ABSTRACT
Bacterial biofilms have been linked with protracted and recurring human infections, and considering that biofilms shed microorganisms into their environment it becomes pertinent to examine their role in waterborne diseases through consumption of treated packaged water. To evaluate the consortia of bacteria in packaged water for the ability to form biofilms. Using membrane filtration technique polythene sachet and pet bottles water randomly collected were examined for bacterial contamination and biofilm forming ability of isolated bacteria. A pH range of 5.0-8.5 was at 25°C while 32% had residual chlorine of less than 0.6 mg/L. Eighty-two percent of the 130 bottled water brands and 71% of the 170 sachet water conformed to the World Health Organization (WHO) standards. Biofilm formation was demonstrated in 70% of the 112 bacterial isolates. This study identifies the presence of a biofilm-forming microorganism in packaged water sold within Lagos metropolis.
Key word: Bacteria, membrane filtration, bottle water, sachet water, biofilms.
Any potable water manufactured, distributed and offered for sale in food grade containers is referred to as packaged water (Tagoe et al., 2011). The consumption of packaged water in Lagos continues to upsurge due to the increasing concern regarding various health challenges associated with consuming contaminated water. In addition to the growing disquiet among the public rising disposable income of Lagosians has also influenced packaged water business significantly resulting in a boom. In Lagos sachet water popularly referred to as pure water is sold in every county and widely patronized by all socio-economic groups because it is cheap and presumed safe since major bottling companies also produce water in sachet packs. Several studies have reported poor bacteriological quality of packaged water and many have attributed the deplorable condition to poor handling, storage and residual chlorine (Olayemi, 1999; Egwari et al., 2005). Furthermore, poor bacteriological quality of packaged water could be linked to the presence of biofilms in the water distribution systems which eventually result in cross-contamination in the final product (Simpson, 2008).
Practically all motile bacteria have the propensity to form biofilms in an aquatic environment (Sekhar et al., 2009), the formation and growth of this heterogeneous community encapsulated and protected by an exopolysaccharide matrix attached to a solid surface is usually a response by bacteria to environmental pressures like the presence of antimicrobial substances (Mah and George, 2001). It has been shown that bacteria present within the community can number over 500 taxa and are usually more resistant to antimicrobial substance than the free-living planktonic cells (Mah and George, 2001). As a matter of fact, according to Prosser et al. (1987) and Costerton et al. (1985) bacteria living within the matrix increases microbial resistance against the deleterious effects of antimicrobial agents by 10-1000 times compared to planktonic cells. Furthermore, Chlorine which is the most common water guard against recontamination in water distribution system has been shown in the laboratory to penetrate less than 20% of biofilm (Stewart et al., 1994; Mah and George, 2001). When biofilms are allowed to reach the advanced level, they often release an array of bacteria into the water supply at various stages (Stewart et al., 1994; Kaplan, 2010). Bacteria present in packaged water may arise from the detachment of biofilm which may cause serious public health challenges (Simpson, 2008).
Unlike the United State of America which has regulations addressing water quality in distribution systems through rules covered by the Safe Drinking Water Act (SDWA) (Bitton, 2014), and the Stages 1 and 2 disinfectant and disinfection byproducts rules (EPA, 2009). At the moment drinking water regulations in Nigeria are mostly concern with water quality at the end of water treatment. This in addition to the ineffective and inefficient monitoring system which does not address deterioration during storage and transportation the final quality of water reaching the consumer is doubtful. This study determined the strength of biofilm formed by bacteria isolated from packaged water with a view to assessing the possible health implication in the consumption of these waters.
Location
Lagos is located on the southwest coast of Nigeria approximately between latitudes 6° 27' 11'' N and longitude 3°23'45''E. Lagos sprawls inland from the Gulf of Guinea, of which 22% of its 3,577 km2 are creeks. It is characterized by a wet equatorial climate with average annual rainfall above 1800 mm and temperature of about 27°C. It is divided politically into 3 senatorial districts (Figure 1) (George, 2009).
Sample collection
Water samples were purchased randomly from retail stalls across the three senatorial districts of Lagos metropolis. A purposive random sampling technique was adopted in the selection of sampling locations. The sample collection form was filled appropriately and samples were immediately placed in a lightproof insulated box containing melting ice to ensure rapid cooling and transported to the laboratory where they were kept at 4°C prior to analysis in less than 24 h. Three hundred samples comprising 130 pet bottled brand water and 170 polythene bag sachet water were studied. Sampling was done over a period of 3 months ensuring sampling was not repeated on the same batch (Egwari et al., 2005).
Isolation and identification
Each water sample (100 ml) was filtered through a sterile membrane filter of pore size 0.22 μm; thickness 150 μm; the filter was then removed with sterile forceps and placed aseptically onto a prepared nutrient based medium (Nutrient agar, MacConkey agar and Cetrimide agar plates). Plates were then labeled appropriately and incubating at 37°C for 24-48 h (WHO, 2006). Isolates were identified by conventional methods (biochemical test) and the use of the API ID kits Biomerieux, France.
Determination of pH
The samples were allowed to thaw and cool to room temperature before all analyses were done. pH value was measured electrometrically with a glass electrode at 25°C (Onweluzo and Akuagbazie, 2010).
Testing for chlorine residual
The residual chlorine was determined by the Diethyl paraphenylenediamine (dpd) indicator test using a comparator. A tablet reagent was placed in the test chamber and few drops of the chlorinated water were added, colouring it red. The strength of the colour determines the concentration of chlorine in the water (WHO, 2007).
Biofilm formation detection (Tube method)
Biofilm formation by isolates was determined by the tube method as previously described by Christensen et al. (1982). A test bacterium was inoculated into 10 ml trypticase soy broth with 1% glucose. After 24 h of incubation at 37°C, content was discarded and tube washed with phosphate buffer saline (pH 7.4) and dried at room temperature in an inverted position. After drying the tubes were stained with 0.1% crystal violet which was allowed to stay for 60 s after which excess stain was washed off with deionized water. Tubes were dried and biofilm formation was considered positive when a visible film creased the wall and the bottom of the tube and was scored based on the strength of the film formed (Hassan et al., 2011; Patel et al., 2016).
Ethical consideration
As some information and result might be commercially sensitive (e.g. poor water quality and sanitary results etc.) results were kept confidential.
Statistical analysis
Studies were conducted in triplicates and mean values calculated. For statistical analysis, all data were analyzed using Microsoft Excel 2010 and results represented in charts and tables.
A total of 300 samples were collected from the 3 senatorial districts of Lagos metropolis. Of this, 32% had a pH below 6.5. Nonetheless, the pH of the 300 samples varied at ± 3.5 (Table 1). These findings are similar to those of Ajayi et al. (2008) who earlier documented a pH range of 5.6-9.7 for 127 samples of packaged water sold within Ibadan metropolis. However, a shorter pH range of 7.11-7.25 was reported for sachet water sold in selected local government areas of Kano, northern -west Nigeria (Sheshe and Magashi, 2014).

Optimum pH of drinking water show discrepancy base on the nature of the material used in the distribution system however, it is usually between the ranges of 6.5-9.5 (WHO, 2007). Water with pH < 6.5 like the once recorded for 32% of the samples could be as a result of metal ions contamination. The human body through the nervous and endocrine system has the ability to maintain a constant internal environment in response to environmental changes, as such, a direct relationship between the pH of drinking water and human health cannot be adduced since enzymes activity is pH dependent also recognizing that disinfection byproducts formation in chlorinated water can be influenced by pH (WHO, 2011a). Also, because pH can affect the degree of corrosion of metallic pipes, favour the proliferation of microbes and alter the activity of disinfectant in water (WHO, 2007); regulation of pH in water becomes paramount to ensure consumers’ health safeguards. It is also important to note at this point that of the 32% that had pH < 6.5, 78% of them were sachet water. These results suggest that the storage and packaging in plastic bags probably interfere with the water integrity in some ways thus, altering the chemical composition which eventually affects the pH, nonetheless, further investigation is needed to substantiate this claims.
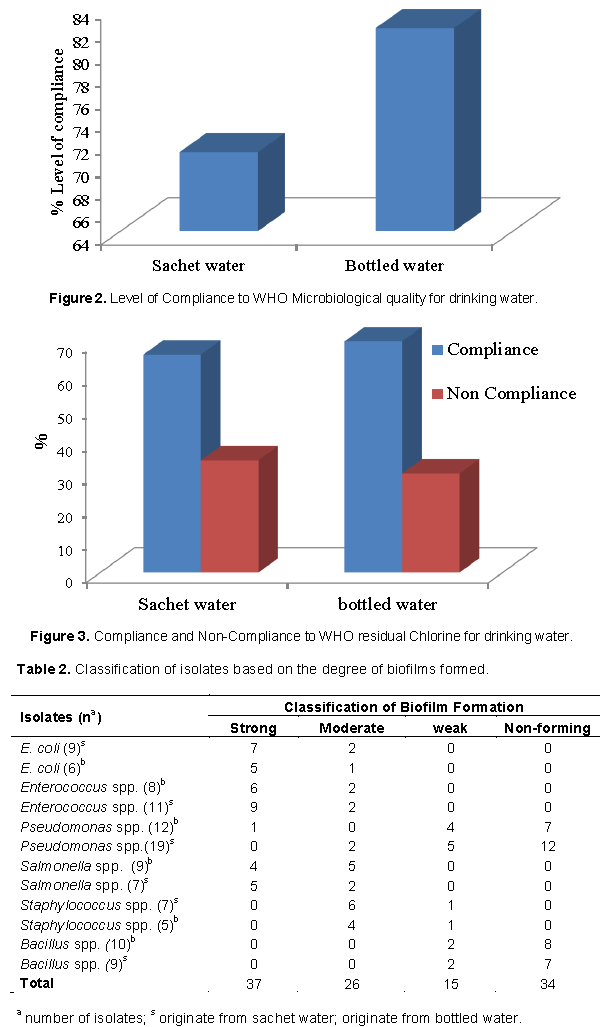
The results in Figure 2 revealed that 82% of the bottled water as against 71% of the sachet water were of good microbiological quality. The samples that fell below the WHO microbiological standard for drinking resulted from high heterotrophic counts and/or the presence of Escherichia coli or coliform. Olayemi (1999) had earlier reported that 40% of packaged water sold in Ilorin did not meet safe drinking water standard and this was further confirmed by Sule et al. (2017). Sasikaran et al. (2012) provided a quantitative estimate of the heterotrophic count (800 cfu/ml) for 22 bottled water brands sold in the Jaffna Peninsula. Overall this study found that bottle water conforms more to drinking water standard than sachet water probably due to the hypothesis held by some other author (WHO, 2011b; Raj, 2005), that packaging and storage in bottles tend to preserve water integrity better than other packaging materials. We are of the opinion that the protective seal is able to protect packaged water integrity, and any issue regarding microbiological quality is likely going to be from the factory.
Chlorine application is an essential drinking water property as such WHO recommends residual chlorine of 0.6-1.0 mg/L for drinking water (WHO, 2006). The results in this study showed that the residual chlorine of approximately 32 % of the samples was below 0.6 mg/L Figure 3 which is inadequate for disinfection, explaining the reason for the poor bacteriological quality of approximately 36 % of the samples Figure 2. Chlorine is unstable and generally photosensitive in nature as such it is subjected to degradation in the distribution system thus giving way for bacterial proliferation and biofilm formation in the distribution network (WHO, 2011). Furthermore, apart from transporting packaged water in open trucks most stalls simply keep it in a cage outside the stall without protecting it from sunlight, this practice could also be responsible for the poor residual chlorine recorded in this study. These findings are in agreement with Ahmad et al. (2015) who reported a gradual decrease in residual chlorine and increase in coliform count as water flow in the distribution system; the further it flows the less the residual chlorine and the higher the coliform count. Egorov et al. (2002) also documented a relationship between the decline in residual chlorine and the bacteriological quality of drinking water.
In the present study, 112 bacteria were isolated from packaged water. Fifty-five percent of the isolates were from sachet water while 45% were from bottled water. Table 2 shows the classification of the isolates based on their ability to form biofilms. The Enterobacteriaceae were strong biofilm formers; biofilm formation by Pseudomonas was moderate to weak with most strains unable to form biofilms. Majority of the Gram-positive bacteria did not form biofilms. Many authors are of the view that all bacteria have the capacity to form biofilm if the conditions that necessitate their formation are present (Olayemi, 1999). However, in the present study, 70% of the isolates were able to form biofilm in three categories: Strong, moderate and weak formers (Hassan et al., 2011; Patel et al., 2016), while approximately 30% of the isolate did not form biofilms at 37°C after 24 h incubation. Shorter time of 30 s for biofilm formation has earlier been reported for Pseudomonas aeruginosa on the surface of Stainless steel (Vanhaecke et al., 1990).
Ninety-seven percent of the 37 isolates (Salmonella spp. = 24%, Enterococcus spp. = 41% and E. coli = 32%) in the category of strong biofilm formers in the present study are active motile bacteria; a requirement for facilitated biofilm formation (Beloin et al., 2008). Pratt and Kolter (1998) in their study concluded that motility itself and not direct surface contact by flagella was required to form a biofilm. Wood et al. (2006) validated these claims using several motile E. coli mutants. They found that E. coli K-12 capacity to form biofilms is largely dependent on its ability to propel itself through the environment. Although the presence of flagella assists bacteria to offset hydrodynamics and static forces at the surface, preliminary attachment to solid surfaces is strongly influenced by certain environmental conditions such as pH, temperature and electrostatic interactions between the bacterial outer membrane and the nature of the surface (Dunne, 2002; Beloin et al., 2008).
Furthermore, the capacity of members of the family Enterobacteriaceae to form strong biofilms in the hydrophilic surface like glass is strongly associated with their ability to produce thin aggregative fimbriae (curli fimbriae) which aggregate at the cell surface. The curli adhesive fibres stimulate biofilm formation on abiotic surfaces by aiding cell-surface interactions and ensuing cell-cell interactions (Cookson et al., 2002; Uhlich et al., 2006; Beloin et al., 2008).
One isolate from bottle water sample out of 31 Pseudomonas spp. which was later identified as P. aeruginosa was classified as strong biofilm former, 2 Pseudomonas isolates from sachet water samples were categorized as moderate biofilm former while, 60% of the 15 weak biofilm formers were Pseudomonas sp. Other studies have reported a varying capacity of Pseudomonas spp. to form biofilms; 47% Ghadaksaz et al. (2015), and 32% Demoliner et al. (2015). Biofilm formation by Pseudomonas was recorded 32% of 50 isolates recovered from a function of the surface involved whether hydrophilic or hydrophobic (Cookson et al., 2002; Demoliner et al. 2015).
The results in Table 2 also revealed that only 4 Bacillus spp. 2 each from bottled and sachet water samples were able to form biofilm in the weak category. Whereas, 79% of the 19 Bacillus spp. isolates could not form a biofilm. Bacillus spp. is known to produce biosulfactant that has anti-adhesive properties. This could be the reason why biofilm formation by Bacillus spp. in the present study was very poor. Rivardo et al. (2009) observed an anti-adhesion activity and reduction of biofilm of up to 97% for E. coli and 90% for Staphylococcus aureus biofilms for two biosurfactants produced by Bacillus spp.; other workers have also demonstrated the anti-adhesive properties of certain biocides against gram-negative bacteria (Carsenti-Etesse et al., 1993; Houari and Di Martino, 2007).
This study identifies the presence of biofilm-forming bacteria in packaged water circulating within the 3 senatorial districts of Lagos metropolis. The high heterotrophic count and presence of faecal coliform indicate either unsatisfactory treatment of the water or poor quality of the packaging material or a combination of both. To safeguard consumer’s therefore routine checks on water treatment facility is mandatory for conformity to standards.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmad MR, Ahmad AF, Sharma HK (2015). Assessment of microbiological quality of drinking water treated with chlorine in the Gwalior city of Madhya Pradesh, India. African Journal of Environmental Science and Technology 9(5):396-401.
Crossref
|
|
|
|
Ajayi AA, Sridhar MK, Adekunle LV, Oluwande PA (2008). Quality of packaged waters sold in Ibadan, Nigeria. African Journal of Biomedical Research11:251-258.
Crossref
|
|
|
|
|
Beloin C, Roux A, Ghigo JM (2008). Escherichia coli biofilms. Current Topics in Microbiology and Immunology 3(22):249-289.
Crossref
|
|
|
|
|
Bitton G (2014). Microbiology of Drinking Water Production and Distribution. John Wiley and Sons, Inc., Hoboken, New Jersey.
Crossref
|
|
|
|
|
Carsenti-Etesse H, Durant J, Entenza J, Mondain V, Pradier C, Bernard E, Dellamonica P (1993). Effects of subinhibitory concentrations of vancomycin and teieoplanin on adherence of staphylococci to tissue culture plates. Antimicrobial Agents and Chemotherapy 37(4):921-923.
Crossref
|
|
|
|
|
Christensen GD, Simpson WA, Bisno AL, Beachey EH (1982). Adherence of slime producing strains of Staphylococcus epidermidis to smooth surfaces. Infection and Immunity 37:318-326.
|
|
|
|
|
Cookson AL, Cooley WA, Woodward MJ (2002). The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. International Journal of Medical Microbiology 72(292):195-205.
Crossref
|
|
|
|
|
Costerton JW, Nickel JC, Ruseska I, Wright JB (1985). Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrobial Agents and Chemotherapy 27(4):619-624.
Crossref
|
|
|
|
|
Demoliner F, de Souza KD, Pacheco DO, Duval EH, de Mello JF, Rodrigues KL, Gandra EA (2015). Resistance to disinfectants and antibiotics of Pseudomonas spp. and Listeria spp. biofilms on polystyrene and stainless steel. African Journal of Microbiology Research 9(27):1706-1715.
Crossref
|
|
|
|
|
Dunne WM (2002). Jr Bacterial adhesion: seen any good biofilms lately. Clinical Microbiology Reviews15:155-166.
Crossref
|
|
|
|
|
Egorov A, Ford T, Tereschenko A, Drizhd N, Segedevich I, Fourman V (2002). Deterioration of Drinking water quality in distribution system and gastrointestinal morbidity in a Russian city. International Journal of Environmental Research 12(3):221-223.
Crossref
|
|
|
|
|
Egwari LO, Iwuanyanwu S, Ojelabi CI, Uzochukwu O, Effiok WW (2005). Bacteriology of sachet water sold in Lagos, Nigeria. East African Medical Journal 82(5):235-240.
Crossref
|
|
|
|
|
George CK (2009). The Challenges of Urbanisation in Nigerian Urban Centres: The Lagos Mega city Situation-A Town Planner's Perspective. Libro-Gem Books Ltd., Lagos.
|
|
|
|
|
Ghadaksaz A, Imani AA, Hossein MF, Amin M (2015). The prevalence of some Pseudomonas virulence genes related to biofilm formation and alginate production among clinical isolates. Journal of Applied Biomedicine13:61-68.
Crossref
|
|
|
|
|
Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M (2011). Evaluation of different detection methods of biofilm formation in the clinical isolates. The Brazilian Journal of Infectious Diseases 15(4):305-311.
Crossref
|
|
|
|
|
Houari A, Di Martino P (2007). Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Letters in Applied Microbiology 45(6):652-656.
Crossref
|
|
|
|
|
Kaplan JB (2010). Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic use. Journal of Dental Research 89(3):205-218.
Crossref
|
|
|
|
|
Mah T-FC, George AO (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology 9(1):34-39.
Crossref
|
|
|
|
|
Olayemi AB (1999). Microbial potability of bottled and packaged water hawked in Ilorin metropolis. International Journal of Environmental Research and Public Health 9:245-248.
Crossref
|
|
|
|
|
Onweluzo JC, Akuagbazie CA (2010). Assessment of the Quality of Bottled and Sachet Water Sold in Nsukka Town. Journal of Tropical Agriculture, Food, Environment and Extension 9(2):104-110.
Crossref
|
|
|
|
|
Patel FM, Goswami PN, Khara R (2016). Detection of Biofilm formation in device associated clinical bacterial isolates in cancer patients. Sri Lankan Journal of Infectious Diseases 6(1):43-50.
Crossref
|
|
|
|
|
Pratt LA, Kolter R (1998). Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis, and type I pili. Molecular Microbiology 30:285-293.
Crossref
|
|
|
|
|
Prosser BL, Taylor D, Barbara AD, Cleeland R (1987). Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrobial Agents and Chemotherapy 31(10): 1502-1506.
Crossref
|
|
|
|
|
Raj SD (2005). Bottled water: How safe is it? Water Environment Research 77(7):3013-3018.
Crossref
|
|
|
|
|
Rivardo F, Turner RJ, Allegrone G, Ceri H, Martinotti MG (2009). Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Applied Microbiology and Biotechnology 83(3):541-53.
Crossref
|
|
|
|
|
Sasikaran S, Sritharan K, Balakumar S, Arasarahman V (2012). Physical, chemical and microbial analysis of bottled drinking water. Ceylon Medical Journal 57(3):111-116.
Crossref
|
|
|
|
|
Sekhar S, Kumar R, Chakraborti A (2009). Role of biofilm formation in the persistent colonization of Haemophilus influenza in children from northern India. Journal of Medical Microbiology 58:1428-1432.
Crossref
|
|
|
|
|
Sheshe MU, Magashi AM (2014). Assessment of physicochemical quality of sachet water produced in selected local government areas of Kano metropolis, Kano state - Nigeria. Bayero Journal of Pure and Applied Sciences 7(2):31-35.
Crossref
|
|
|
|
|
Simpson D (2008). Biofilm processes in biologically active carbon water purification. Water Research 42(12):2839-2848.
Crossref
|
|
|
|
|
Stewart PS, De Beer D, Srinivasan R (1994). Direct measurement of chlorine penetration into biofilms during disinfection. Applied and Environmental Microbiology 60(12):4339-4344.
|
|
|
|
|
Sule IO, Agbabiaka TO, Saliu BK, Bello AB, Adeboye AB (2017). Bacteriological and physicochemical assessment of selected brands of bottled water in Ilorin, Nigeria. Al-Hikmah Journal of Pure and Applied Sciences 4:15-22.
|
|
|
|
|
Tagoe DN, Nyarko H, Arthur SA, Birikorang EA (2011). Study of antibiotic susceptibility pattern of bacterial isolates in sachet drinking water sold in the Cape coast metropolis of Ghana. Research Journal of Microbiology 6:153-158.
Crossref
|
|
|
|
|
Uhlich GA, Cooke PH, Solomon EB (2006). Analyses of the red-dry-rough phenotype of an Escherichia coli O157:H7 strain and its role in biofilm formation and resistance to antibacterial agents. Applied and Environmental Microbiology 72:2564-2572.
Crossref
|
|
|
|
|
United States Environmental Protection Agency (EPA) (2009). National Primary Drinking Water Regulations. EPA 816-F-09-004.
|
|
|
|
|
Vanhaecke E, Remon JP, Moors M, Raes F, de Rudder D, Van Peteghem A (1990). Kinetic of Pseudomonas aeruginosaeadhension to 304 and 316-L stainless steel, role of cell surface hydrophobicity. Applied and Environmental Microbiology 41:518-527.
|
|
|
|
|
World Health Organization (WHO) (2006). Guidelines for Drinking Water Quality, 1st Addendum VI. 1. Recommendations 3rd Edition, Electronic version. Available at:
View
|
|
|
|
|
World Health Organization (WHO) (2007). Revised background document for development of WHO Guidelines for Drinking-water Quality. Available online:
View
|
|
|
|
|
World Health Organization (WHO) (2011a). Manganese in Drinking Water. Background document for development of WHO guideline for drinking water quality.
|
|
|
|
|
World Health Organization (2011b) Guidelines for drinking-water quality. Geneva: World Health Organization. 632 p.
|
|
|
|
|
Wood TK, Gonzalez AF, Herzberg M, Lee J (2006). Motility influences biofilm architecture in Escherichia coli. Applied Microbiology and Biotechnology pp. 1-7.
Crossref
|
|