron from farm to factory in Iran. World Academy of Science, Engineering and Technology 77 p.
|
|
|
|
|
Kumar S, Chaturvedi M, Kumar V, Singh D (2013). Assessment of Microbial load of some common Vegetables among two different Socioeconomic grounds. International Food Research Journal 20(5):2927-2931.
|
|
|
|
|
Miracle Trees, September 2014.
View
|
|
|
|
|
Monica A Valdez-Solane, Veronica Y Mejia- Garcia, Alfredo Tellez- Valencia, Guadalupe Garcia-Arenas, Jose Salas-Pachco, Jose J Albe- Romero, Brick Sierra-Campos (2015). Nutritional Content and Elemental and Phytochemical Analyses of Moringa oleifera Grown in Mexico. Journal of Chemistry Article ID 860381, 9 pages.
Crossref
|
|
|
|
|
Moyo B, Masika PJ, Hugo A, Muchenje V (2011). Nutritional characterization of Moringa (Moringa oleifera Lam) leaves. African Journal of Biotechnology 10(60):12925-12933.
Crossref
|
|
|
|
|
Ntuli V, Bekele M, Molebatsi N, Makotoko M, Chatanga P, Asita A (2013). Microbial and Physicochemical characterization of Maize and wheat flour from a milling company, Lesotho. Internet Journal of Food Safety 15:11-19.
|
|
|
|
|
Osuagwu OS, Ega RIA, Okoh T, Oyerinde AA (2014). Comparative studies of the physicochemical properties and mineral elements of Moringa oleifera Lam. leaves in the Guinea Savannah of Nigeria.
|
|
|
|
|
International Journal of Agricultural and Biosciences 3(6):266-270.
|
|
|
|
|
Paulsamy S, Jeeshna MV (2011). Preliminary Phytochemistry and antimicrobial studies of an endangered Medicinal herb Exacum bicolor Roxb. Research Journal of Pharmaceutical, Biological and Chemical Science 2(4):447-457.
|
|
|
|
|
Pinky K, Nishant R (2015). bacteriological analysis of fresh vegetables from main market of Dehradun. International Journal of PharmTech Research 8(3):415-425.
|
|
|
|
|
Prescott L, Harley J, Klein DA (2002). Microbiology (5th edn). New York, USA: McGraw-Hill companies 41:964-976.
|
|
|
|
|
Singla R, Kamboj N (2017). Enumeration of Microbial load in Vegetables irrigated with Sewage water. International Journal of Advanced Research in Science and Engineering 6(1).
|
|
|
|
|
Sapkota R, Dasgupta R, Nancy, Rawat DS (2012). Antibacterial effects of plants extract on human microbial pathogens and microbial limit tests. International Journal of Research in Pharmacy and Chemistry 2(4):926-936.
|
|
|
|
|
Vivas A, Gelaye B, Aboset N, Kumie A, Berhane Y, Williams M (2010). Knowledge, attitudes, and practices (KAP) of hygiene among school children in Angolela, Ethiopia. Journal of Preventive Medicine and Hygiene 51(2):73.
|
|
|
|
|
Wilhelm L, Suter D, Brusewitz G (2004). Drying and Dehydration. Food and Process Engineering Technology, St. Joseph, Michigan: ASAE: American Society of Agricultural Engineers.
View
|
|
|
|
|
Witthuhn R, Engelbrecht S, Joubert E, Britz T (2005). Microbial content of commercial South African high- moisture dried fruits. Journal of Applied Microbiology 98:722-726.
Crossref
|
|
|
|
|
Xiaompin Z, Daniel M, John NA, Arthur G, Eric K, Godelieve M (2011). Comparison of Volatile profile of Moringa oleifera leaves from Rwanda and china, using HS-SPME. Pakistan Journal of Nutrition, 10(7):602-608.
Crossref
|
|
|
|
|
Yanishlieva NV, Marinova E, Pokorny J (2006). Natural antioxidants from herbs and spices. European Journal of Lipid Science and Technology 108(9):776-793.
Crossref
|
|