ABSTRACT
The objective of this study was to determine the potential of using Malaysian silica sand as the SiO2 raw material in producing leucite (SiO2-Al2O3-K2O) glass-ceramics. The crystallization, mechanical and biological properties of the glass-ceramic was studied. A starting glass composition in the system of leucite was melted in an electric furnace, quenched in deionized water and dry milled to obtain glass powder. The glass powders were ball milled and compressed to form 13 mm x 10 mm pellet. The thermal analysis, phase composition, microstructure, flexural strength and cytotoxicity of the glass-ceramics were investigated. Thermal analysis showed that crystallization of the glass occurred at the range of 650 and 850°C. The pellets were sintered at 650, 700, 750, 800 and 850°C for 1.0 h. The effect of sintering time on crystallization was also studied through five different soaking time at 3.0, 6.0, 9.0 and 12.0 h. The crystallization depends on the temperature and time of sintering. At 700°C, leucite began to form with minor phase of sanidine. The peak intensity increased as the temperature was increased up to 850°C. For sintering time 3.0 to 12.0 h, the peak intensity of leucite and sanidine were increased but microcline was formed as a minor phase. The microstructure analysis showed that the dendritic leucite and prismatic sanidine. The leucite glass-ceramics appeared translucent. The flexural strength values (80 to 175 MPa) were comparable with commercial product (112 to 140 MPa). The in vitro bioactivity results prove that the leucite glass-ceramics sample can be classified as a bio-inert and non-cytotoxity material and can be used for restorative dental products.
Key words: Silica, glass-ceramics, leucite, sanidine, dental, bioactivity, bio-inert, cyctotoxicity.
Silica sand is the main raw material for production of silicates glass. Glasses in general term are not advanced materials but through refining in composition, heat treatment and manufacturing, glasses can be transformed into a new class of material called glass-ceramics. Glass-ceramics are polycrystalline materials and produced through controlled crystallization during heat treatment process of glass. Currently, the application of glass-ceramics as biomaterial for dental products is growing. For example, leucite glass-ceramics are widely used in dentistry as restorative dental material to fabricate dental inlays, crowns, bridges and veeners prostheses. Leucite glass-ceramics can be produced by sintering with surface crystallization of the glass powder. This processing route involves melting, quenching, milling of glass frit, and sintering in order to promote crystallization of glass-ceramics. Glass-ceramics based on leucite show exceptional biocompatibility, and good physical, chemical and mechanical properties (Ahmad, 2006; Cattel et al., 2005).
Since the 1990s, efforts to develop biomaterials for restorative dentistry have been concentrated on producing metal-free systems. An important milestone in this respect was reached in the development of glass-ceramics containing leucite (K2O -Al2O3- SiO2) (Rheinberger, 1997). Glass-ceramics have been successfully used for many years in dentistry to construct crowns and fixed partial bridges due to the properties of high mechanical strength, chemical inertness, wear resistance, aesthetics and low density. Presently, leucite, mica and lithium disilicate glass-ceramics are widely used as restorative materials (Apel et al., 2007). These materials are particularly suitable for fabricating single units such as dental inlays crowns and veneers because of its special optical properties (Lee et al., 1997). The aim of this project was to investigate the effect of using natural Malaysian silica sand as the SiO2 raw material on the phase crystallization, microstructure, flexural strength, in vitro bioactive and cytotoxicity of leucite glass-ceramics.
Natural silica sand sample was taken from Terengganu. The sample was wet screened and a fraction 75< to <150 µm was prepared in order to comply with the requirement of traditional glass production technology for glass melts. Chemical composition of the silica sand sample was determined by X-ray fluorescence analysis (Rigaku, Japan). The composition weight percent of the starting glass selected for the study was; 64.2% SiO2, 16.1% Al2O3, 11.9% K2O, 5.1% Na2O, 1.7% CaO, 0.5% TiO2 and 0.5% LiO2. All oxides used were with purity in the range of 90 to 99% from Merck, Germany; Unilab, Australia and Hamburg Chemical. The batches were placed in plastic bottles and mixed using a Heldoph Reax 2 Shaker, Germany for 8.0 h in order to make sure the raw materials were homogenized. Zirconia balls were used as the media in the mixing process. Samples were melted in alumina crucibles in a bottom-loading high temperature furnace (Modutemp, Australia) at 10°C/min to 1450°C and held for 3.0 h, and then quenched in cool water to produce glass frit. The glass powders were milled (Retsch PM-400, Germany) for 1 h and sieved to the required size of less than 75 µm. The crystallization temperature of the glass powder was studied using Differential Thermal Analysis, DTA (Linseis, Germany) with a 10°C/min heating rate at temperature from 25 to 1100°C in a dry air atmosphere. The glass powder was cold pressed using a laboratory hydraulic hand press (Carver, USA) to obtain green compact in the form of cylindrical pellet of 13 mm x 10 mm. Pellets were heat treated in electric furnace (Termo Temp, UK) at 700, 750, 800 and 850 °C at a heating rate of 2 °C/min and 1.0 h soaking time to study the crystallization behaviour of glass. The effect of sintering time on crystallization was also studied through five different soaking time at 3.0, 6.0, 9.0 and 12.0 h.
XRD (D8 Advanced, Bruker, Germany) was used for the identification of the crystalline phases in the glass-ceramics using Cu Kα radiation at a scan speed of 2°/min for 2θ from 10 to 80°. Microstructure studies were done using field emission scanning electron microscopy (FESEM) (Supra 40 VP, Germany). Before viewing with FESEM, glass-ceramics specimens were embedded, polished and etched with 30% hydrofluoric acid for 7 s. The specimens were platinum coated and viewed using the secondary electron detector. The flexural strength of sintered glass-ceramics was measured using three points bending test with bars of 20 mm x 5 mm x 5 mm (INSTRON, UK, 0.5 mm/min displacement).
The biocompatibility of glass-ceramics sample after heat treatment at 850°C for 9.0 h was examined by in vitro bioactivity and cytotoxicity test. The in vitro bioactivity test was conducted in Kokubo's simulated body fluid, SBF, which contains almost the same inorganic constituent as human body plasma. The sample was brought into contact with SBF fluid for 10 and 20 days. XRD was used to characterize the formation of apatite on the glass-ceramic surfaces. Cytotoxicity of the leucite glass-ceramics was evaluated by testing on extracts of leucite glass-ceramics according to ISO 10993-5:2009(E) and ISO 10993-12:2012(E). American Type Culture Collection L-929 mouse subcutaneous connective tissue fibroblast cells (Mus musculus, NCTC clone 929, CCL-1) were used in this test. Zinc sulphate at 240 µg/ml and complete growth medium were used as the positive and negative control, respectively. The test material was tested in triplicate at concentration of 6.25, 12.5, 25, 50, 100 and 200 mg/ml.
Chemical analysis result of the silica sand is presented in Table 1. The result reveals that the natural silica sand sample is highly pure and contains very low concentrations of impurities. The content of Fe2O3 is low. Generally, typical acceptable value of Fe2O3 in silicate glass product is between 0.02 to 0.03% (Malaysian Standard MS 701:1981).

The XRD pattern as shown in Figure 1 identifies the main mineralogical content of the silica sand sample to be quartz. Quenching the glass melts in water at room temperature resulted in transparent and colourless glass frit. The XRD pattern of the glass sample as shown in Figure 1 indicates the present of amorphous glass phase and did not show any evidence of crystalline phase in the glass. The DTA result of the quenched glass powder is shown in Figure 2. The DTA was used to identify the glass transition and crystallization temperature of the glass sample. The temperatures behaviour of a glass can provide information of glass-ceramics fabrication.
According to Sooksaen et al. (2010), the glass transition value of SiO2-Al2O3-K2O glass system is at about 594 to 638°C (Sooksaen et al., 2010). The DTA curves show that no clear endothermic peak was observed related to glass transition.
However, an exothermic peak associated with the crystallization temperature of glass to form glass-ceramic was in the range of at about 650 to 850°C. Consequently, heat treatment was performed in this study at 700, 750, 800 and 850°C, respectively to form the glass-ceramics, assuming phase evolution was completed at each isothermal hold of 1.0 to 12.0 h.
The glass-ceramics appearance after sintering at 700°C was slightly translucent and white. Increasing the sintering temperature up to 800°C has transformed the samples to become more white and opaque. The XRD patterns of glasses that were heat-treated at 700, 750, 800 and 850°C for 1.0 h are shown in Figure 3. The results indicated the presence of cubic leucite, KAlSi2O6, (ICDD: 00-038-1423) and sanidine, (K,Na)(Si3Al)O8, (ICDD: 00-019-1227). It is also proved that the amount of leucite and sanidine phase for each heat-treated glass composition was increased with the increasing of heat treatment temperature.
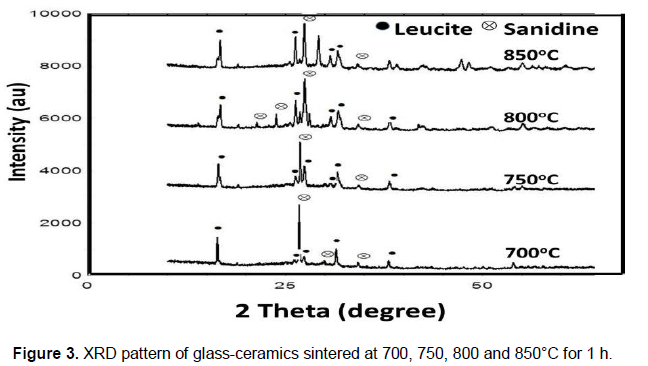
The effect of sintering time on the crystallization is shown in Figure 4. As the sintering time was increased, all peaks associated with cubic leucite and sanidine became stronger. However, for sintering temperature at 700 and 750°C and soaked 6 h, microcline (ICDD: 00-019-0932) started to form as a minor phase. While for 800 and 850°C microcline started to crystallize within 3.0 h soaking time. The cry stallization of microcline was previously identified in leucite reinforced glass-ceramics in SiO2-Al2O3-K2O glass system (Chen et al., 2010). Figure 5 shows the FESEM micrographs of glass-ceramics sample sintered at 850°C for 1.0 h and 9.0 h. The formation of dendritic cubic leucite and prismatic sanidine phases is clearly observed. A previous study by Holand and Beall (2002) on crystallization mechanisms in glass-ceramics showed that the early stages of bulk leucite growth have been due to dendrites growing in preferred crystallographic directions (Holand and Beall, 2002). The micrographs also show there were no obvious micro cracks.
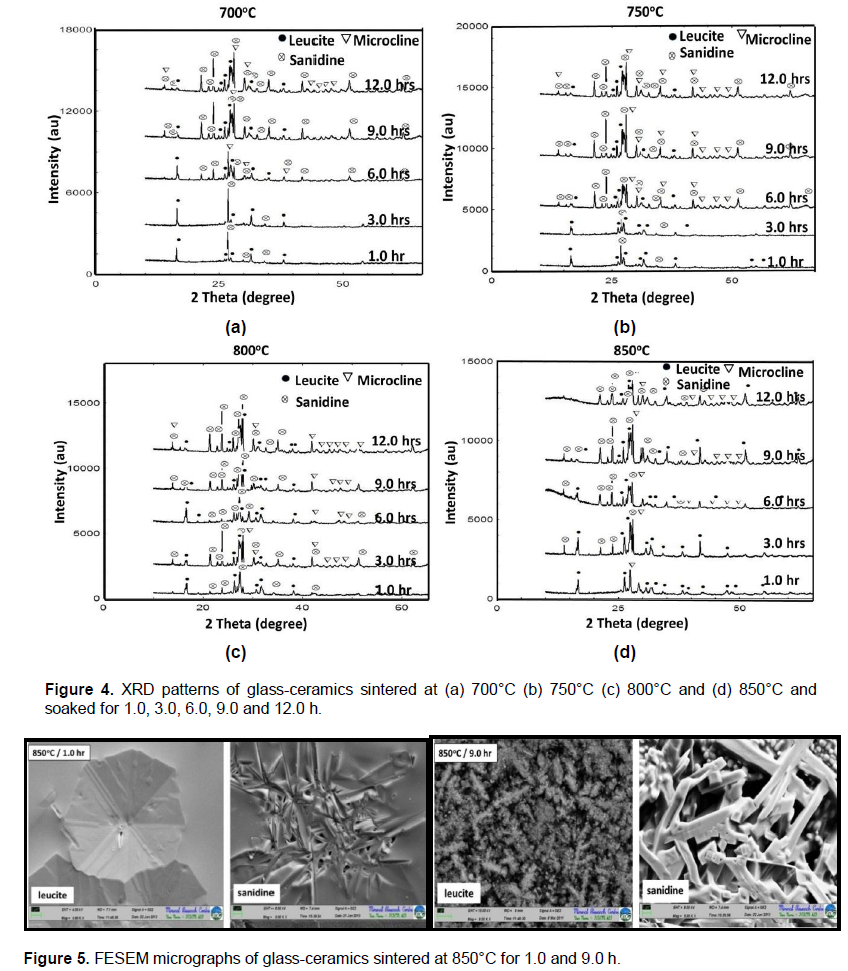
Figure 6 shows the graph of the flexural strength results of the glass-ceramics sintered at 700, 750, 800 and 850°C and soaked at 1.0, 3.0, 6.0, 9.0 and 12.0 h. The flexural strength of leucite glass-ceramics was increased as the time and sintering temperature was increased. It could be due to the high volume of crystalline phases and the existence of prismatic sanidine. The higher flexural strength, 175 MPa was achieved for sample sintered at 850°C for 9.0 h. However, the flexural strength decreased after soaking for 9.0 h at all temperatures. This is probably due to the glass-ceramics which started to melt and part of crystallite began to dissolve in the residual glass phase. The flexural strength values of glass-ceramics samples were comparable with commercial product of which is 112-140 MPa (El-Meliegy and Noort, 2011).
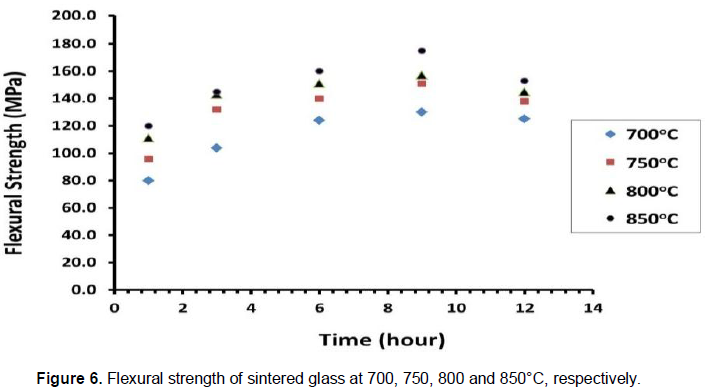
Glass-ceramics for restorative dental applications must fulfill the standard tests for biomaterial use, such as compatibility with the oral environment. Bioactivity on the surface of the dental restoration did not occur in XRD patterns of sintered glass-ceramics before and after soaking in SBF for several days as shown in Figure 7. Patterns show that there is no presence of apatite layer on the surface of glass-ceramics sample after 20 days of immersion in SBF and that indicated the sample is inert bioactive materials.
In this study, the MTT assay is used to evaluate the toxic characteristics of the leucite glass-ceramics and the yellow tetrazolium salt (MTT) is reduced in metabolically active cells to form insoluble purple formazan crystals which are solubilized by the addition of a solvent (dimethyl sulfoxide). Cell viability is qualified by colorimetric enumeration whereby a low optical density (OD) reading corresponds to low cell viability which is associated with a loss in mitochondrial dehydrogenase activity.
The test material leucite glass-ceramics did not inhibit the viability of L929 cells at all concentrations following 24-hour treatment as shown in Table 2 and Figure 8. Table 2 also shows that at all concentrations of test material, the viability (%) of active cell is ≥90 and this means that the leucite glass-ceramics did not demonstrate a cyctotoxic effect at all concentrations under the condition of the study. Both negative and positive controls performed as anticipated. In MTT assay, the well with the highest absorbance indicates the highest cell viability.
The highly pure natural Malaysian silica sand can be used as SiO2 source for producing leucite glass-ceramics without any further chemical upgrading. Sintering of SiO2-Al2O3-K2O glass powder at 700 to 850°C for duration of 1.0 to 12 h contributed to the crystallization of cubic leucite and sanidine with minor phases of microcline. The leucite glass-ceramics has a flexural strength comparable with commercial product. The absence of apatite layer on the surface indicated that it is an inert bioactive material. The leucite glass-ceramics is ranked non-cytotoxic in terms of in vitro cellular response to human cell lines under the prevailing test conditions.
The authors would like to thank the Director of Mineral Research Centre, Department of Minerals and Geoscience Malaysia and all staff of Mineral Research Centre who were involved and had contributed in this research work.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmad N (2006). Production of high purity silica from Malaysian silica sand, Phd. Thesis, University of Leeds, UK.
|
|
|
|
Cattel MJ, Knowles JC, Clarke RL (2005). The crystallization of an aluminosilicate glass in the K2O-Al2O3-SiO2 system, J. Dent. Mater. 21:811-822.
Crossref
|
|
|
|
|
Rheinberger V (1997). Perspectives in dental ceramics. Glastech Ber. Glass Sci. Technol. 70C:339-400.
|
|
|
|
|
Apel E, Hoen CV, Rheinberger V, Holand W (2007). Influence of ZrO2 on the crystallization and properties of lithium disilicate glass-ceramics derived from a multi-component system. J. Eur. Ceramic Soc. 27:1571-1577.
Crossref
|
|
|
|
|
Lee HH, Kon M, Asaoka K (1997). Influence of modification of Na2O in a glass matrix on the strength of leucite-containing poercelains. Dent. Mater. 16:134-143.
Crossref
|
|
|
|
|
Sooksaen P, Boonmee J, Witpathomwong C, Likhitlert S (2010). Effect of K2O/SiO2 ratio on crystallization of leucite in silicate based-glasses, J. Metals Mater. Minerals 20(1):11-19.
|
|
|
|
|
Chen X, CT, Wilson R, Hill R, Cattell M (2010). Crystallization of high-strength fine-sized leucite glass-ceramics. J. Dent. Res. 89:1510-1516.
Crossref
|
|
|
|
|
Holand W, Beall GH (2002). Glass-ceramic technology. American Ceramic Society, Ohio, USA.
|
|
|
|
|
El-Meliegy EM, Noort R (2011).Glasses and glass-ceramics for medical applications, Springer.
|
|