ABSTRACT
The endeavor of this paper was to review cancer immunotherapy which means the modulating and using of the patient’s own immune system to target the cancer cells rather than using an extrinsic means of therapy. The best way to prevent and remove infections is through the natural 'sterilising' action of the immune response that combines elements of both innate and adaptive immunity to ward off foreign pathogens without medical intervention. The use of immunostimulants, non-specific approach, for cancer therapy is one of the earliest approaches in immunotherapy that aims to enhance the activity of the lymphocytes that are already attacking to the tumour cells but are insufficient to produce a full-powered immune response. In this review, radioimmunotherapy (coupling a radioactive atom to a monoclonal antibody (mAb)), immunotoxins (generated by coupling plant-derived or bacterial toxins to mAbs), antibody-directed enzyme prodrug therapy (an antibody is used as a vector to transfer an enzyme) and immunomodulators were among the discussed approaches to use mAbs as an anti-cancer. A new and promising immunotherapy that is especially highly effective against metastatic melanoma, adoptive cell therapy (ACT), and different cancer vaccines were also reviewed in detail.
Key words: Adoptive cell therapy, cancer immunotherapy, cancer vaccines, immunostimulants, monoclonal antibodies.
Cancer immunotherapy, also called biological therapy of cancer, means the modulating and using of the patient’s own immune system to target the cancer cells rather than using an extrinsic means of therapy. In that manner, cancer immunotherapy focuses on developing agents that activates or enhances the immune system’s recognition and killing of the cancer cells (Sharma et al., 2011).
Cancer immunotherapies can be either passive or active (Todar, 2008). Passive therapy is based on the adoptive transfer of immunomodulators (including cytokines), tumor-specific antibodies, or immune cells (Rescigno et al., 2007). These substances, or cells, are then administered to the patient to initiate an antitumor action. In general, this therapy does not generate immunologic memory and, therefore, require chronic infusion-based treatment (McNeel et al., 2007). Several passive immunotherapies have been approved for use in breast cancer, melanoma, renal cell carcinoma, leukemia, and various other hematologic and solid tumors (Dudley et al., 2005).
Active immunotherapy, on the other hand, stimulates the patient’s immune system, with the intent of promoting an antigen-specific antitumor effect using the body’s own immune cells (McNeel et al., 2007). In addition, active immunotherapy seeks to create a durable antitumor response that can protect against minimal residual disease and tumor recurrence (Marrari et al., 2007).
Immunotherapy can be further divided into nonspecific and specific types: nonspecific immunotherapy involves the administration of cells or substances that are not targeted to a specific antigen. Lymphokine activated killer (LAK) cell therapy is an example of a nonspecific cellular immunotherapy currently being investigated for the treatment of cancers such as melanoma, renal cell carcinoma, and non-Hodgkin’s lymphoma (Clark et al., 1990).
This approach is based on the concept that leukocytes activated ex vivo by interleukin-2 can lyse tumor cells that are resistant to natural killer cells. Other types of non-specific approaches have been approved for renal, bladder, and other cancers, and may exert a wide range of effects on the immune system (Emeryville, 2008).
Conversely, active specific immunotherapy involves the priming of the immune system in order to generate a T-cell response against tumor-associated antigens (Kipp and McNeel, 2007). One example of the active specific approach is adoptive T-cell therapy, which involves the ex vivo cultivation of T cells with demonstrated activity against a specific target cancer antigen. The goal is to increase the frequency of these T cells to achieve therapeutic levels and then infuse them back into the patient. This approach is highly specific and has been investigated for the treatment of melanoma (Wrzesinski et al., 2007).
The use of immunostimulants for cancer therapy is one of the earliest approaches in immunotherapy. It is a non-specific approach that aims to enhance, in general, the activity of the lymphocytes that are already attacking to the tumour cells but are insufficient to produce a full-powered immune response. In this manner, this strategy uses the patient’s own immune system as the effecting factor. In the late 1980s and early 1990s, the most important cytokines for cancer therapy, interleukin-2 (IL-2) and alpha-interferon (IFN-α), demonstrated their anti-cancer properties and were approved by the U.S. Food and Drug Administration (FDA) for the treatment of diverse types of cancers including metastatic melanoma and renal cell carcinoma (Kirkwood et al., 2008).
Alpha-interferons are proteins belonging to the type-I IFN family which was discovered decades ago for its anti-viral properties (Belardelli, 1995). The human IFN-α family consist of at least 13 functional subtypes which share the same receptor system and very similar biological functions (Mogensen et al., 1999). These diverse biological functions include the activation and regulation of both innate and adaptive immune system by enhancing the effects of macrophage and natural killer (NK) cells, increasing the expression of major histocompatability (MHC) class-I antigens and regulating the proliferation and survival of both helper and cytotoxic T-cells.
Alpha-interferon (IFN-α) has also direct effects on cancer cells by its apoptotic, antiangiogenic and antiproliferative properties (Belardelli et al., 2002). In today’s immunotherapy regimes, IFN-α is the most used cytokine for the treatment of more than a dozen types of cancer, such as hairy cell leukemia, chronic myeloid leukemia, B and T cell lymphomas, melanoma, renal carcinoma and Kaposi’s sarcoma (Pfeffer et al., 1998 ).
Inteleukin-2 (IL-2) is a glycoprotein which is a strong T-cell and NK-cell growth factor that plays a key role in immune regulation and lymphocyte proliferation. Unlike IFN-α, IL-2 has only indirect anti-cancer effects through the activation of the effector lymphocytes which are also called lymphokine-activated killer cells (Fang et al., 2008). The drawbacks of this immunotherapy are its high cost and its severe but reversible adverse effects. Nevertheless, to this date, IL-2 remains to be an indispensible immunotherapeutic agent for the treatment of metastatic melanoma (Fang et al., 2008).
Apart from IL-2 and IFN-α, Bacillus Calmette-Guerin (BCG), Levamisole and Granulocyte-macrophage colony-stimulating factor (GM-CSF) are also being used as immunostimulants over the years for immunotherapy, but mostly in combinations with other immunotherapies or other strategies for anti-cancer therapeutics (Waller, 2007).
CANCER THERAPY WITH MONOCLONAL ANTIBODIES
A century ago, an idea was set forth by Paul Ehrlich that suggested the use of antibodies to selectively target tumours. Over the years, this concept became applicable with the development of hybridoma technology by Kohler and Milstein and further by the generation of chimeric and humanized monoclonal antibodies (mAbs) to increase their immunogenicity and their ability to activate and channel the effector immune mechanisms (Adams and Weiner, 2005). Today, the monoclonal antibodies play a crucial role in cancer immunotherapy through their diverse range of effects and targets. The mechanisms of mAb actions include direct toxicity which consists of antibody-dependent cellular cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC), directing and enhancing the activity of effector immune cells, slowing tumour growth and delivering radioactive isotopes, toxins or chemotherapeutic drugs to tumour cells (Bisht et al., 2010) Figure 1. Antibody-dependent cellular cytotoxicity (ADCC) and CDC outline one of the common mechanisms of the mAbs. ADCC can be considered as a mechanism to directly provoke an acute tumour destruction in variable levels which also leads to antigen presentation and the activation of adaptive immune components against cancer cells (Adams and Weiner, 2005).
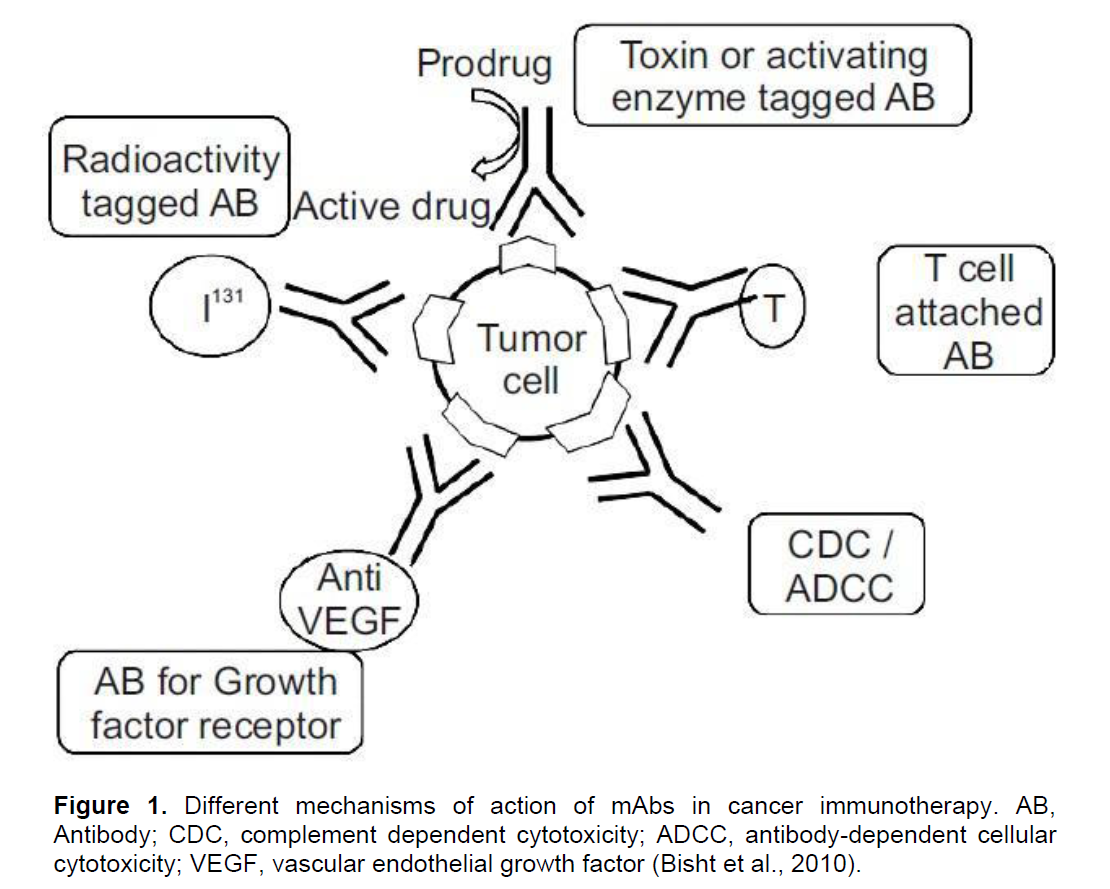
CDC acts on cell membranes where the complement cascade ends up forming a 100 Å pores that result in cell death because of the uncontrolled passage of contents into and out of the cell (Gelderman et al., 2004). The applications and efficacy of the mAbs for anti-cancer therapies can be further improved by administrating them in combination with other anti-cancer therapies such as chemotherapy, radiotherapy, targeted therapy agents and cancer vaccines (Weiner et al., 2010).
Radioimmunotherapy
One of the techniques to extend the use of monoclonal antibodies is to couple a radioactive atom to a mAb which is targeting a tumour specific antigen. This approach is called radioimmunotherapy in which, the goal is to limit the application of the deadly radiation to those of tumour cells and keep the toxicity at minimal for the healthy cells (Dimberu and Leonhardt, 2011).
There are currently two FDA approved radioimmunotherapy agents that are being used for the treatment of B-cell malignancies; the radioactive isotope yttrium-90 with an IgG1 mAb against CD20 antigen on B-cells, 90Y-ibritumomab tiuxetan (Zevalin®), and the radioactive isotope iodine-131 with an IgG2a mAb that is also against CD20, 131Itositumomab (Bexxar®) (Waldmann, 2003).
Immunotoxins
Immunotoxins are generated by coupling plant-derived or bacterial toxins to mAbs that target specific antigens on the surface of cancer cells. The first developed toxins for this purpose included gelonin, ricin, abrin, pokeweed antiviral protein, Pseudomonas exotoxin and diphtheria toxin. However, due to several drawbacks of these techniques such as rapid clearance from blood stream and immunogenicity led to the generation of the second cohort immunotoxins such as BL22 and moxetumomab pasudotox (Teicher and Chari, 2011). Both immunotoxins are anti-CD22-Pseudomonas exotoxins that are recently being tested in clinical trials for the treatment of B-cell malignancies and other hematological malignancies (Kreitman and Pastan, 2011).
Antibody-directed enzyme prodrug therapy
Another approach to use mAbs as an anti-cancer therapy is called antibody-directed enzyme prodrug therapy (ADEPT) where an antibody is used as a vector to transfer an enzyme that is capable of activating an initially nontoxic drug, called a "prodrug," to a potently cytotoxic agent for tumour cells (Melton and Sherwood, 1996).
In this method, an antibody-enzyme conjugate is injected and allowed to localize at the tumour cells depending on the specificity of the antibody. Then, the prodrug that should be converted to a cytotoxic agent is administered only within the tumour tissue where the activating enzyme resides even though the initial reaction towards the ADEPT technology was promising; this approach has not been further developed due to its drawbacks, such as immunogenicity of the enzyme components, short half-life of the conjugates and the observed little anti-tumour activity from in vivo studies (Teicher and Chari, 2011).
Immunomodulators
There are several key regulatory elements, also called ‘immune-checkpoints’, in the immune system that manages the level of immune response by the means of down regulation and inhibition to restore the homeostasis. These critical elements are absolute necessity for the development of self-tolerance and to prevent autoimmunity, however, tumour cells constantly benefit from this property of the immune system in order to escape from its destructive power (Dimberu and Leonhardt, 2011).
There are several approaches to prevent this inhibition of immune response and to enhance the duration and activation of the T-cell mediated immunity; increasing the expression of co-stimulatory factors on the surface of dendritic cells (DC) by the CD-40 or toll-like receptor 9 (TLR-9) stimulation of DCs (Krieg, 2006), and enhancing and prolonging the T-cell activation by inhibiting the cytotoxic T-lymphocyte antigen-4 (CTLA-4) or programmed death-1 (PD-1) binding to B7 or to PD1 ligand (PDL-1), respectively (Ribas et al., 2005).
CTLA-4, a homolog of CD-28 that functions as an inhibitory receptor for B7 co-stimulatory molecules on mature antigen presenting cells (APC), is the main negative regulatory element of the T-cell mediated anti-tumour immune response since its binding to B7 down regulates the T-cell activation (Kirkwood et al., 2008). Two anti-CTLA-4 mAbs that are in clinical trials now, Ipilimumab and Tremelimumab, therefore were developed to block the CTLA-4 binding to B7 through their higher affinities for the CTLA-4 than the B7 and so, competitively inhibiting the down regulation of T-cells (Ribas et al., 2005). Ipilimumab binds and blocks CTLA-4 and has recently shown striking clinical successes against metastatic melanoma that has led to its FDA approval in 2011 (Dimberu and Leonhardt, 2011) Figure 2.
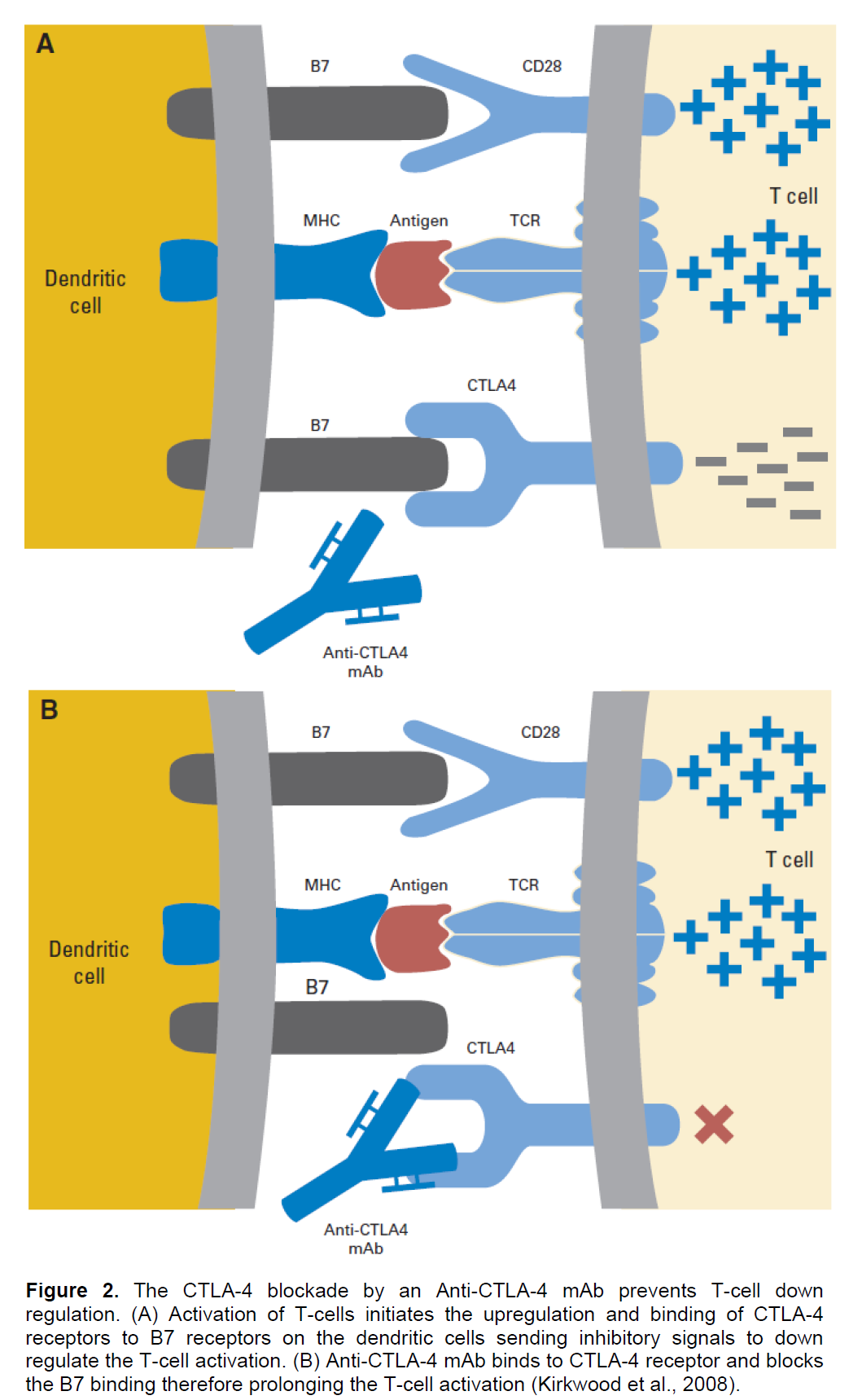
Apart from the CTLA-4 and PD-1 blockade, another recently developed approach to avoid the suppression and to enhance the activation of immune response is the increase of the expression of co-stimulatory factors on the surface of DCs by a TLR-9 agonist. Toll like receptor-9 (TLR-9) is an intracellular receptor that recognizes un-methylated cytosine-guanine (CpG) dinucleotides which are frequently found in viral and bacterial DNAs (Krieg, 2002).
Stimulation by TLR-9 agonist induces the activation/maturation of the DCs by resulting in an increase in surface expression of co-stimulatory molecules, secretion of cytokines/chemokines, activation of NK-cells, and antigen presentation (West et al., 2004; Pashenkov et al., 2006) Figure 3.
Adoptive cell therapy (ACT) is a new and promising immunotherapy that is especially highly effective against metastatic melanoma (Rosenberg and Dudley, 2009). In ACT, the T cells of a patient that have anti-tumour activity are identified, isolated, grown ex vivo, further stimulated by the tumour-APCs and infused back to the same patient (Dimberu and Leonhardt, 2011).
Before this infusion of the high amounts of tumour-infiltrating lymphocytes (TILs), the host can be manipulated in order to increase the effectiveness of the transferred cells. Patients normally undergo a lymphodepletion with either chemotherapy or body irradiation before the infusion that does not only provides the elimination of the regulatory T cells which have immunosuppressive activities, but also eliminates the other lymphocytes that can compete with the transferred cells for cytokines that are essential for T-cell survival such as interleukin 7 (IL-7) and interleukin-15 (IL-15) (Rosenberg and Dudley, 2009). For the same purpose, vaccines or growth factors, such as IL-2, can also be infused along with the transferred cells (Rosenberg et al., 2008) Figure 4.
Cancer vaccines probably create one of the most diverse classes in the immunotherapeutic approaches where it is also the case for the use ofmonoclonal antibodies. The development of cancer vaccines can be divided into two groups; preventative, also called prophylactic, and therapeutic. These groups are also further sub-grouped and some examples of each are briefly discussed here (Table 1).
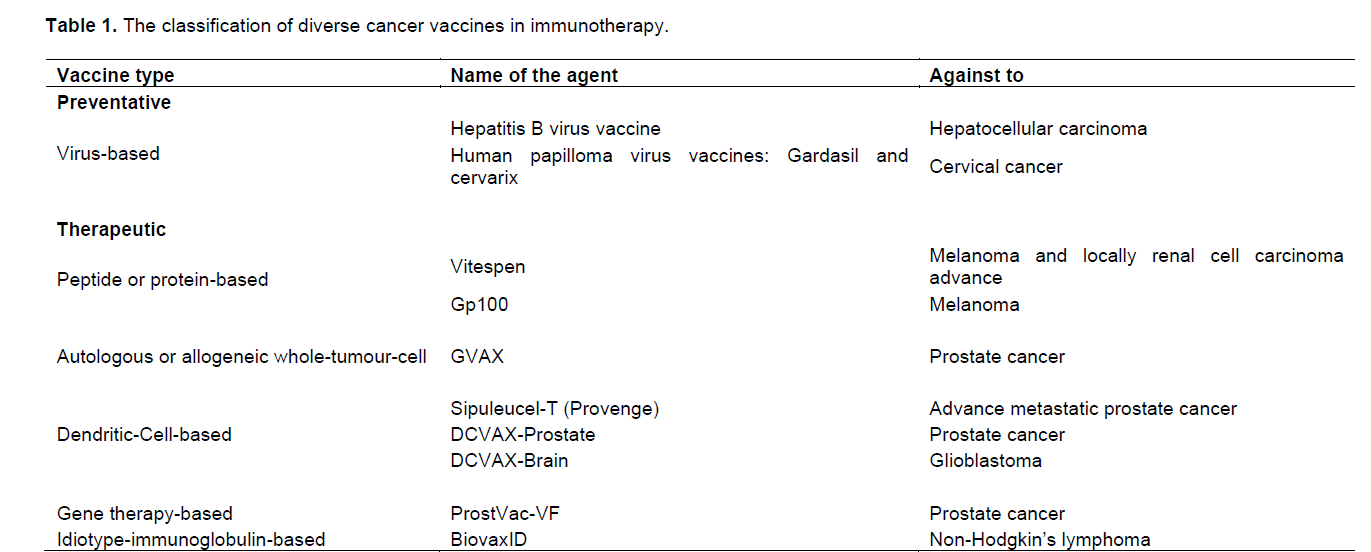
Preventative cancer vaccines are being used with relative success for more than 30 years to prevent the increased chance of tumorigenesis caused by various viral infections. Currently, there are six human viruses identified which are indicated as carcinogenic to humans: hepatitis B virus (HBV), human papilloma virus (HPV), Epstein–Barr virus (EBV), human immunodeficiency virus type-1 (HIV-1), hepatitis C virus (HCV) and Kaposi’s sarcoma-associated herpes virus (KSHV) (Sarid and Gao, 2011). However, there are currently no vaccines against these viruses with the exception of first two viruses. The very first such preventative cancer vaccine was the HBV vaccine in which, it was approved by FDA in 1981 and since then it has been used as one of the standard agents in scheduled routine vaccinations for infants (Dimberu and Leonhardt, 2011). The common use of this HBV vaccine not only dramatically reduced the rates of HBV infections but also reduced the number of incidences of Hepatocellular Carcinoma (HCC) where the immunization provided by this vaccine continued well for vaccinated individuals even in later ages (Chang et al., 2009). The second preventative cancer vaccine is the human papilloma virus vaccine. In the 1980s, it was demonstrated (by Harald zur Hausen) that certain HPV types, HPV16 and HPV18, were present in most cervical cancer biopsies and also in cervical cancer-derived cell lines (Hausen, 2009). Nowadays, HPV is known to be responsible for virtually all cases of cervical cancer in which the HPV16 and HPV18 are the high-risk HPVs that consist the almost 80% of the cervical cancer incidences (Sarid and Gao, 2011).
Apart from preventative vaccines, the therapeutic cancer vaccines aim to raise an immune response to an existing cancer rather than trying to prevent it from forming. This approach has been developed due to realization that the cancer patients can indeed produce both cytotoxic and helper T cells specific to antigens expressed in their tumours (Boon et al., 2006). Therapeutic cancer vaccines intent to trigger or enhance these pre-existing T cell responses against the tumour cells and there are several different approaches in the making of these vaccines (Mellman et al., 2011).
Peptide or protein-based vaccines
This type of cancer vaccines use a whole protein or short peptide derived from the tumour cells as a tumour cell-specific antigen for the immunization. A vaccine belongs to this type, called Vitespen, is a peptide-based vaccine which uses an autologous tumour-derived heat shock (chaperone) protein; glycoprotein (gp) 96-peptide complex (HSPPC-96) as an antigen (Hammerstrom et al., 2011). In phase-III clinical studies against melanoma and locally advance renal cell carcinoma, Vitespen has failed to provide a significant increase in overall survival rates and showed no overall benefit in recurrence-free survival (Wood et al., 2008; Testori et al., 2008). However, these studies showed an insignificant benefit with patient in earlier stages and also the subgroup analysis indicated that patients with higher doses of Vitespen survived longer than ones with the lower doses (Hammerstrom et al., 2011).
Another peptide-based therapeutic cancer vaccine is called Gp100 (or Gp100-based) that uses peptides from this glycoprotein 100 as a melanoma associated antigen for the vaccination. Even though this vaccine has succeeded to demonstrate its ability to establish an immune response against the tumour cells, no reduction in tumour size was observed (Hodi et al., 2010). However, a recent study, where Gp100 was co-administrated with the Immunostimulant IL-2, showed an anti-cancer immune response with a prolonged progression-free survival rate in patients with advanced melanoma (Schwartzentruber et al., 2011).
Even though there are some potential in the future of peptide or protein-based cancer vaccines, these primary studies clearly indicate the difficulties associated with the use of them. These difficulties may arose from the fact that short and free peptides are likely to be discarded rather quickly from the body without having the chance to associate with a dendritic cell to cause an immune response. Following up from the same problem, another issue can be the lack of effective dendritic-cell-activating adjuvant that is supposed to assist the peptides to be loaded to dendritic cells and promote their activation and maturation. Circumventing these issues can indeed improve the therapeutic benefits provided by these cancer vaccines (Rosenberg et al., 2004).
Autologous or allogeneic whole-tumour-cell vaccines
Whole-tumour-cell cancer vaccines are prepared from either autologous tumour cells or allogeneic tumour cell lines. Even though the use of autologous tumour cells eliminates the antigen selection problem by providing the advantage of targeting the individual’s own tumour associated antigens, this approached has been abandoned due to the motion that this kind of vaccine would not raise an effective anti-cancer immune response since it was not pre-existing in the first place (Hammerstrom et al., 2011). Furthermore, the high complexity of the vaccine preparation for each individual patient additionally instigated the abandoning of this approach (Mellman et al., 2011). In the other hand, the use of allogeneic tumour cell lines for the whole-tumour-cell vaccination was favored because of its ability to introduce multiple antigens and therefore to stimulate a better immune response. An example to this class of cancer vaccines is called GVAX which uses Allogeneic Prostate Cancer Cell Lines VITAL-1 and VITAL-2 that are manipulated to secrete GM-CSF (Higano et al., 2008). Despite its success in phase I and II clinical trials, the application of GVAX was terminated in phase III clinical trials against prostate cancer due to the increased rate of deaths and the low chance of reaching to its end point (Drake, 2009).
Gene therapy-based vaccines
Gene therapy-based vaccines are also called vector or viral-vector vaccines since they use viruses to insert the vaccine. In this approach, these viral vectors are engineered to encode for specific tumour antigens for the purpose of stimulating and enhancing the immune responses against cancer cells that carry the particular antigens. While advantages of using viruses as a delivery vehicle includes the easy gene insertion, low cost and ability to induce persistent immune response, the viruses belonging to the poxvirus family create an attractive candidate for this treatment due to their safe applications since the 1960s (Madan et al., 2009). The recombinant poxvirus vaccine, belonging to this class of cancer vaccines, is called ProstVac-VF that encodes for a prostate-specific antigen (PSA) and the adhesion molecules B7-1, Intercellular Adhesion Molecule-1(ICAM-1) and Lethal Factor Antigen-3 ( LFA-3) to boost the T-cell activation by resembling a specialized dendritic cell (Mellman et al., 2011).
Additionally, GM-CSF is administrated along with the vector to further stimulate the immune response. In a phase II clinical trial against minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC), ProstVac-VF failed to improve progression-free survival but succeeded to demonstrate a significant increase in overall survival rates and more than 40% of decrease in death rates, leading to its schedule to be used in a large phase III clinical trial (Kantoff et al., 2010).
Idiotype immunoglobulin-based vaccines
This type of cancer vaccines are prepared by fusing patient’s malignant B lymphoma cells with a myeloma cell line in which the resulting heterohybridoma expresses antibodies that consist of patient’s tumour-specific antigens called idiotypes (Hammerstrom et al., 2011). Then the idiotypes are isolated from the produced antibodies from these heterohybridoma B cells, purified and are coupled to keyhole limpet hemocyanin (KLH) to enhance their immunogenic properties by providing specific T-cell responses (Reinis, 2008). The vaccine called BiovaxID was developed in such way as a cancer vaccine against the B-cell lymphomas. Three phase III clinical trials were performed with this vaccine in which one of them was for patients with follicular non-Hodgkin’s lymphoma that the BiovaxID showed increased progression-free survival rates when administrated with GM-CSF (Schuster et al., 2009). Unfortunately in the other two phase III clinical studies, BiovaxID failed to provide a significant clinical benefit which may be due to the differences between the populations of patients or due to the time and labour intensive manufacturing method of the BiovaxID (Mellman et al., 2011).
Dendritic-cell-based vaccines
Among all the cancer vaccines discussed here, perhaps dendritic-dell-based vaccines hold of the highest potentials in the field of therapeutic vaccination that still needs to be explored. Considering the amount of information accumulated in the recent decades, the importance of dendritic cells is now known for a potent T-cell stimulation and therefore a persistent anti-cancer immune response (Mellman et al., 2011). One of the dendritic-dell-based vaccines is called DCVAX-Prostate which is an autologous dendritic cell vaccine, however it does not use a whole protein as in peptide or protein-based vaccines and it does not include GM-CSF in its administration.
Its manufacturing follows an incubation of the patient’s dendritic cells with a prostate-specific membrane antigen (PSMA) before it is infused back into the same patient (Hammerstrom et al., 2011). The phase I and II clinical trials in patients with prostate cancer, DCVAX-prostate proved to be able to induce an anti-cancer immune response against the prostate cancer cells (Fishman, 2009). Another dendritic-cell-based vaccine is called DCVAX-Brain which uses the exact same concept as in DCVAX-prostate but instead of PSMA the autologous dendritic cells are loaded with the patient’s tumour cell lysates. The DCVAX-Brain vaccine is used in patients with glioblastoma multiforme which the most aggressive, malignant and common brain tumour in humans (Van Meir et al., 2010). As in the case of DCVAX-Prostate, the phase I and II clinical trials of the DCVAX-Brain vaccine also showed low toxicity and successful stimulation of an anti-tumour immune response (Wheeler and Black, 2009).
Sipuleucel-T (Provenge)
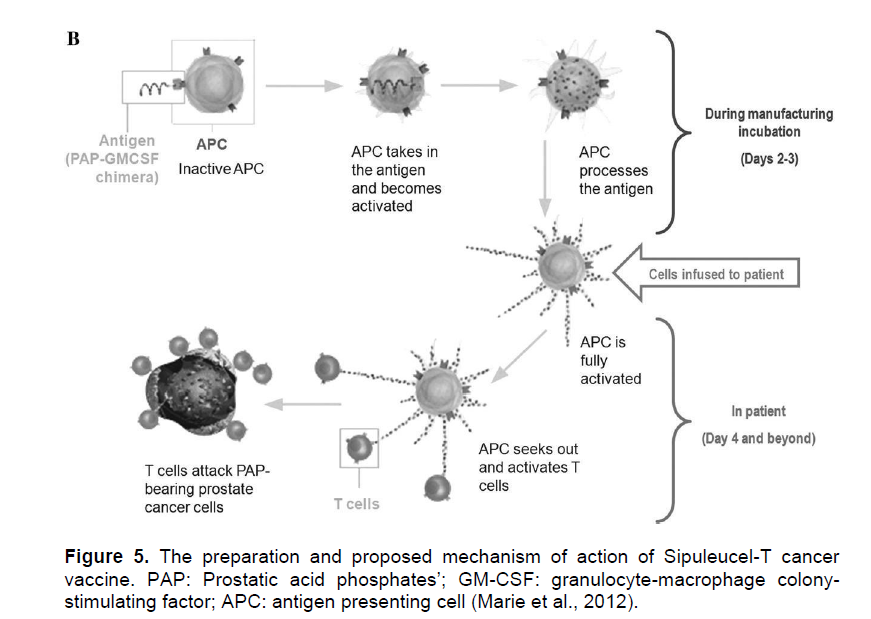
The use of Sipuleucel-T for advance metastatic prostate cancer was approved by FDA in 2010; making Sipuleucel-T the first FDA approved therapeutic cancer vaccine (Hammerstrom et al., 2011). It is an autologous personalized vaccine that is prepared from the patient’s own peripheral blood mononuclear cells. After discarding platelets, monocytes, low-density lymphocytes and erythrocytes by leukapheresis, the remaining dendritic cells, T cells, B cells, and natural killer cells are incubated from 36 to 44 hour ex vivo with a fusion protein PA2024 which is composed of a prostate cancer antigen, prostatic acid phosphates (PAP) and GM-CSF (Dimberu and Leonhardt, 2011). After the ex vivo incubation, the cells are infused back into the same patient where the cells are thought to effectively present the antigen to host immune system and activate the cytotoxic T-cell responses against the tumour cells (Drake, 2010).
Even though the Sipuleucel-T vaccine is considered as an autologous dendritic-cell-based vaccine, its mechanism of action is not fully comprehended since it has not been clearly demonstrated yet whether the complex mixture of the ex vivo incubated cells indeed contain the PAP-loaded dendritic cells or that the induction of PAP-specific T-cells by the infusion indeed exists (Pardoll and Drake, 2012). Therefore, there is still a need for further characterization of the incubated cells to fully understand the mechanism of this vaccine. Although the phase III clinical studies of Sipuleucel-T did not show reduction in tumour size or reduction in disease progression rate, it succeeded to provide a significant increase in the median survival rates that led to its FDA approval. This appearance of increase in overall survival provided by the Sipuleucel-T vaccine without demonstration of an observable anti-tumour effect has led to the discussion that the tumour response criteria in clinical trials might be in need of modification for this kind of immunotherapeutic approaches (Kantoff et al., 2010) Figure 5.
Cancer immunotherapy focuses on developing agents that activates or enhances the immune system’s recognition and killing of the cancer cells. It can be either passive or active. Passive therapy is based on the adoptive transfer of immunomodulators (including cytokines), tumor-specific antibodies, or immune cells while active immunotherapy, on the other hand, stimulates the patient’s immune system, with the intent of promoting an antigen-specific antitumor effect using the body’s own immune cells. Immunotherapy can be further divided into nonspecific and specific types: nonspecific immunotherapy involves the administration of cells or substances that are not targeted to a specific antigen. In contrary to this, active specific immunotherapy involves the priming of the immune system in order to generate a T-cell response against tumor-associated antigens.
Radioimmunotherapy (coupling a radioactive atom to a monoclonal antibody (mAb)), immunotoxins (generated by coupling plant-derived or bacterial toxins to mAbs), antibody-directed enzyme prodrug therapy (an antibody is used as a vector to transfer an enzyme) and immunomodulators were among the discussed approaches to use mAbs as an anti-cancer.
Peptide or protein-based vaccines (use a whole protein or short peptide derived from the tumour cells), autologous or allogeneic whole-tumour-cell vaccines (prepared from either autologous tumour cells or allogeneic tumour cell lines), gene therapy-based vaccines (use viruses to insert the vaccine), idiotype immunoglobulin-based vaccines (prepared by fusing patient’s malignant B lymphoma cells with a myeloma cell line), dendritic-cell-based vaccines and sipuleucel-T (provenge) (prepared from the patient’s own peripheral blood mononuclear cells) were among the cancer vaccines in the immunotherapeutic approaches.
The authors have not declared any conflict of interests.
REFERENCES
|
Adams GP, Weiner LM (2005). Monoclonal antibody therapy of cancer. Nat. Biotechnol. 23:1147-1157.
Crossref
|
|
|
|
Belardell F (1995). Role of interferons and other cytokines in the regulation of the immune response. APMIS, 103:161-79.
Crossref
|
|
|
|
Belardelli F, Ferrantini M, Proietti E, Kirkwood JM (2002). Interferon-alpha in tumor immunity and immunotherapy. Cytokine and Growth Factor Rev. 13:119-134.
Crossref
|
|
|
|
Bisht M, Bist SS, Dhasmana DC (2010). Biological response modifiers: Current use and future prospects in cancer therapy. Indian J. Cancer, 47(4):443-451.
Crossref
|
|
|
|
Boon T, Coulie PG, Van-den Eynde BJ, van der Bruggen P (2006). Human T cell responses against melanoma. Annu. Rev. Immunol. 24:175-208.
Crossref
|
|
|
|
Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS (2009). Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J. Natl. Cancer Inst. 101(19):1348-55.
Crossref
|
|
|
|
Clark JW, Smith JW, Steis RG (1990). Interleukin 2 and lymphokine-activated killer cell therapy: Analysis of a bolus interleukin 2 and a continuous infusion interleukin 2 regimen. Cancer Res. 50:7343-7350.
|
|
|
|
Dimberu PM, Leonhardt RM (2011). Cancer immunotherapy takes a multi-faceted approach to kick the immune system into gear. Yale J. Biol. Med. 84:371-380.
|
|
|
|
Drake CG (2009). Immunotherapy for prostate cancer. J. Clin. Onco. 27:4035-4037.
Crossref
|
|
|
|
Drake CG (2010). Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 10:580-593.
Crossref
|
|
|
|
Dudley ME, Wunderlich JR, Yang JC (2005). Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 23:2346-2357.
Crossref
|
|
|
|
Emeryville CA (2008). Proleukin. Bayer HealthCare Pharmaceuticals, Inc.
|
|
|
|
Fang L, Lonsdorf AS, Hwang ST (2008). Immunotherapy for advanced melanoma. J. Invest. Dermatol. 128:2596-2605.
Crossref
|
|
|
|
Fishman M (2009). A changing world for DCVax: A PSMA loaded autologous dendritic cell vaccine for prostate cancer. Expert Opin. Biol. Ther. 9:1565-1575.
Crossref
|
|
|
|
Gelderman KA, Tomlinson S, Ross GD, Gorter A (2004). Complement function in mAb-mediated cancer immunotherapy. Trends Immunol, 25:158-164.
Crossref
|
|
|
|
Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P (2011). Cancer Immunotherapy: Sipuleucel-T and Beyond. Pharmacotherapy 31(8):813-828.
Crossref
|
|
|
|
Hausen HZ (2009). Papillomaviruses in the causation of human cancers: A brief historical account. Virol. 384(2):260-265.
Crossref
|
|
|
|
Higano CS, Corman JM, Smith DC, Centeno AS, Steidle CP, Gittleman M, Simons JW, Sacks N, Aimi J, Small EJ (2008). Phase 1or 2 dose escalation study of a GM-CSF secreting, allogeneic and cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 113:975-984.
Crossref
|
|
|
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711-723.
Crossref
|
|
|
|
Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR (2010). Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28: 1099-10105.
Crossref
|
|
|
|
Kipp RT, McNeel DG (2007). Immunotherapy for prostate cancer: Recent progress in clinical trials. Clin. Adv. Hematol. Oncol. 5:465-479.
|
|
|
|
Kirkwood JM, Tarhini AA, Panelli MC, Moschos SJ, Zarour HM, Butterfield LH, Gogas HJ (2008). Next generation of immunotherapy for melanoma. J. Clin. Oncol. 26(20):3445-3455.
Crossref
|
|
|
|
Kreitman RJ, Pastan I (2011). Antibody-fusion proteins: Anti-CD22 recombinant immunotoxin: Moxetumomab pasudotox. Clin. Cancer Res. 17:6398-63405.
Crossref
|
|
|
|
Krieg AM (2002). CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760.
Crossref
|
|
|
|
Krieg AM (2006).Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 5:471- 484.
Crossref
|
|
|
|
Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL, Prostvac VF (2009). A vector-based vaccine targeting PSA in prostate cancer. Expert Opin. Investig. Drugs 18:1001-1011.
Crossref
|
|
|
|
Marie LH, Haynes L, Parker C, Iversen P (2012). Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J. Natl. Cancer Inst. 104:1-7.
|
|
|
|
Marrari A, Iero M, Pilla L (2007). Vaccination therapy in prostate cancer. Cancer Immunol. Immunother. 56:429-445.
Crossref
|
|
|
|
McNeel DG (2007). Active specific immunotherapy in cancer: Cellular immunotherapies for prostate cancer. Biomed. Pharmacother. 61:315-322.
Crossref
|
|
|
|
Mellman I, Coukos G, Dranoff G (2011). Cancer immunotherapy comes of age. Nature 480(7378):480-489.
Crossref
|
|
|
|
Melton RG, Sherwood RF (1996). Antibody enzyme conjugates for cancer therapy. J. Natl. Cancer Inst. 88(3-4):153-165.
Crossref
|
|
|
|
Mogensen KE, Lewerenz M, Reboul J, Lutfalla G, Uze G (1999). Type-I IFN receptor: Structure, function and evolution of a family business. J. Interferon Cytokine Res. 19:1069-1098.
Crossref
|
|
|
|
Pardoll D, Drake C (2012). Immunotherapy earns its spot in the ranks of cancer therapy. J. Exper. Med. 209(2):201-209.
Crossref
|
|
|
|
Pashenkov M, Goëss G, Wagner C, Hörmann M, Jandl T, Moser A, Britten CM, Smolle J, Koller S, Mauch C, Tantcheva-Poor I, Grabbe S, Loquai C, Esser S, Franckson T, Schneeberger A, Haarmann C, Krieg AM, Stingl G, Wagner SN (2006). Phase II trial of a toll-like receptor 9-activating oligonucleotides in patients with metastatic melanoma. J. Clin. Oncol. 24(36):5716-5724.
Crossref
|
|
|
|
Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, Walter MR, Nagabhushan TL, Trotta PP, Pestka S (1998). Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 58:2489-2499.
|
|
|
|
Reinis M (2008). Biovax ID, a personalized therapeutic vaccine against B-cell lymphomas. Curr. Opin. Mol. Ther. 10:526-534.
|
|
|
|
Rescigno M, Avogadri F, Curigliano G (2007). Challenges and prospects of immunotherapy as cancer treatment. Biochim. Biophys. Acta 1776:108-123.
Crossref
|
|
|
|
Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J (2005). Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP. J. Clin. Onco. 23(35):8968-8977.
Crossref
|
|
|
|
Rosenberg SA, Dudley ME (2009). Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opinion Immunol. 21:233-240.
Crossref
|
|
|
|
Rosenberg, SA, Yang JC, Restifo NP (2004). Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 10(9):909-915.
Crossref
|
|
|
|
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME (2008). Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8:299-308.
Crossref
|
|
|
|
Sarid R, Gao SJ (2011). Viruses and human cancer: From detection to causality. Cancer Lett. 305(2):218-227.
Crossref
|
|
|
|
Schuster SJ, Neelapu SS, Guase BL, Muggia FM, Gockerman JP, Sotomayor EM, Winter JN, Flowers CR, Stergiou AM, Kwak LW (2009). Idiotype vaccine therapy (Biovax ID) in follicular lymphoma in first complete remission: phase III clinical trial results. J. Clin. Oncol. 27:18.
|
|
|
|
Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P (2011). gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 365(8):771.
Crossref
|
|
|
|
Sharma P, Wagner K, Wolchok JD, Allison JP (2011). Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nature reviews Cancer 24:(11):805-812.
Crossref
|
|
|
|
Teicher BA, Chari RVJ (2011). Antibody conjugate therapeutics: Challenges and Potential. Clin. Cancer Res. 17:6389-6397.
Crossref
|
|
|
|
Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, Yuh L, Gupta R, Srivastava PK (2008). C-100-21 study group phase III comparison of vitespen: An autologous tumor derived heat shock protein gp96 peptide complex vaccine with physician's choice of treatment for stage IV melanoma. J. Clin. Oncol. 26(6):955-962.
Crossref
|
|
|
|
Todar K (2008). Immune defense against bacterial pathogens: Adaptive or acquired immunity. Todar's Online Textbook of Bacteriology.
View
|
|
|
|
Van Meir EG, Hadjipanayis CG, Norden AD, Shu Hui-Kuo Wen PY, Olson JJ (2010). Exciting new advances in Neuro-oncology: The avenue to a cure for malignant glioma. CA. Cancer J. Clin. 60(3):166-193.
Crossref
|
|
|
|
Waldmann TA (2003). Immunotherapy: Past, present and future. Nat. Med. 9(3):269-277.
Crossref
|
|
|
|
Waller EK (2007). The Role of Sargramostim (rhGM-CSF) as Immunotherapy. The Oncologist 12(2):22-26.
|
|
|
|
Weiner LM, Surana R, Wang S (2010). Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 10:317-327.
Crossref
|
|
|
|
West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C (2004). Enhanced dendritic cell antigen capture via toll-like receptor induced actin remodeling. Sci, 305 (5687):1153-1157.
Crossref
|
|
|
|
Wheeler CJ, Black KL (2009). DCVax-Brain and DC vaccines in the treatment of GBM. Expert Opin. Investig. Drugs 18(4):509-519.
Crossref
|
|
|
|
Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, Isakov L, Flanigan R, Figlin R, Gupta R, Escudier B (2008). C-100-12 RCC study group an adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: A multicentre, open label, randomised phase III trial. Lancet 372:145-154.
Crossref
|
|
|
|
Wrzesinski C, Paulos CM, Gattinoni L (2007). Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8+ T cells. J. Clin. Invest. 117:492-501.
Crossref
|