ABSTRACT
Chitosan is a natural polymer gotten from shrimp and crab skeleton; chemical structure of chitosan is similar to cellulose where hydroxyl (OH) in glucose molecule in cellulose is replaced by amine (–NH2) in chitosan. From the chemical structure and its properties, chitosan can be used as a filler and adhesive to replace the traditional materials and it will improve several properties of papers such as to increase strength, reduce water absorption, increase smoothness, and probably anti bacteria and anti fungus of the papers. The use of chitosan in paper production can also reduce impact on environment, because chitosan is a natural polymer that is degradable. In this research, chitosan will be applied into paper processing to replace filler and also as an adhesive to increase the properties of the paper. The result shows that low molecular weight chitosan, 1%, can affect the decrease of water absorption property and increase paper strength and the smoothness of the paper.
Key words: Chitosan, paper, water absorption, strength, smoothness, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermogravimetric analyzer (TGA).
Paper and carton are wrapping materials that are very versatile to break; they are produced from cellulose fibers which are renewable materials. However, they have to consider the use of environmental friendly materials. Wrapping papers are used for food packaging, they have the required strength and they are resistant to water and grease (Butkinaree et al., 2008; Reis et al., 2013). Paper has some disadvantages with some of its properties as regard moisture and grease. Therefore, paper is often coated to increase its resistant to moisture, oxygen, aroma and grease with hydrophobic material such as paraffin wax and polyethylene (Butkinaree et al., 2008).
Although, paraffin wax and polyethylene will be difficult to separate in packaging. Used paper will be recycled or decomposed after uses. Several kinds of polymers were developed to increase fiber properties such as urea and melamine formaldehyde, epoxy containing polyamide, cationic polyamide and polyethyleneimine (PEI). PEI is the most interesting due to its amine primer, secondary and tertiary content (Atlan et al., 1975). Pulp cellulose contains more acid site because of the oxidation from polymer, or the presence of residue lignin or hemicelluloses that can be efficient to form ionic and hydrogen bonds based on PEI (Struszczyk, 2002; Hudson and Smith, 1998).
The association of biopolymers with paper provides interesting functionalities while maintaining the environment-friendly nature of the material and its recyclability. They can be applied in-line and the coated material can easily be re-pulped (Kuusipalo et al., 2005). As an effort to produce environment friendly materials and biodegradable, biopolymer like chitosan can be used as coating materials (Hudson and Smith, 1998; Pitaloka et al., 2013).
Application of chitosan into paper production was explained by Struszczyk (2002). Paper is a collection of cellulose fibers and fibers take place in hydrogen bonds to form bulk, but the bond is weakened by a large amount of water molecule which competes with hydrogen bond in fibers. He said that chitosan with unique molecular structure contain hydroxyl and amine groups all at once; it can be used in many applications, such as additive.
Chitosan can be used as crosslinking agent between fibers in paper, so it can increase paper strength. Fibers in paper are stringed by hydrogen bonds, and this bond affects the distance between fibers crosslinking. The presence of more water can reduce paper strength. To increase paper strength, several resins and polymers has been used such as urea, fenol, melamine and formaldehyde (Nada et al., 2005).
According to Puvvada et al. (2012), many biochemists have found that chitosan such as biocompatible, biodegradable and non toxic make wide applicability. Houbin et al. (2004) found that chitosan is almost all absorbed by cellulose fibers, especially on flat surface in various system of cellulose at low concentration that is normally used in industry. Its absorption increase as its degree of deacetylation increase (DD). Several polymers can be used to increase the brightness of paper; one of which is chitosan (Gavhane et al., 2013).
Chitosan has been reported to be used as a wet end additive in paperboard. It is used to improve the mechanical properties of the product or in the development of paper barrier properties or for bending strength by its inclusion in paper coating formulas (Kuusipalo et al., 2005).
CHITIN AND CHITOSAN POLYMER
Chitin is a linear chain polysaccharide with formula β(1-4) 2-asetamide-2-deoxy-D-glucopyranose (Muzzarelli,1977) and chitin is chitosan precursor. Chitosan was first found in 1811 in France by Henri Braconnot as isolation of fungi, while chitin is from an insect shell found in 1820 (Rismana, 2004).
Chitin is one material that is abundant in nature (Puvvada et al., 2012), this material is extracted from animal skin especially crustacean, mollucan and insect, and it is also a part found in fungi (Muzzarelli, 1977; Gopakumar, 2002). Chitin generally consists of b-(1®4)-2-acetamido-2-deoxy-D-glucopiranose (Anonim, 2008), and chitin from its structure is a derivative of cellulose. Chitin has a structure where hydroxyl groups (OH) bunch at C-2 in cellulose is replaced by acetamide groups (NHCOCH3) in chitin.
Waste of crab and shrimp shells was examined by (Gopakumar, 2002; Hardjito, 2006) as shown in Table 1. Table 1 show that the protein content of shrimp waste is about 35 to 40% which is a bit higher than crab shell, 30 to 35%. Chitin content in shrimp waste is about 15 to 20%, which is also a bit higher than crab shell, that is, 13 to 15%. However, component of ash in crab shell is higher, 45 to 50%, than in shrimp shell, about 30 to 35%.
Chitosan was first discovered by a France scientist named Ojier in 1823. Ojier examined chitosan produced from extraction of strong animal shell, such as shrimp, crab and insects (Sugita, 2009). Chitosan serves many purposes, such as flocculation agent, wound healing, medicine, cosmetic and biomaterial for immobilization.
Chitosan is a derivative compound of chitin with molecule structure (1,4)-2-Amino-2-Deoksi-β-D-Glukosa. The potential resources of chitosan are Crustaceae shell (Muzzarelli, 1977). Chitosan is a natural polymer with molecular structure similar to cellulose, but their differences are hydroxyl groups in C-2 chain in cellulose replaced by amine (NH2) in chitosan (Tiyaboonchai, 2003). Chitosan, poly[b-(1®4)-2-amino-2-deoxy-D-glucopyranose] with molecule structure (C6H11NO4)n has a structure where acetamide groups in chitin substituted by amine groups in chitosan, so deacetylated chitin is chitosan (Anonim, 2008). Chitosan is also found naturally in some organisms (Struszczyk, 2002).
Figure 1 shows the comparison among chitin, chitosan and cellulose structures where chitin consists of acetamide groups (-NHCOCH3) in C-2, chitosan consists of amine groups (-NH2) and cellulose consists of hydroxyl groups (-OH). The availability of these groups can cause and chitosan has effect in their solubnilities. Chitin and the difference in their solubility (Habibie, 1996). In chitosan structure, not all acetamide groups are replaced by amine groups, in fact, several chitosan polymers may have various degree of deacetylation depending on acetamide groups in the structure, although in small quantity (Habibie, 2014).
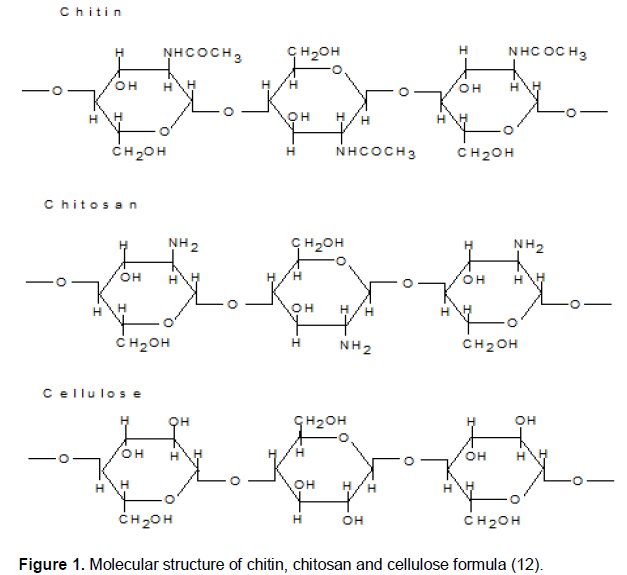
The different amount of amine groups between chitin chitosan are not soluble in water but chitosan is soluble in acid solution with a degree of deacetylation higher than 60%. On the contrary, chitin does not dissolve (Habibie, 2014). Chitin and chitosan generally do not dissolve in alkali and organic solution. Generally, the higher percentage of amine (NH2) in chitosan determines the degree of deacetylation (DD). The DD is an important parameter affecting solubility, chemical reactivity, and biodegradability. Depending on the source and preparation procedure, DD may range from 30 to 95% (Martino et al., 2005).
Chitin does not dissolve in many solvents; however, chitosan dissolves in many organic acid solutions at pH lower than 6.5 including formic acid, acetic acid, tartrate acid and citric acid. Chitosan does not dissolve in formic acid and sulphuric acid. Chitosan appears in various molecular weight and degree of deacetylation. Molecular weight and degree of deacetylation are prime factors that affect particle size, particle formation and aggregation (Tiyaboonchai, 2003).
The mechanical and biological properties of chitosan scaffolds depend on the properties of chitosan, such as molecular weight, DD and degree of crystallinity, and the scaffold preparation method used (VandeVord, 2002; Thein and Kitiyanant, 2006; Dhiman et al., 2004; Chalonglarp et al., 2007). The higher DD chitosan scaffolds have smaller pore sizes, ranging from 50 to 100 µm, greater mechanical strength, moderate water absorption properties and higher cellular activities, than lower DD chitosan scaffolds (Thein and Kitiyanant, 2006; Dhiman et al., 2004; Amaral et al., 2006).
Materials used in this research include wood pulp. Chitosan samples were purchased, aldrich low molecular weight and medium molecular weight chitosan (CAS 901276-4). Wood pulp used in this research is leaf bleached kraft pulp (LBKP) from Acasia pulp plant that was found from Pulp and Paper Research Bureau, and calcium carbonate (CaCO3) powder.
Characterization of chitosan polymer
Degree of deacetylation measurement
The deacetylation degree of chitosan samples was measured using acid-basic method by Czechowska-Biskup et al., 2012. Dried chitosan (0.2 g) was dissolved in 20 cm3 0.1 M hydrochloric acid and 25 cm3 deionized water. After 30 min continuous stirring, next portion of deionized water (25 cm3) was added and stirring continued for 30 min. When chitosan was completely dissolved, the solution was titrated with 0.1 mol·dm-3 sodium hydroxide solution using automatic burette (0.01 cm3 accuracy). The degree of deacetylation (DA) of chitosan was calculated using this formula:
where m is the weight of sample, V1 and V2 are the volumes of 0.1 mol·dm-3 sodium hydroxide solution corresponding to the deflection points, 2.03 is the coefficient resulting from the molecular weight of chitin monomer unit, and 0.0042 is the coefficient resulting from the difference between molecular weights of chitin and chitosan monomer units.
Molecular weight measurement
The molecular weight was measured using Viscosity-Average Molecular Weight Determination method. Viscosity-average molecular weight was identified by viscometric measurements using an Ubbelohde Capillary Viscometer type 531/10. This value was calculated from [η] = KMα equation, where K= 9.66×10-5 (dm3/g) and a = 0.742 determined in 0.15 M ammonium acetate and 0.2 M acetic acid solution at 25°C.
Ash content
Ash content was measured using the following method: Chitosan ash content was determined by combustion using a constant weight crucible. The crucible was repeatedly placed in an oven at 550 ± 20°C for 30 min and then removed; after cooling for 30 min in dessicator, and then weighed until a constant weight W0 ± 0.3 mg was obtained. Chitosan, 2 to 5 g, was placed in the constant weight crucible and repeatedly heated in an oven at 100°C for 1 h to find constant weight (W1). Then, chitosan in a constant weight crucible was combusted in an oven at 550 ± 20°C for 3 h. The crucible was removed, cooled in a desiccator for 30 min, and re-weighed (W2). The ash percentage was calculated by the equation:
Where W0 is the constant weight of crucible, W1 is the weight of sample and crucible, W2 is the weight of ash and crucible. The ash content was determined from each of the two samples.
Method of paper production and its testing
Material preparation
The moisture of pulp is the first measured, then separated with beater without weighing. Pulp paper is prepared with a consistency of 3 to 4%. The machine used to separate pulp fibers was valley beater.
Furnish production to make paper sheet
Chitosan solutions were prepared with concentrations of 1 and 2% using 2% solvent of acetic acid. Pulping paper was weighted as needed, and then it was prepared in a beaker and stirred. Chitosan solution was added to pulping paper with a dose of 1 and 2% of dry pulp weight. Other pulping paper was prepared for pure paper (blanko) without adding chitosan solutions. After which the furnish produced was stirred for 3 min. Apart from chitosan based pulping paper, filler of calcium carbonate with dose of 10 and 20%, was added and stirred for 2 min.
Paper sheet production
Pulping paper already prepared was taken and weighted as needed to make paper sheet with 80 g weight. Every sample variation was made with 5 sheets. Hand sheet former machine was used for paper production.
Sheet testing
Paper sheets were subjected to grammature measurement using the standard of SNI ISO 536:2010. Paper and carton were subjected to stiffness test using SNI 0935.1:2009, hardness test using SNI 0932.1:2008, and water adsorption using SNI 0499:2008. The testing apparatus were used as follows: Taber stiffness tester was used to measure the stiffness of paper, Bendtsen roughness tester was used to measure the roughness of paper, and Cobb tester was used to measure water adsorption of paper. Other test done include strength test (outsoursing in Balai Besar Tekstil Bandung with no data of apparatus found).
Characterization of treated paper
Thermogravimetric analysis (TGA)
A Shimazu Model TG-DTA (DTG-60A) thermogravimetric system with a microprocessor driven temperature control unit and a TA data station was used. The weight of the samples was generally in the range of 3 to 6 mg. The sample pan was placed in the balance system equipment and the temperature was raised from 28 to 550°C at a heating rate of 5°C per min. The weight of the sample pan was continuously recorded as a function of temperature.
Scanning electron microscopy (SEM)
The SEM micrographs were used to compare the surface of untreated (blank) and treated papers. For this purpose, the FE-SEM FEI INSPECT F50 was used. The surfaces of three samples treated with Chitosan LMW (1 and 2%) were compared using Scanning Electron Microscope enlarged 10,000 times. The surface of the paper samples were observed visually.
Application of chitosan in paper making
Chitosan samples (low molecular weight and medium molecular weight) were used to process paper production to characterize their ash content, molecular weight and degree of deacetylation. The specifications are shown in Table 2, where their ash content was about 1 and 3%, respectively. The molecular weight was 0.768 × 105 and 2.375 × 105 (g/mol), and the degree of deacetylation was about 91%.
Treated sample properties
Then, chitosan polymer (LMW and MMW) with concentration of 1 and 2% (%/wt) were applied separately into paper production, using wood pulp and also combination with filler (CaCO3) to produce paper sheet. Papers produced were then subjected to testing such as gramature, roughness, stiffness, and water adsorption tests as shown in Tables 3 and 4.
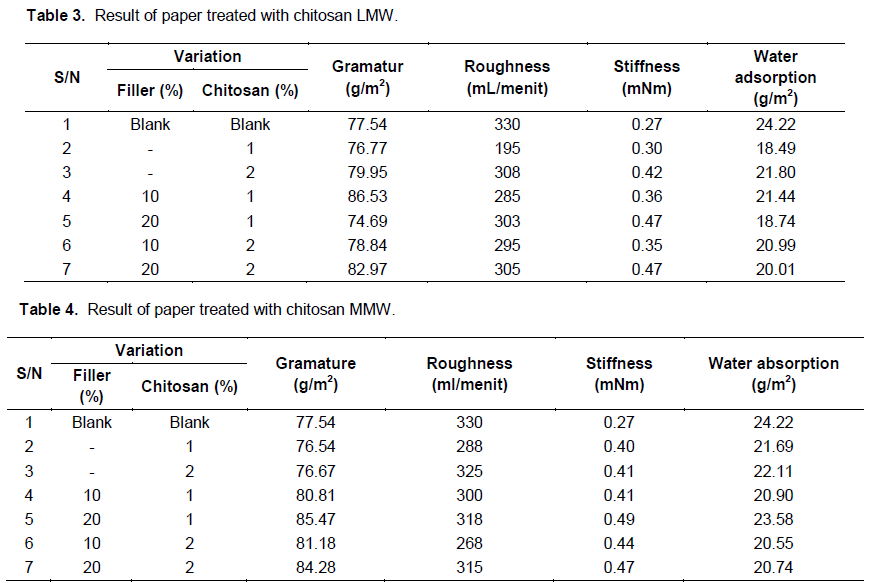
Chitosan LMW with a concentration of 1% (%/wt) can reduce water absorption of paper for about 23.66 to 18.49% compared to other variation (Figure 2). Almost the same result was achieved by sample treated with combination of chitosan 1 and 20%filler. However, the increase of chitosan concentration (2%) in turn increased its water absorption again, although it was still below the absorption blank sample (24.22%). This indicates that chitosan LMW (1%) can be used as sizing agent in paper production. On the other hand, chitosan MMW (1 and 2%) can also reduce water absorption of paper, but not as much as chitosan LMW (1%). Thus, chitosan LMW (1%) also reduces the roughness level of paper almost half from 330 ml/menit (blank sample) to 195 ml/menit (sample of chitosan LMW 1%), this shows that the sample of paper treated with chitosan LMW 1% is smoother compared with sample of blank paper.
Table 3 also shows that all treated samples give higher stiffness value than the blank sample has. Also, all treatments either chitosan or combination with filler can reduce the hardness of paper. However, the reduction of roughness value in sample treated with chitosan LMW 1% (C-1) is the lowest.
As shown in Table 4, chitosan MMW 1% gives lower water absorption value of paper (21.69 g/m2) than blank sample (24.22 g/m2), but it is higher than chitosan LMW by 1%. This indicates that the molecular weight can also have an effect on the water absorption of paper. Figure 3 shows the comparison of water absorption in treated samples. Sample treated with chitosan MMW 1% (Ch-1) has similar gramature with blank sample but it has lower water absorption (21.69). However, sample treated with 1% chitosan and 20% filler gives high gramature (85.47 g/m2) but with slight reduction of water absorption value (23.58 g/m2).
Table 4 also shows that all treated samples give higher stiffness value than blank sample do. Also, all treatments, either chitosan or combination with filler, have an effect to reduce hardness of paper.
The use of chitosan has increased the dry strength of paper, especially chitosan LMW 1%. The increase of concentration however reduced its dry strength. It is known that standard paper is a group of cellulose fibers that are connected with hydrogen bond (Allan and Neogi, 1974; Corte, 1965; Nissan, 1967). This study is supported by Miranda et al. (2013). They found that the low molecular weight native chitosan is more efï¬cient than the medium molecular weight chitosan in all cases when they applied chitosan into paper making process. Bond has to be suitable with distance between segments at fibers, in order for them to form area of inter-fibre bonding together (Vainio, 2007). Even though water molecule is separated from or not of integral relationship with fibers, the presence of water in immense disturbs the cohesion state in the paper. It can be explained that hydrogen bond in the fibre surface is monopolized mainly by water molecule than fiber forms macroscopic fluid bridge. The weakness of this bridge shows wet tensile strength in paper (Yamauchi, 1987). However, water absorption of the composite is mainly due to the cellulosic material. This increases the rates of water uptake by forming the hydrogen bonding between water and the hydroxyl group from cellulosic cell wall fiber (Martino et al., 2005; Reis et al., 2013).
Table 5 shows the result of the dry strength test of papers. The higher tensile strength of paper was reached at chitosan concentration 1% in MMW and LMW. Chitosan 2% concentration tends to reduce paper strength, and this may be caused by the increase of stiffness in paper that affects the reduction of strength. The roll of filler in paper production contributed to the increase of gramature and reduces paper roughness but it did not have much effect to the reduction of water absorption and paper strength. On the contrary, the increase in concentration of filler tends to reduce paper strength and increase water absorption of paper.
Table 5 shows the result of dry strength test in papers. A higher tensile strength of paper was reached at chitosan concentration 1% in MMW and LMW and it got to 2% at chitosan concentration which reduced paper strength. The reduction in strength may be caused by the increase of stiffness in the paper. The roll of filler in paper production contributed to the increase of gramature and reduces the paper roughness though it did not have much effect on the reduction of water absorption and paper strength. On the contrary, the increased concentration of filler tends to reduce paper strength and increase water absorption in paper.
Table 5 also shows that the sample treated with chitosan LMW 1% displays a higher tensile strength 59.97 (Nm/g) compared to blank the sample 49.01 (Nm/g). However, the sample treated with 1% chitosan MMW (60.10 Nm/g) gives a slightly higher tensile strength than that of the sample treated with 1% chitosan LMW. The higher concentration of (2%) chitosan LMW tends to reduce the dry strength of paper. It also occurred in the sample treated with chitosan MMW (2%). This may explain why the more chitosan in the paper, the more the stiffness and reduce elasticity that affects sample brittle will increase.
The role of filler (CaCO3) in paper production tends to increase gramature and smoothness of the paper, but do not have much effect on water absorption and stiffness. Actually, filler tends to reduce the dry strength of the paper. This idea is supported by Hubbe et al. (2016) with their work on filler for paper making. During their research they found that some paper properties, such as folding endurance, appear especially vulnerable to the effects of filler addition. Also, the degree of strength loss depends on many variables, including the properties of the filler.
Scanning electron microscope (SEM) measurement
Several samples treated with chitosan were subjected to
SEM observation, as shown in Figure 4. This observation is focused on sample treated with chitosan LMW (1 and 2%), to analyze the effect of chitosan LMW that was found to give good improvement to paper properties. If these three samples are compared - blank, paper treated with chitosan LMW 1% and paper treated with chitosan LMW 2%- their surface can be seen.
By using SEM apparatus with enlargement of about 10,000 times (10 µm size) (Figure 4a), the surface of fibers in blank sample is coarse (Figure 4b). The surface of fibers in samples treated with 1% chitosan LMW shows smooth and clean (Figure 4c), and the surface of fibers in samples treated with 2% chitosan LMW shows there are deposit of white color.
Chitosan is used as sizing agent in paper (Figure 4). Chitosan forms hydrogen bond formation with fibres, and also also places pores area in fibers and between fibers. As a result of this condition, equilibrium will be created, where functional groups of both cellulose fibers and chitosan reach saturation (Figure 4b). This can cause the absorption of treated sample reduces and the surface is smoother. This can be concluded that chitosan LMW 1% gave optimum saturated into treated sample where functional groups in both cellulose fibers and chitosan forming hydrogen bonds reach equilibrium stochiometry, as a result water absoption of treated sample reduced.
In Figure 4c however, while chitosan reached equilibrium condition in samples, the increase of chitosan concentration will affect chitosan polymer deposited in the surface of the fibres as shown in Figure 4c. As a result, the sample treated with 2% chitosan LMW will increase in water absorption. It will also increase the stiffness properties of paper and reduce the strength.
Thermogravimetric analysis
Thermogravimetric Analysis is used to measure the change in polymer behavior when it is subjected to thermal exposure. In TGA analysis, chitosan polymer is exposed to an increasing temperature slowly until it gets to 550°C. Then the weight loss of polymer during exposure is recorded in the computer. This TGA test is used to measure chitosan polymer and chitosan treated samples.
Figure 5 shows TGA result of blank paper (a) and chitosan polymer (b). In Figure 5a, TGA curve of blank paper sample shows that its moisture is about 8% and the sample starts to degrade at about 300°C and reaches a peak point at 348.04°C, and residue at 515°C about 11.95%. On the other hand, in Figure 5b, TGA curve of chitosan sample shows that its moisture is about 12%, while the sample starts to degrade at 250°C and reaches peak point at 296.68°C, and its residue at 515°C about 37.08%.
By applying chitosan polymer into paper production, it may affect the thermal properties of paper samples. As shown in Figure 6, 1% chitosan LMW does not have effect on the moisture content of treated papers anddegradable temperature but 1% chitosan LMW has increased residue of treated paper about 54% from initial.
This can be attributed to the greater interaction of amino groups in chitosan and the hydroxyl groups in cellulose (Lin et al., 2012). So it may be affected by the nitrogen content present in chitosan.
As shown in Figure 6, paper treated with a combination of chitosan and filler did not give much change on moisture, degradation temperature and residue at 515°C.
The application of chitosan on paper as an additive has several conclusion as the following.
Chitosan low molecular weight with 1% (%/wt) concentration produces paper with low water absorption, smooth and with higher dry strength compared to chitosan medium molecular weight with 1% concentration. Therefore, molecular weight of chitosan has an effect on treated paper properties.
The increase of chitosan concentration (2%) in both LMW and MMW tends to increase water absorption in paper. It may be concluded that the equilibrium stoichiometry reaction occurred between chitosan and cellulose paper in the sample treated with chitosan LMW 1%. This argument is supported by SEM result of chitosan treated paper, where the picture of sample treated with 1% LMW is very clear, and sample treated with 2% chitosan LMW has some white spots that is expected as unreacted chitosan in paper. It may be concluded that chitosan low molecular weight is much better than medium molecular weight which can be used to increase some properties of treated paper. This is in line with what was found by Miranda et al. (2013) whenthey applied chitosan into paper making.
However, the use of filler (CaCO3) in combination with chitosan tends to increase water absorption of samples and no significant change in other properties of sample. CaCO3 filler is hygroscopic material, so it affects water absorption of the treated paper.
It may be settled that chitosan low molecular weight with 1% (%/wt) concentration can be used as sizing agent in paper production.
The authors have not declared any conflict of interests.
The authors would like to express their deepest appreciation to the Ministry of Research Technology and Higher Education, Republic of Indonesia for giving them the chance to carry out the research on the “Process of Chitosan Production and Its Application on Production of High Quality Paper” in 2015. Their appreciation also goes to the Director of Centre for Technology of Industry Process for facilitating and supporting the research. A special thanks to all team members.
REFERENCES
|
Allan GG, Neogi AN (1974). Fundamental of Fiber Assemblages: A Unifying Theory for the Tensile Strength of Paper and Nonwoven. Cellul. Chem. Technol 8:297-318.
|
|
|
|
Amaral IF, Sampaio P, Barbosa MA (2006). Three-dimensional culture of human osteoblastic cells in chitosan sponges: the effect of the degree of acetylation. J. Biomed. Mater. Res. 76A:335-346.
Crossref
|
|
|
|
|
Atlan GG, Crosby GD, Sarkanen KV (1975). Proceedings of the 1975 International Paper Physics Conference, Technical Association of the Pulp and Paper Industry, Inc., Atlanta, IJSA pp. 109-115.
|
|
|
|
|
Anonim (2008). Chitosan: Apakah manfaat chitosan? Naturakos III(7):10-12.
|
|
|
|
|
Butkinaree S, Jinkarn T,Yoksan R (2008). Effects of Biodegradable Coating on Barrier Properties of Paperboard Food Packaging, J. Metals Mater. Miner. 18:219-222.
|
|
|
|
|
Chalonglarp T, Sorada K, Neeracha S, Rath P, Tanom B, Yasuhiko T, Siriporn D (2007). The influence of molecular weight of chitosan on the physical and biological properties of collagen/chitosan scaffolds. J. Biomater. Sci. Polym. Ed. 18:147-163.
Crossref
|
|
|
|
|
Corte HK (1965). "Composite Materials" (Ed. L. Holiday), Elsevier Publishing Co., Amsterdam pp. 490-495.
|
|
|
|
|
Czechowska-Biskup R, JarosiÅ„ska D, Rokita B, UlaÅ„ski P, Rosiak JM (2012). Determination of Degree Of Deacetylation Of Chitosan – Comparision Of Methods, Prog. Chem. Appl. Chitin and Its Deriv. 17:5-20.
|
|
|
|
|
Dhiman HK, Ray AR, Panda AK (2004), Characterization and evaluation of chitosan matrix for in vitro growth of MCF- 7 breast cancer cell lines. Biomaterials 25:5147-5154.
Crossref
|
|
|
|
|
Gavhane YN, Gurav AS, Yadav AV (2013), Chitosan and its applications: A Review of Literature, Int. J. Res. Pharm. Biomed. Sci. 4(1):312-331.
|
|
|
|
|
Gopakumar K (2002). Textbook of fish processing technology, Indian Council of Agricultural Research New Delhi, pp. 467-483.
|
|
|
|
|
Habibie S (1996), PhD Theses, in University of Leeds.
|
|
|
|
|
Habibie S (2014). Chelation and Metal-ion Complex Formation of Chitosan Treated Cotton, Majalah Pengkajian Industri, 8(3):93-100
|
|
|
|
|
Hardjito L (2006). Chitosan lebih awet dan aman (online).
View
|
|
|
|
|
Houbin L, Du Y, Xu Y (2004). Adsorption and complexation of chitosan wet-end additives in papermaking systems, J. Appl. Polym. Sci. 91(4):2642-2648.
Crossref
|
|
|
|
|
Hubbe MA, Gill RA (2016). "Mineral fillers for paper," BioResources, 11(1):2886-2963.
|
|
|
|
|
Hudson SM, Smith C (1998). Polysaccharide: chitin and chitosan: chemistry and technology of their use as structural materials, Biopolymers from renewable resources, edited by DL Kaplan (Springer-Verlag, New York) 96-118.
|
|
|
|
|
Kuusipalo J, Kaunisto M, Laine A, Kellomäki M (2005). Chitosan as a coating additive in paper and paperboard, TAPPI J. 4(8)17-21.
|
|
|
|
|
Lin S, Chen L, Huang L, Cao S, Luo X, Liu K, Huang Z (2012), Preparation and characterization of chitosan/cellulose blend films using ZnCl2.3H2O as a solvent, peer-reviewed article, BioResources 7(4):5488-5499.
Crossref
|
|
|
|
|
Martino AD, Sittinger M, Risbud MV (2005). Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26:5983-5990.
Crossref
|
|
|
|
|
Miranda R, Nicu R, Latour I, Lupei M, Bobu E, Blanco A (2013). Efï¬ciency of chitosans for the treatment of papermaking process water by dissolved air flotation, Chem. Eng. J. 231:304-313.
Crossref
|
|
|
|
|
Muzzarelli RAA (1977). Chitin, Pergamon. Oxford, Chapter 6.
|
|
|
|
|
Nada AMA, El-Sakhawy M, Kamel S, Eid MAM, Adel AM (2005). Effect of Chitosan and Its Derivates on the Mechanical dan Electrical Properties of Paper Sheets, Egypt. J. Solids 28(2):359-377.
|
|
|
|
|
Nissan AH (1967). "Surface and Coatings Related to Paper and Wood" (Eds. R. H. Marchessault, C. Skaar), Syracuse University Press, Syracuse, NY pp. 221:228.
|
|
|
|
|
Pitaloka AB, Saputra AH, Nasikin M (2013). Water Hyacinth for Superabsorbent Polymer Material, World Appl. Sci. J. 22(5):747-754.
|
|
|
|
|
Puvvada YS, Vankayalapati S, Sukhavasi S (2012). Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry, Int. Curr. Pharm. J. 1(9):258-263
Crossref
|
|
|
|
|
Reis AB, Yoshida CMP, Vilela ESD, Nascimento RD, Melo IS, Franco TT (2013). Biodegradability Kraft Paper Coated with Films Emulsified Chitosan and Palmitic Acid, J. Res. Updates Polym. Sci. 2:122-131.
Crossref
|
|
|
|
|
Rismana E (2004). Chitin dan Chitosan.
|
|
|
|
|
Struszczyk MH (2002), Applications of chitosan, Polymery 47:396-340
|
|
|
|
|
Sugita P (2009). Chitosan: Sumber Biomaterial Masa depan, IPB, Bogor.
|
|
|
|
|
Thein HWW, Kitiyanant Y (2006). Chitosan scaffolds for in vitro buffalo embryonic stem-like cell culture: An approach to Tissue Engineering. J. Biomed. Mater. Res. 80:92-101.
|
|
|
|
|
Tiyaboonchai W (2003). Chitosan nanoparticles: A promising system for drug delivery, Naresuan University J. 11(3):51-66.
|
|
|
|
|
Vainio A (2007). Interfibre bonding and fibre segment activation in paper – observations on the phenomena and their influence on paper strength properties, Doctoral Thesis - Helsinki University of Technology, Laboratory of Paper and Printing Technology, Reports, Series A29, Nov, 30, 2007.
|
|
|
|
|
VandeVord PJ, Matthew HWT, DeSilva SP, Mayton L, Wu B, Wooley PH (2002). Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 59: 585-590.
Crossref
|
|
|
|
|
Yamauchi T (1987). Measurement of paper thickness and density. Appita 40(5):359-369.
|
|