ABSTRACT
Several materials such as tuff and Tripoli which is naturally occurring and industrial by-products wastes as high calcium ash and slag were investigated as cement substitutes in this paper. Compressive strength of various standard mortar samples have been tested at 7 and 28 days. The obtained results show that these materials have improved the properties Ordinary Portland Cement (OPC) concrete. It was found that an increased compressive strength of 22% were attained at an optimum of 10% addition of ash. Meanwhile, the compressive strength increases by 30% when the content of Tripoli is of 10%. The slag and Tuff have adverse effect on the strength. The reason may be attributed to the mode and original of crystals. The finding of this research work also show that using these materials will reduce the use of OPC. This reduction will reduce some of the adverse environmental effect due to OPC production and consequently, reduces the consumption of energy in Jordan.
Key words: Ordinary Portland Cement (OPC), cement substitutes, tuff, Tripoli, ash, environmental.
Mineral admixtures are materials used as an ingredient of concrete or mortar and added to the batch immediately before or during mixing mainly to improve or modify several properties of Portland cement concrete PCC. Mineral admixtures are used in conjunction with Portland or blended cements as a supplementary cementing material (SCM) through hydraulic or pozzolanic activity or both. PCC mixtures are multiphased, particle-reinforced composites that consist of irregularly shaped and randomly oriented aggregate particles embedded in an inelastic matrix. PCC mixtures generally exhibit complicated mechanical behavior and multiple modes of damage. Although precise identification and prediction of the inelastic damage modes of the PCC is extremely difficult, it is important to seek out simpler approaches of predicting mechanical behavior including damage characteristics of the mixture in place of expensive and time-consuming laboratory experiments where possible.
Recently, the use of reinforced concrete in multi store structure and industrial plants in the vicinity of the capital Amman and surrounding towns is continuously increasing in the last decade. There is shortage in production and huge demand on OPC which the local cement factories cannot satisfy the local market needs. This has led to an increase in the demand energy. On the other hand, there is a negative environmental impact of cement production. For instance the production of one ton of OPC releases one ton of CO2 and these issues are considered the most important challenges facing construction sector.
Some of the local cement factories in Jordan have investigated the use of bituminous limestone ash as a source of raw material after direct combustion and reducing its energy in the kiln system. This may be considered as a good approach for energy saving, but it will lead to complicated environmental problems due to the evolved SOx and COx gases.
To achieve sustainability of the OPC, changes in working practices are required. This means that low energy, low carbon, and low waste techniques have to be developed to replace more intensive techniques. For example, in situ remediation of contaminated land may become a more attractive than a ‘dig-and-dump’ approach, where the material is removed and disposed of to landfill and then replaced with new material. These techniques are not only high in energy consumption and produce large amounts of contaminated waste, but also highly expensive. Alternative methods however, have been recently developed to reuse several types of industrial waste materials in civil engineering construction by many researchers such as Tay (1987), Churchill (1994), Perez et al. (1996), Stefanov (1986), Pereira et al. (2000), Pavlova (1996) and Maaitah (2012).
In this paper, some materials such as Tripoli, ground tuff, high calcium ash and air cooled slag will be added to the mortar. These materials have been investigated as cement substitutes. The aim of this work is to investigate the enhancement of mechanical properties of the PCC by the addition of mineral admixtures to the concrete mixtures as cement substitutes. The optimum percent or fixation value for each additive will be investigated. The weight of cement content decreases but the strength is increased that is required to produce a certain class of reinforced concrete with improved quality and durability through adding self cementitious materials as mineral admixtures. This may reduce the consumption of OPC which in turn may help reduce all the adverse effect to the cement production.
Materials
The naturally occurring material such as ash and Tripoli are abundant in Jordan. Bituminous limestone as a source of ash and Tripoli are available as millions of tones in the vicinity of Al-Karak city about 120 km to the south of the capital Amman. Slag is available at many steel factories located at and around Amman. The material that produces waste as high calcium ash and slag were used as cement substitutes.
In the present work the Karak ash (from various outcrops from El-Lajjun) was obtained by direct combustion of Karak bituminous limestone at a temperature of (900 to 1000)°C. The sample was allowed to cool down to the ambient temperature which can be considered as fast cooling. This means that the crystal is micro crystalline. Then, the sample was ground under dry conditions to obtain the possible minimum grain size. Small ball mills and Los Angles machine were used. The sample was crushed using a jaw crusher to obtain bituminous limestone aggregates of 9 mm nominal size particles.
Ash could be the solid waste product of possible utilization of the Karak bituminous limestone. The ash used in this study is a high calcium ash that has been produced by direct combustion of the bituminous limestone at 950°C (Maaitah, 2012). The chemical properties of Karak ash are summarized in Table 1. The combusted bituminous rocks in Karak/Jordan have indicated the presence of two groups of minerals; high temperature which is equivalent to clinker cement (Khoury, 1993; Al-Hamaiedh, 2010) and low temperature which is similar to the hydrated cement products (Khoury and Nasser, 1982). The low temperature mineral group has a similar composition to the hydrated cement products and has been precipitated from high alkaline circulating water (pH > 12.5). This naturally occurring alkaline water is analogous to the cement percolating water (Khoury, 1993).
Tuff can be obtained from the northern province of Jordan. Tuff is a natural volcanic material that is characterized by very high porosity, low density, rich in SiO2, and has a considerable content of Al2O3 in addition to Fe2O3. Huge quantities of this material are available in the eastern and north eastern provinces of the Kingdom; these natural recourses are utilized in various engineering and agricultural aspects.
About 20 kg of reddish crushed sand size was sampled from Amani quarry at Tall Hassan in the vicinity of Al-Azraq area. The sample was ground to get the maximum possible passing #200 sieve fraction, the Loss Angles abrasion machine was used for this purpose to produce a bulk fine tuff sample that will be used later to investigate the cement-tuff mortars. The chemical composition of each material was determined using the XRF technique as shown in Table 2.
Slag can be obtained from the United Iron and Steel MFG. Co., 30 km to the south of Amman. Huge quantities of blackish, tough aggregation stockpiles of iron slag wastes are available as by-product of iron production at different steel factories in the vicinity of Amman and surrounding areas. The sample was obtained from Al Manaseer Steel Factory about 30 km south of Amman. The sample was ground to fine powder and passing sieve No. 200 was used.
The chemical composition of each material was determined using the XRF technique as shown in Table 2. Conplast C3O as a super plasticizer was used in constant dosage in all trials and with Specific Gravity of 3.3. The unit weight is 1750 kg/m3 and the Absorption is up to 2.32. The slag crystal is not compatible, some crystal is glassy because of the cooling is very fast in the surface and some fine. The fine crystal is constituted because it is cooled slowly somehow at the down of the stack. Therefore, the production is out of control and the composition varies from time to time. It is difficult to find similar sample among the slag stack after manufacturing.
Tripoli is microcrystalline silica. It is a form of silica, earthy, light colored, light weight, friable, very fine grained chalcedonic and opaline silica of cryptocrystalline, and it is commercially classified as "Soft Silica" or "Amorphous Silica", having a 90 to 94% Silica content with high purity and high melting point. The Tripoli can be used as filler material in paints and rubber, plastic industries, mild abrasive, insecticides. The occurrences are found in Karak District, 168 km Southwest of Amman, in the following areas:
i) Around thirteen million metric ton in El-Adnanieh (8 km South of Karak);
ii) Around seven million metric ton in El-Shahabiyeh, 4 km Southwest of Karak;
iii) Undetermined huge millions metic tonnage in Wadi Rakin and Wadi Ben Hamad 4.5 km Northwest of Karak;
iv) Undetermined millions of metic tonnage in Wadi Falqa, 5.5 km Southwest of Karak;
v) Undetermined huge millions metic tonnage in Ainun, 5.5 km South-Southwest of Karak along the west bank of Wadi Daba.
The chemical composition of Tripoli was determined using the XRF technique as shown in Table 3. The Adnanieh Tripoli were used in this work.
Approach
The combusted limestone ash, slag, tuff and Tripoli were ground using Loss Angles machine for one and half an hour each, followed by sieving the ground material on No. 200 sieve. The fraction passing #200 sieve was collected in tight plastic bags. The silica sand was sieved to prepare standard sand for mortar preparation. A reference mortar mix composed of 1500 gr of the standard silica sand was mixed with 500 gr of OPC at a w/c ratio of 0.485. The mortar was mixed well and cast into 5x5x5 cm cubes, two layers into each cube and 10 blows on each layer using a standard rod with 2x5 cm cross sectional area. Six cubes were prepared and de-molded after 24 h. The samples were cured in water until testing at 7 and 28 days. Compressive strengths presented are the averages for 3 cubes at each of the (7 and 28 days). The same procedures were repeated for the other mortars- admixtures with substituting cement by various percents of that admixture. ASTM C109 was followed strictly for the whole procedure. The compressive strength of various standard mortar samples have been tested at 7 and 28 days.
Mortar composition
The proportions of materials for the standard mortar shall be one part of cement to 2.75 parts of graded standard sand by weight.
The water/cement ratio of 0.485 was used for all Portland cements. The water/cement ratio for other than Portland and air entraining Portland cements shall be such as to produce a flow of 110 +/- 5.
The specimen mold preparation was conducted using Mortar Mixing Procedure in accordance with (ASTM C305). This was performed by applying a thin coating of mold release to the interior surfaces of the molds and base plates. Surfaces were wiped with a cloth to remove any excess, with dry paddle and bowl placed in the mixing position of the mixer. The strength for native sample (without any additives) after 7 days is 21 kg/cm2 and after 28 days is 27 kg/cm2. An increase in strength of 28.5%, possibly due to curing time.
The bond strength between cement substitutes and concrete materials is related to the interface properties, interface fracture mechanics and most likely to the crystal. The mode of crystal (that is, phanertic, aphanertic and glassy) play a significant role in determining the success of the substitutes. The loadings will impart both tensile and compressive normal and shear stresses at the interface, and thus failure will be under multi-component stresses.
The development of the self cementaceous properties and strength are controlled by the additive content and the curing period. The additive is similar to a great extent to the Portland cement. The results are obtained through the standard mixtures of each sample (Table 4). The mixtures are prepared and cured under the same standard procedures and conditions. Figure 1 show the effect of curing time on Tripoli which improve that the Tripoli reacts with OPC. This is because it has micro crystalline original and a high content of SiO2 and CaO reacts with cement (Table 5).
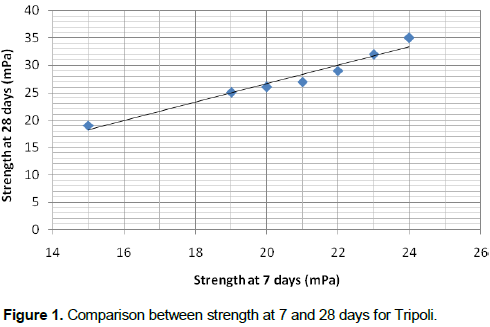
The strength of mortar increases as the Tripoli content increases up to specific value (that is, 20%) and then it has no effect on strength up to 40% content. The content of Tripoli more than 40% has adverse effect because the strength decreases as shown in Figure 2. At fixation point (10% of Tripoli content) the strength improved 30% from native sample after 28 days. On the other hand, the strength increases by 14% after 7 days for the same Tripoli content. This is an improvement on the fact that the Tripoli reacts with time. The well known reaction between the elite (C3S) and water is
The extra Portlandite Ca(OH)2 will react with SiO2 from Tripoli to produce Belit which will cause an increase in the strength by 30% as shown in Figure 2.
The extra Belit (2CaOSiO2) which is due to the addtion of Tripoli is appearantly resposible for the increase in compressive strength.
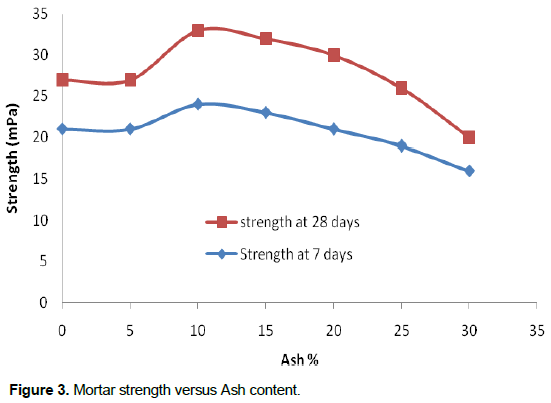
From the obtained results in Figure 3, it is clear that the mortar strength has increased by 22% of ash fixation point after 28 days, whilst, at 7 days an increase of 14% were observed. The fixation point is at 10% ash content.
The ash and Portland cement are essentially composed of lime (CaO). Silica (SiO2) and alumina (Al2O3) are present at higher concentrations in the Portland cement and react with CaO at about 1425°C to form alite. Heat treatment of the bituminous rocks and Portland cement raw material involves dehydration, thermal decomposition of clay minerals (300 to 650°C), decomposition of calcite (greater than 800°C), the formation of belite (C2S), tricalcium aluminate (C3A), and tetracalcium alumina ferrite (C4AF). The liquid phase and sintering at about 1425°C form alite (C3S) which is responsible for the strength of concrete (Khoury, 1993).
The variable content of SO3, the alkali CaO, and pozzolanic content (SiO2 +Al2O3+ Fe2O3), are found in both the ash samples and OPC raw material. The strength build up in all the samples is related to the setting reactions of lime (CaO) with the pozzolanic constituents to produce calcium silicate hydrate (CSH) and calcium aluminate hydrate (CAH). High pH solution is highly reactive with amorphous Al-Si rich phases at normal room temperature. Sulfate minerals as ettringite are expected to form because of the availability content of SO3. The CaO content plays an important role in the alkali–pozzolanic reaction.
The reaction products leave the kiln as a clinker. The clinker leaves the kiln here to be cooled, mixed with gypsum, and then ground into a fine powder (cement). The setting of cement involves a number of stages at different rates. It is generally known that a complex series of reactions do take place as the cement reacts with water. Setting of C2S involves slow hydration reactions and the formation of Portlandite Ca (OH2) and calcium silicate hydrate.
Portlandite Ca (OH)2 plays an important role in the setting reaction. Portlandite reacts with silicates and aluminum rich phases to form insoluble compounds which contribute to the strength formation (pozzolanic reactions). Excess portlandite reacts with atmospheric CO2 to precipitate calcium carbonate that helps in strengthening the product after aging.
The combusted bituminous rocks in central Jordan have indicated the presence of two groups of minerals; high temperature which is equivalent to clinker cement (Khoury, 1993) and low temperature that is similar to the hydrated cement products (Khoury and Nasser, 1982). The low temperature mineral group has a similar composition to the hydrated cement products and has been precipitated from high alkaline circulating water (pH > 12.5). This naturally occurring alkaline water is analogous to the cement percolating water (Khoury, 1993).
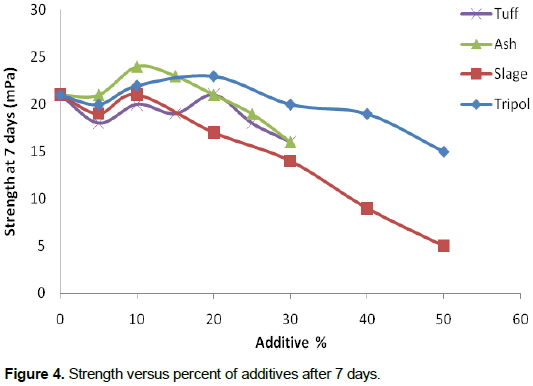
Figure 4 and Table 5 show that the tuff has an adverse effect on strength. This is possibly, because the Tuff is poured rock and the powder has high surface area. The Tuff powder absorbs extra water, in turn affecting the water/cement ratio for reaction. This could be related to the kind of crystal of tuff that shows no reaction with OPC. Figure 4 and Table 5, also, show that the slag has an adverse effect on strength. The slag production is out of control. The slag contains strange materials and impurities. The properties of slag vary from sample to sample. The slag durability may be low due to the rust.

The effect of curing time can be seen Figure 5 and Table 5. Curing period of 28 days and more has influenced the compressive strength results. High compressive strength values obtained for intact samples indicate no disintegration features under fully saturated conditions. All hydrated samples have shown a similar behavior to the hydrated OPC products but with lower compressive strength.
Table 5 summaries the results of this research and illustrates that an addition of 20 and 10% Tripoli and ash, respectively, lead to an improvement of the strength with respect to native sample by 19 and 22%. It is also, apparent that any increases in the additive content more than the fixation point will cause a reduction in the compressive strength. The obtained results suggest that the presence of Tuff and slag additives have determined effect on the compressive strength, which can be explained as a result of the mode and original of crystal (that is, phanertic, aphanertic and glassy). The crystalline plays a significant role in determining the success of the substitutes. Curing period of 28 days and more has a good influence on the compressive strength results.
The authors have not declared any conflict of interests.
REFERENCES
|
Churchill M (1994). Aspects of sewage sludge slime utilization and its impact on brickmaking, Global Ceram. Rev. 1:18. |
|
|
|
Khoury H (1993). Mineralogy and Isotopic Composition of the Metamorphic Rocks in the Bituminous Limestone of the Maqarin Area, Jordan, Dirasat 20B(2). |
|
|
|
Khoury H, Nasser S (1982). A discussion of on the origin of Daba-Siwaqa marble, Dirasat. 9:55-66. |
|
|
|
Maaitah ON (2012). Evaluation of Al-Karak Ash for Stabilization of Marl Clayey Soil, 17[2012], Bund. G, EJGE. |
|
|
|
Pavlova L (1996). Use of industrial waste in brick manufacture, Tile and Brick Int. 12:224. |
|
|
|
Pereira DA, Couto DM, Labrincha JA (2000). Incorporation of aluminum-rich residues in refractory bricks, CFI – Ceramic Forum International, 77:21. |
|
|
|
Perez JA, Terradas R, Manent MR, Seijas M, Martinez S (1996). Inertization of industrial wastes in ceramic materials. Ind. Ceram. 16(7):571-584. |
|
|
|
Stefanov S (1986). Use of industrial wastes in the brick and tile industry, Ziegelindustrie Int. 3:137. |
|
|
Tay JH (1987). Bricks manufacture from sludge slime. J. Environ. Eng. 113:278.
Crossref |