ABSTRACT
The objective of this work was to study the epidemiology and antibiotic resistance of strains of Acinetobacter spp. in the University Hospital of Yopougon Abidjan. This work studied the Acinetobacter strains isolated from humans and environment of the hospital; they were preserved in the culture collection of the Laboratory of Bacteriology from January 2007 to December 2011. Isolation and identification were made by conventional bacteriological methods, and antibiotic susceptibility was studied by the method of agar diffusion. Interpretation was made according to the standards of the CA-SFM. 110 strains of Acinetobacter spp. have been studied (61% of human strains and 39% of strains isolated from the hospital environment). Acinetobacter baumannii was the most isolated in 66% of cases. 52. 8% of strains were resistant to ceftazidime; 5.6% to imipenem; 21.2% to gentamicin and 35.2% to ciprofloxacin. 12.5% of human strains of A. baumannii were multi-resistant bacteria. Acinetobacters spp. are present in the hospital environment and patients with a predominance of A. baumannii species. The presence of imipenem-resistant strains is a major public health problem because their disclosure could lead to therapeutic impasse in hospital.
Key words: Acinetobacter spp., epidemiology, antibiotic resistance.
Acinetobacter spp. are emerging opportunistic pathogenic bacteria that play an important role in hospitals worldwide (Munoz-Price and Weinstein, 2008; Schreckenberger et al., 2007). Indeed, in recent years, these bacteria have become a concern in hospital services in different countries.
Thus, they are responsible for nosocomial infections manifested by septicemia, urinary tract infections, secon-dary meningitis and especially lung infections in patients under mechanical ventilation in intensive care units (Balkhy et al., 2014; Meite et al., 2011). Moreover, in recent years, Acinetobacter infections of the central nervous system, skin, soft tissue and bone have been observed (Bergogne-Berezin and Towner, 1996; French et al. 1980; Jaffar et al., 2007).
These infections are difficult to treat, due to the increasing resistance of Acinetobacter strains growing on different families of antibiotics. Several studies have shown an appearance and an increase in the resistance of Acinetobacter spp. to carbapenem, choice antibiotics for the treatment of infections caused by these bacteria (Balkhy et al., 2014; Boni-Cisse et al., 2011; Chaisathaphol and Chayakulkeeree, 2014; Halstead et al., 2007; Unal and Garcia-Rodriguez, 2007).
In addition, antibiotic resistant strains of Acinetobacter spp. have a great ability to survive for several days in hospital environment, inert material or dust, thereby increasing the likelihood of transmission of the inter-human bacteria via a human or tank material (Boni-Cisse et al., 2011).
In Côte d'Ivoire, although Acinetobacter is responsible for nosocomial infections (Boni-Cisse et al., 2011; Meite et al., 2010, 2011), few data are available on circulating bacteria including species, the resistance profile of strains to antibiotics and the main hospital tank bacteria.
The objective of this work is to study Acinetobacter baumanii complex circulating in patients and hospital environment as well as their antibiotic resistance profile.
The authors studied 110 strains of Acinetobacter spp. kept in the culture collection of the Laboratory of Bacteriology Virology of CHU Yopougon, from January 2007 to December 2011. These strains of Acinetobacter spp. were isolated from Biologic patients and hospital environment (sink, handle of door, sick bed and respirators).
Isolation of strains
Acinetobacter strains were revived in Brain Heart broth for 3 h at 37°C and then re-plated on nutrient agar, sheet blood agar and non selective lactose agar; they were incubated at 37°C for 18 to 48 h. The colonies were identified by standard bacteriology tests (culture on minimal agar) as the genus Acinetobacter ssp. which are Gram negative bacilli, non-motile; strictly aerobic, oxidase-negative and glucose non fermentative. Reference strains, Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used for the validation of rapid biochemical tests used.
Biotypique study
For identification of Acinetobacter calcoaceticus-A. baumanii com-plex, the following characters were investigated: the ability to grow at 41 and 44°C, using citrate as sole carbon source, the production of an ornithine decarboxylase (ODC) and the production of arginine dihydrolase (ADH). Reference strains ATCC 13883 Klebsiella pneumoniae, Shigella sonnei ATCC 25931 and ATCC 27853 Pseudomonas aeruginosa were used to validate the research of ODC and ADH.
Studies of antibiotic susceptibility
The search for antibiotic susceptibility was performed by the method of agar diffusion. The following antibiotics disk of biorad were tested: ticarcillin/clavulanate (75/10 μg); ceftazidime (30 μg); imipenem (10 µg); aztreonam (30 μg); gentamicin (10 IU); amikacin (30 μg); netilmicin (30 μg); tobramycin (10 µg) and ciprofloxacin (5 μg). Interpretation of results was done according to the standards committee of the susceptibility of the French society of Microbiology (CA-SFM) recommendations 2012.
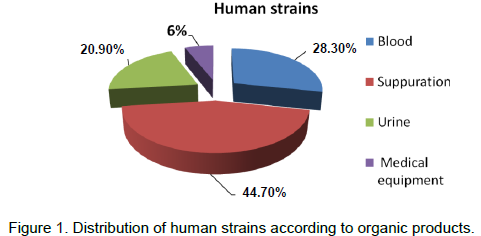
A total of 110 strains of Acinetobacter were analyzed, of which 61% were strains of human origin and 49% of environmental strains. 44.8% of the human strains were from suppurations, 28.3% from blood, 20.9% from urine and 6% from medical equipment (Figure 1). In the environment surface, 38.1% were isolated from sink, 23.8% from the patient bed, 21.4% from the handle of door and 12% from respirators. 100% of the strains gave positive cultures on isolation media used. About biotypique study, none of the strains yielded positive test for glucose fermentation.
ADH test was negative for all the strains and hemolysis test in the sheet blood agar. 94% of cultures at 41°C were positive (Table 1). For the identified species, 94% were A baumanii-calcoaceticus complex and 6% of Acinetobacter johsonii. 62% A. baumanii–calcoaceticus complex were of human origin.
The antibiogram for beta-lactam revealed that, 39.4, 52.8 and 05.6% of tested strains were resistant to the combination of respectively Ticarcillin-clavulanic acid, and preview Ceftazidime, Imipenem. Regarding amino-glycosides, 21.2 and 10.3% of the strains tested were resistant to Gentamicin and Amikacin. 35.2% of tested strains were resistant to ciprofloxacin regarding quinolones. The high proportion of antibiotic resistance was found in strains of human origin and in A. baumanii complex species.
Thus, 100% of resistant strains were imipenem essentially A. baumanii species. 05.2% of tested strains were resistant to three antibiotics families tested. This was the case of human stem A. baumanii. Resistance to two different antibiotics family was not observed with environmental strains, except the three strains resistant to imipenem.
In this study, in humans, Acinetobacter was found in suppuration from blood and urine. This is consistent with some data from the literature (Van Looveren et al., 2004). Predominantly, the suppuration is linked to the fact that Acinetobacter is from commensal skin bacterium (Nordmann, 2004).
The environments handled by the nursing staff and visitors (sinks, beds, door handle) were colonized with Acinetobacter. This presents a risk of nosocomial infec-tion (Oie et al., 2002). This is also the problem of hygiene in our hospitals.
A. baumanii-calcoaceticus complex is a predominance
species in this study. These results are in agreement with those of literature. Indeed, several studies in different countries have shown a predominance of A. baumanii species. It is found in two thirds of infections caused by Acinetobacter spp. In a study conducted in Kosovo (Raka et al., 2009), A. baumanni represented 81.2% of Acinetobacter spp. In 1993, Seifert et al. (1993) identified 73% of Acinetobacter strains from clinical isolates as A. baumannii.
Acinetobacter lwoffi, Acinetobacter hemolyticus and Acinetobacter junni species were not isolated in this study. These bacteria are rarely clinically isolated; the isolation of A. lwoffi suggests a port rather than an infection. For resistance to beta-lactam antibiotics, the rate of resistance to Ceftazidime and Cefsulodin was respectively, 52.8 and 89.6%. This resistance was greater with A. baumanii complex strains of human origin. The rate of resistance to Ceftazidime was higher than that observed in our previous studies (Meite et al., 2010, 2011). This is in favor of a steady increase in antibiotic resistance of Acinetobacter in our health facility. These results corroborate those of Balkhy et al. (2014) in Saudi Arabia and Rahbar et al. (2010) Iran, who found 84.1 and 99% rates of resistance to ceftazidime. Regarding resistance to carbapenems, it was 5.6%, including 75% of the strains of environmental origin. A baumannii complex resistance to carbapenems has appeared in many parts of the world and is clearly increasing (Balkhy et al., 2014; Chaisathaphol and Chayakulkeeree, 2014; Gootz and Marra, 2008; Perez et al., 2008).
The main mechanism of resistance is the acquisition of carbapenemases Class B and D (Gootz and Marra, 2008). Low levels of resistance (3 and 4.5%) of A. baumannii to imipenem were reported in Saudi Arabia by Jaffar et al. (2007) and in Iran by Rahbar et al. (2010). However, more recent studies in these countries have shown a marked increase in resistance to carbapenems by Acinetobacter spp., especially in Saudi Arabia where it is grown to over 80% (Sameera et al., 2010). Asencio et al. (2010)’s study carried out in Spain showed a resistance rate of 83% by A. baumanii to Imipenem, while that of Cisneros et al. (2005) was 43% resistance rate. These high rates of resistance to Imipenem are found in hospitals. The variations in time of the resistance of A. baumanii to Imipenem are related to increased use of the molecule and use not mastered by some prescribers, sometimes. Monotherapy is not recommended and should be used in combination synergistically, taking into account the bioavailability of each molecule, the site of action, the causative organism and risk factors related to patients. Strains tested were sensitive enough to aminoglycosides. Resistance was 21.2% for Gentamicin and 10.3% for Amikacin. These results are quite close to that of Asencio et al. (2012), in which the rates were very low (12% for Gentamicin and 2% for Amikacin). These low levels of resistance are due to the fact that aminoglycosides are rarely used in treating infections in our country, generally. Oral forms are rare and injectable forms available are used only in association with other molecules and in hospitals.
In this study, the rate of resistance to ciprofloxacin was 49%. Our results corroborate those of Ben Haj et al. (2010) in Tunisia who regained a resistance rate of 50%. These results, however, are contrary to those found in Iran by Rahbar et al. (2010) which was 90.9%. Described for the first time in Taiwan in 1998 and defined as being resistance to more than three classes of antibiotics, the incidence of strains of multi-resistant A. baumannii (MDR) continues to grow in recent years (Appleman et al., 2000). In a study realized in the United States by Dent et al. (2010), it involved almost 72% of A. baumannii studied. In our present study, it was 5.2% for all strains and reached 12.5% for strains isolated from humans.
A. baumannii with P. aeruginosa are frequently pan-resistant bacteria of antibiotic. In effect, these bacteria may be resistant to all antibiotics, including the aminoglycosides, cephalosporins, carbapenems, carboxypénicillines and fluoroquinolones. Dent et al. (2010) in USA found about 46% in their series. This profile was not found in our study. However, the phenotypes of resistance to at least two families of antibiotics have been observed. This resistance was generally observed as regards the lactams and amino-glycosides. Strains involved were the cases of A. baumanii especially of human origin. The proportion of resistance to these two antibiotic families is linked to therapeutic habits of our health facilities. The combination of these two antibiotic families is the first therapeutic choice in many empirical studies in Côte d'Ivoire.
Acinetobacter remains an environmental bacterium whose species A. baumanii complex is the most involved in human infections in hospitals. It presents a profile of increasingly resistant to conventional antibiotics in our health facility and its main concern remains the appearance of resistant strains to imipenem. This multidrug-resistant Acinetobacter to imipenem requires the implementation of policy microbiological monitoring and control to limit dissemination.
The authors did not declare any conflict of interest.
REFERENCES
|
vitro activities of non-traditional antimicrobials against multiresistant Acinetobacter baumannii strains isolated in an intensive care unit outbreak. Antimicrob. Agents Chemother. 44:1035-1040.
Crossref
|
|
|
|
Asencio MÁ, Carranza R, Huertas M (2012). Resistencia a antimicrobianos de los microorganismos más frecuentemente aislados en el Hospital General La Mancha Centro entre junio de 2009 y mayo de 2010. Rev Esp Quimioter. 25:183-188.
|
|
|
|
|
Balkhy HH, El-Saed A, Maghraby R, Al-Dorzi HM, Khan R, Rishu AH, Arabi YM (2014). Drug-resistant ventilator associated pneumonia in a tertiary care hospital in Saudi Arabia. Ann. Thorac. Med. 9(2):104-111.
Crossref
|
|
|
|
|
Ben Haj KA, Khedher K M (2010). Frequency and antibiotic susceptibility profile of bacteria isolated blood cultures CHU Mahida. Tunis. J. Infect. Dis. 3:92-95.
|
|
|
|
|
Bergogne-Bérézin E, Towner KJ (1996). Acinetobacter spp. as Nosocomial Pathogens: Microbiological, Clinical and Epidemiological Features. Clin. Microbiol. Rev. 9:148-165.
PMid:8964033 PMCid:PMC172888
|
|
|
|
|
Boni-Cissé C, Méité S, N'douba Kacou A, Koffi P, Faye Ketté H, Dosso M (2011). Colonization of catheters by bacterial in the intensive care unit in Abidjan. Sch. J. Med. 2(3):38-42.
|
|
|
|
|
Chaisathaphol T, Chayakulkeeree M (2014). Epidemiology of infections caused by multidrug-resistant gram-negative bacteria in adult hospitalized patients at Siriraj Hospital. J. Med Assoc. Thai. 97(3):35-45.
|
|
|
|
|
Cisneros JM, Rodrνguez-BJ, Fernαndez-Cuenca F, Ribera A, Vila J, Pascual A, et al. (2005). Risk-factors for the acquisition of imipenem-resistant Acinetobacter baumannii in Spain: a nationwide study. Clin. Microbiol. Infect. 11(11):874-879.
Crossref
|
|
|
|
|
Dent LL, Marshall DR, Pratap S, Hulette RB (2010). Multidrug resistant Acinetobacter baumannii: a descriptive study in a city hospital. BMC Infect. Dis. 10:196.
Crossref
|
|
|
|
|
French GL, Casewell MW, Roncoroni AJ, Knight S, Phillips I (1980). A hospital outbreak of antibiotic-resistant Acinetobacter anitratus: Epidemiology and control. J. Hosp. Infect.1:125-131.
Crossref
|
|
|
|
|
Gootz TD, Marra A (2008). Acinetobacter baumannii: Multidrug resistant emerging threat. Expert Rev. Anti Infect. Ther. 6:309-325.
Crossref
|
|
|
|
|
Halstead DC, Abid J, Dowzicky MJ (2007). Antimicrobial susceptibility among Acinetobacter calcoaceticus-baumannii complex and Enterobacteriaceae collected as part of the tigecycline evaluation and surveillance trial. J. Infect. 55:49-57.
Crossref
|
|
|
|
|
Jaffar A, Alâ€Tawfiq MD, Thangiah X, Mohandhas BS (2007). Prevalence of antimicrobial resistance in Acinetobacter calcoaceticusâ€baumannii complex in a Saudi Arabian Hospital. Hosp. Infect. Control 28:870-872.
Crossref
|
|
|
|
|
Meite S, Boni-Cisse C, Monemo P, Babo JC, Mlan Tanoa AP, Faye-Ketté H, Dosso M, Amonkou A (2011) . Resistance patterns of bacteria isolated from bronchial samples protected in patients receiving mechanical ventilation in the ICU CHU Yopougon. Méd Afr Noire. 58 (8/9):416-422.
|
|
|
|
|
Meite S, Boni-Cissé C, Monemo P, Mlan Tanoa AP, Faye-Ketté H, Dosso M (2010). Microbiological monitoring of surfaces at a tertiary hospital: CHU Yopougon, Abidjan, Côte d'Ivoire. J. Sci. Pharm. Biol.11 (1):73-81.
|
|
|
|
|
Munoz-Price LS, Weinstein RA (2008). Acinetobacter infection. N. Engl. J. Med. 358:1271-1281.
Crossref
|
|
|
|
|
Nordmann P (2004). Acinetobacter baumannii, the nosocomial pathogen par excellence. Pathol. Biol. 52(6): 301-303.
Crossref
|
|
|
|
|
Oie S, Hosokawa I, Kamiya A (2002). Contamination of room door handles by methicillin-sensitive / methicillin resistant Staphylococcus aureus. J. Hosp. Infect. 51:140-143
Crossref
|
|
|
|
|
Perez F, Endimiani A, Bonomo RA (2008). Why are we afraid of Acinetobacter baumannii? Expert Rev. Anti Infect. Ther. 6:269-271.
Crossref
|
|
|
|
|
Rahbar M, Mehrgan H, Aliakbari NH (2010). Prevalence of Acinetobacter baumannii antibiotic-resistance in bed tertiary care hospital in Tehran 1000. Iran J. Pathol. Microbial. 53:290-293.
Crossref
|
|
|
|
|
Raka L, Kalenc S, Bošnjak Z, Budimir A, Katic S, Šijak D, Mulliqi-Osmani G, Zoutman D, Jaka A (2009). Molecular Epidemiology of Acinetobacter baumannii in Central Intensive Care Unit in Kosova Teaching Hospital. Braz. J. Infect. Dis.13:408-413.
|
|
|
|
|
Sameera M, Johani Al, Javed A, Hanan B, Ayman ES, Mousaad Y, Ziad M (2010). Prevalence of antimicrobial resistance among gram negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann. Saudi Med. 30:364-369.
|
|
|
|
|
Schreckenberger PC, Daneshvar MI, Weyant RS, Hollis DG (2007). Acinetobacter, Achromobacter, Chryseobacterium, Moraxella,and other non-fermentative Gram-negative rods. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of Clinical Microbiology, 9th ed. Washington, DC: ASM Press. pp. 770-802.
|
|
|
|
|
Seifert H, Baginsky R, Schulze A, Pulverer G (1993). The distribution of Acinetobacter species in clinical culture materials. Zentralbl. Bakteriol. 279:544-552.
Crossref
|
|
|
|
|
Unal S, Garcia-Rodriguez JA (2005). Activity of meropenem and comparators against Pseudomonas aeruginosa and Acinetobacter spp. isolated in the MYSTIC Program, 2002-2004. Diagn. Microbiol. Infect. Dis. 53:265-271.
Crossref
|
|
|
|
|
Van Looveren M, Goossens H, the ARPAC Steering Group (2004). Antimicrobial resistance of Acinetobacter spp in Europe. Clin. Microbiol. Infect. 10(8):684-704.
Crossref
|
|