Full Length Research Paper
ABSTRACT
About one-third of diabetes mellitus (DM) patients are complicated by diabetic nephropathy. It is even more challenging in type 2 DM in which this complication may occur before presentation. Microalbuminuria which is the traditional marker of early diabetic nephropathy is limited in its usefulness because it is detected when significant renal damage has already occurred. The aim of this study was to evaluate and compare urinary fibronectin and laminin with microalbuminuria as markers of glomerular injury in type 2 DM patients. A cross-sectional study with 120 consenting non-hypertensive type 2 DM, consisting of 57 (47.5%) males and 63 (52.5%) females; and 120 non-diabetic/non-hypertensive controls, consisting of 51 (42.5%) males and 69 (57.5%) females. Urinary albumin, fibronectin and laminin as well as plasma glucose were measured. The mean albumin –creatinine ratio was significantly higher (p<0.001) among type 2 DM (1.990.78 mg/mmol) compared to the control (0.120.01 mg/mmol). Also, urinary fibronectin (1.290.35 µg/mL) and laminin (1251.34+234.61 pg/mL) were significantly higher (p<0.001) in type 2 DM patients than in controls (0.290.07 µg/mL and 127.8114.07 pg/mL respectively). The sensitivity test for urinary fibronectin and laminin was 93.9% each while their specificities were 8.0 and 13.8% respectively. The negative predictive values (NPV) for urinary fibronectin and laminin were 77.8 and 85.7% respectively while the positive predictive values (PPV) were 27.9 and 29.2% respectively. This study suggests that assay for urinary fibronectin and laminin, which showed high sensitivities and high negative predictive values are useful as markers of early diabetic kidney disease and can substitute microalbuminuria in the routine evaluation of type 2 DM patients. The slightly higher PPV and NPV for urinary laminin suggest that it is a better marker than urinary fibronectin.
Key words: Diabetes mellitus, microalbuminuria, urinary fibronectin, urinary laminin.
INTRODUCTION
Globally, diabetes mellitus affects 366 million people and is expected to rise to 552 million by 2030 (Whiting et al., 2011). The prevalence in Nigeria was reported as having increased from 2.0% in 1992 to 5.0% in 2013 (Akinkugbe et al., 1997; Oputa and Chinenye, 2015). It is associated with long-term damage, dysfunction and failure of various organs especially the eyes, kidneys, nerves, heart, and blood vessels (Alwan, 2011).
Diabetic nephropathy is one of the most challenging microvascular complications of type 2 DM, with significant morbidity and mortality (Hellemons et al., 2012). It occurs in about one-third of diabetic patients in the world and is the leading cause of end-stage renal disease in developed and developing countries (WHO, 2008). Death due to renal disease is 17 times more common in diabetes mellitus patients than in non-diabetic patients (Hong and Chia, 1998). In a study, Alebiosu and Ayodele (2005) reported increasing prevalence of diabetic nephropathy as a cause of end stage renal disease in Nigeria.
Screening for diabetic kidney disease helps in early detection, evaluation and treatment. Consequently, multiple biomarkers of kidney injury in serum and urine have been studied. Based on the mechanisms of structural damage to the kidneys, biomarkers are classified as markers of glomerular injury, oxidative stress, endothelial damage and inflammation (Matheson et al., 2010).
The urinary markers of glomerular injury result from either increased permeability to plasma proteins (albumin; transferrin) or increased excretion of extracellular matrix proteins (fibronectin; laminin) (Lehmann and Schleicher, 2000).
Some researchers consider microalbuminuria as the best predictor of end-stage renal disease and cardiovascular events in diabetics (De Zeeuw et al., 2006; Ninomiya et al., 2009). However, microalbuminuria develops when significant kidney damage has occurred (Yamazaki et al., 1991; Mario et al., 1989). This scenario suggests that attempts should be made to detect or diagnose diabetic nephropathy before the microalbuminuric stage. It therefore follows that more sensitive and specific markers of early kidney damage is imperative for early treatment of diabetic nephropathy. Glomerular extracellular basement membrane proteins such as fibronectin and laminin whose appearance in urine precedes micro albuminuria have been investigated in diabetic nephropathy (Remuzzi et al., 2002; Naresh et al., 2009). This study evaluates the usefulness of urinary fibronectin and lamininas sensitive and specific biomarkers of early nephropathy in type 2 diabetics as compared to microalbuminuria.
MATERIALS AND METHODS
This hospital-based cross-sectionalcase-control study was carried out at the diabetic and metabolic clinics of the University of PortHarcourt Teaching Hospital (UPTH) between March 2015 and February 2016. UPTH is a tertiary referral centre and the foremost teaching hospital in the South South geopolitical region of Nigeria.
Study population
The subjects were type 2 diabetics attending the Diabetic and Metabolics Clinic of UPTH. They were selected following review of their clinical history and management. Only uncomplicated cases were included in the study. The controls were age matched non-diabetic, non-hypertensive, and apparently healthy individuals not on any medication and with normal renal function. Controls were recruited from different settings outside the hospital including churches, residential neighbors of the researchers and hospital colleagues. Subjects and controls were subjected to urinalysis, blood pressure measurement and fasting plasma glucose and serum creatinine determination. The eGFR was calculated using the Cockcroft-Gault equation (Lynch and Wu, 2010) from age, weight (kg), sex and plasma creatinine value. This was done to exclude any renal impairment or disease condition.
The minimum sample size required for this study was determined using the formula below (Araoye, 2004).
Where:
n = minimum sample size;
z = 95% confidence interval = 1.96;
p = prevalence in the target population is 6.8%19;
q = 1-p=0.932;
d = degree of accuracy desired (at 0.05); n = 97.4
The calculation was based on a prevalence rate of 6.8% observed for diabetes mellitus in a study done in UPTH (Nyenwe et al., 2003). The confidence level was set at 95% giving a calculated minimum sample size of 97 subjects. The final sample size was adjusted for a 10% non-response rate (Lott et al., 1992). Therefore, a projection of 120 study subjects (type 2 DM patients) and 120 apparently healthy subjects as control were included in the study giving a total of 240 subjects.
A systematic sampling technique was used to select the subjects. This involved the calculation of a sampling interval fraction, 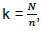 , where N is the total population at the clinic, n is the daily sample size required to cover three (3) months data collection period.
, where N is the total population at the clinic, n is the daily sample size required to cover three (3) months data collection period.
The first subject was selected by simple random sampling via ballot method after which the sixth patient was selected. Approval for this study was obtained from the Ethical Research Committee of the University of Port Harcourt Teaching Hospital. Informed written consent was obtained from the subjects following disclosure of the objectives of the study. The subjects bore no cost for any of the tests. An interviewer administered questionnaire was used to obtain information on the socio-demographic and clinical characteristics of the subjects.
Strict universal precaution and standard operating procedures were observed in collecting 5 ml of venous blood from the antecubital vein of subjects. Samples in fluoride oxalate bottle were used for plasma glucose estimation using glucose oxidase method (Weissman and Klein, 1958) while samples in lithium heparin bottle were used for plasma creatinine estimation using the modified Jaffe kinetic method (Vasiliades 1976). Early morning urine collected was used for urinary creatinine estimation using the modified Jaffe kinetic method (Vasiliades 1976). Urinary albumin and fibronectin were assessed using immunoturbimetric assay (Elving et al., 1989; Brentnall et al., 2014), while laminin was assessed using ELISA technique (Kleinman et al., 1985).
Urinary fibronectin (Brentnal et al., 2014) was quantitatively evaluated with a commercially available human fibronectin ELISA kit produced by Abcam® (Abcam Laboratories, 330 Cambridge Science Park Road, Milton, Cambridge CB4 OFL, UK). Also, quantitative determinationof urinary laminin (Kleinman et al., 1985) was done with a commercially available human laminin ELISA kit manufactured by Abcam® (Abcam Laboratories, 330 Cambridge Science Park Road, Milton, Cambridge CB4 OFL, UK).Quantitative determination of urinary albuminwas done with a commercially available microalbumin (mALB) immunoturbidimetric assay kit produced by BIOLABO SA Laboratories, 02160,Maizy, France (Elving et al., 1989).
Validity tests were carried out to ascertain the usefulness of urinary fibronectin and urinary laminin as early markers of diabetic kidney disease as against microbalbuminuria. The tests include: sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV).
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS) version 16. Assessment of statistical differences between groups was achieved with chi-square test and Student’s independent t test while Pearson’s correlation was used to assess the relationship between urinary fibronectin and urinary laminin, with microalbuminuria. A p-value of less than 0.05 was considered statistically significant.
RESULTS
A total of 240 subjects were studied; 120 were type 2 DM patients who met the inclusion criteria, while the remaining 120 were apparently healthy, non – diabetic and non-hypertensive, age matched controls. Fifty seven (47.5%) of the type 2 DM patients were males while 63 (52.5%) were females giving a male to female ratio of 1:1.1. Also, 51 (42.5%) of the control subjects were males while 69 (57.5%) were females, giving male to female ratio of 1: 1.4. Altogether, 108 (45%) males and 132 (55%) females were studied.
The age of the subjects ranged between 24– 74 years. The mean age was 51.30 ± 11.06for type 2 DM patients and 49.88 ± 11.30 years for the control group. Majority 42 (35.0%) of the diabetics were within 41-50 years age bracket while 41(34.2%) of the control group were in same age bracket. The age and gender distribution of the study participants is presented in Table 1.
Generally, the DM patients had higher values of the tested parameters than the controls. Biochemical characteristics of type 2 DM patients and the controls are shown in Table 2. The mean fasting plasma glucose among type 2DM - 10.35 5.53 mmol/L was significantly higher (p < 0.001) than that of the controls - 4.97 0.81mmol/L. Similarly, the mean plasma creatinine of 89.33 21.75µmol/L for DM patients was higher than that of controls - 71.58 9.10 µmol/L.(p < 0.001). Mean urine creatinine for DM patients was 27.04 5.68mmol/L as against the 19.45 2.44 mmol/L of controls.(p<0.001); microalbuminuria for DM was 59.92 7.07 mg/L while for the controls, it was 2.11 1.06 mg/L.(p < 0.001). The albumin-creatinine - ratio (ACR) was 1.99 0.78 mg/mmol for DM cases and 0.12 0.01 mg/mmol for controls.(p< 0.001); urinary fibronectin for DM was 1.29 0.35 µg/ml and 0.29 0.07µg/ml for controls(p <0.001). For DM cases, urinary laminin was 1251.43 234.61 pg/ml while it was 127.81 14.07 pg/ml for controls (p<0.001). However, no significant difference was found in the mean estimated glomerular filtration rate (GFR) between type 2 DM patients and the control group: (97.35 18.23 ml/min vs 113.13 18.81 ml/min, respectively; p = 0.090). Fifty three (44.2%) of type 2 DM patients had normal GFR (GFR >90 ml/min) compared to 107 (89.2%) of the control group. Forty seven (39.2%) of type 2 DM patients had mildly decreased GFR (GFR of 60 - 89 ml/min) compared to 13 (10.8%) of the control group, while 20 (16.7%) of type 2 DM patients had moderately decreased GFR (GFR of 30 - 59 ml/min) compared to none in the control group, and this difference was statistically significant (p< 0.001). Nostage 4 (severely reduced GFR) or stage 5 (end stage renal failure) was detected in the study (Table 3).
The distribution of urinary fibronectin, laminin and microalbuminuria excretions in type 2 DM patients and controls with stage 1 and 2 GFR is shown in Table 4. Urinary fibronectin, laminin and microalbuminuria were significantly higher in type 2 DM with stage 1 GFR compared to controls with same GFR stage. Similarly, in subjects with stage 2 GFR, urinary fibronectin, laminin and microalbuminuria were significantly higher in type 2 DM compared to the control group.
Table 5 shows the proportion of subjects with microalbuminuria using ACR. Eighty-seven (72.5%) of type 2 DM patients had normal ACR as compared to 120 (100%) of the control, while 33 (27.5%) of type 2 DM had microalbuminuria as compared with none in the control group, and this difference was statistically significant (p<0.001).
The sensitivity, specificity, PPV and NPV of urinary fibronectin as a marker of early glomerular injury were 93.9, 8.0, 27.9 and 77.8% respectively.
The sensitivity specificity, PPV and NPV of urinary laminin as a marker of early glomeralar injury were 93.9, 13.8, 29.2 and 85.7% respectively.
The receiver operator characteristics (ROC) curve for both urinary fibronectin and urinary laminin are shown in Figures 1 and 2, respectively.
Correlation analysis
In the type 2 DM patients, a significant inverse correlation was found between GFR and microalbuminuria (r = - 0.18; p = 0.046) and between GFR and urinary laminin (r = -0.18; p = 0.045) while a significant positive correlation was found between urinary fibronectin and microalbuminuria (r = 0.23; p = 0.013) and between urinary fibronectin and ACR (r = 0.23; p = 0.012).
In the controls, however, a significant positive correlation was found between urinary laminin and microalbuminuria (r = 0.19; p = 0.038), between urinary laminin and ACR (r = 0.18; p = 0.045) and between urinary laminin and urinary fibronectin.
DISCUSSION
This hospital-based study noted higher number of females than males among the study population, this may not be surprising as females have been shown to have better health seeking behavior and higher utilization rate of health care services (Hosseinpoor et al., 2012).The age of the subjects ranged from 24 to 74years with the41-50 year age group constituting the peak. This agrees with the typical age of onset of type 2 DM (Dods, 2010).
Microalbuminuria is a well-recognized marker of early glomerular injury in DM patients. The finding in our study which show that 27.5% of type 2 DM patients showed microalbuminuria is comparable to a similar hospital-based study in Northern India (Kanakamani et al., 2010). However, an earlierhospital-based study carried out in our centre reported that out of the 60 type 2 DM patients, 63.3% had microalbuminuria (Orluwene and Mommoh, 2008). The wide disparity between our study and the previous one of our centre may likely be due to the differences in the eligibility criteria of the two studies. While our DM patients were non hypertensive, those of the previous study included hypertensive patients. It is documented that co-morbid conditions in diabetes like hypertension increase the risk of microalbuminuria (Parchwani and Upadhyah, 2012). Also, in the previous index study, microalbuminuria was determined qualitatively by the use of dipstick while in our present study microalbuminuria was determined quantitatively. Hence, the possible explanations for the higher rate reported in the former study. Another study in Jos – North Central, Nigeria among newly diagnosed type 2 DM patients reported that 49.2% of the patients had microalbuminuria (Agaba et al., 2004). This finding is also higher than that of our current study finding. However, a similar study carried out in Enugu – South Eastern Nigeria reported a much lower rate of 16.1% among type 2 DM patients (Ogbu et al., 2009). This could be due to the smaller population size in the Enugu study. In spite of these differences in the reporting rates of microalbuminuria among type 2 DM patients, the findings of this study along with the aforementioned Nigerian studies reveal that microalbuminuria among type A 2 DM patient is not uncommon; thus, highlighting the need for regular screening for microalbuminuria among type 2 DM patients, in order to detect and treat early glomerular damage. However, it is interesting to note that research has also shown that some type 2 DM patients with glomerular damage may not develop microalbuminuria (An et al., 2009). Thus, the use of other biomarkers of glomerular damage is imperative. Urinary fibronectin excretion as a marker for the detection of early glomerular damage, investigated in our study revealed findings which are in agreement with literature documentation (Takahashi, 1995; Fagerudd et al., 1997). It is worthy of note that, the mean urinary fibronectin excretion levels was significantly higher in the diabetic group than the control group, which is consistent with the report of similar study by Fagerudd et al. (1997). The latter found that the mean urinary fibronectin excretion level was much higher in type 2 DM with micro-vascular complications (retinopathy,neuropathy andnephropathy). However, our study which considered type 2 DM patients without complications still showed higher fibronectin excretion than controls. This implies that with or without complications, the mean urinary fibronectin level is significantly higher in type 2 DM than normal controls.
The 93.9% sensitivity of urinary fibronectin excretion observed in this study highlights the need for the incorporation of fibronectin assessment as a screening tool for glomerular damage. High sensitivity is a necessity for any screening tool as recommended in the World Health Organization (WHO) screening criteria (Andermann et al., 2008). Additionally, the significant positive correlation between urinary fibronectin and microalbuminuria reported in this study reflects the good comparison of this biomarker to microalbuminuria. This study also notes the low specificity (8.0%) of urinary fibronectin. Nonetheless, the high negative predictive value (77.8%) reported also indicates its usefulness as a screening tool for detection of glomerular damage.
Concerning urinary laminin excretion as a marker for glomerular damage, this study noted similar findings with that of urinary fibronectin. The urinary laminin excretion levels was significantly higher in the diabetic group than in the control group, which is also consistent with findings of similar study by Miyake et al. (1993) although the latter study wason children with insulin dependent diabetes mellitus. This possibly indicates that irrespective of age and type of DM, urinary laminin excretion is significantly higher amongDM patients than controls. Urinary laminin was positively correlated with microalbuminuria, thus highlighting the association between these two markers. Although, our study noted that the sensitivity of urinary laminin was the same as urinary fibronectin (93.9%), its specificity was slightly higher than that of urinary fibronectin. It, therefore, implies that urinary laminin is a better biomarker in comparison with urinary fibronectin, as a screening test for the detection of glomerular injury.
The absence of previous studies on urinary fibronectin and laminin as biomarkers for glomerular injury in Nigeria precludes the comparison of the findings reported in present study with such studies in Nigeria. Thus, this study highlights the need for similar studies to be carried out in Nigeria to validate the findings and serve as a comparison to the present study. However, the findings of this study, which reveals the usefulness of urinary fibronectin and laminin as biomarkers for the detection of glomerular injury is in agreement with other studies done outside Nigeria (Hong and Chia, 1998; Fagerudd et al., 1997; Andermann et al., 2008; Miyake et al., 1993).
This study is a hospital based cross-sectional study with relatively small population size. Hence, the outcome of the research may not be extrapolated or generalized for the entire population. Also, the study was limited by high cost of reagents being that it was sponsored by the investigators.
CONCLUSION
This study suggests that assay for urinary fibronectin and laminin, both of which showed high sensitivities and negative predictive values, are useful in the evaluation glomerular injury in type 2 DM patients. Compared to urinary fibronectin, urinary laminin is a better screening biomarker for early diabetic glomerular damage. Although, the specificities of both biomarkers were low, they are good alternatives to microalbuminuria in the routine evaluation of type 2 DM patients for glomerular injuries.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors are grateful to the diabetic patients as well as the non-diabetic volunteers without who this study would not have held, despite not receiving any form of monetary inducement. Authors’ appreciation also goes to the nurses and laboratory scientists who in some ways made the study a success.
REFERENCES
|
Agaba EI, Agaba PA, Puepet FH (2004). Prevalence of microalbuminuria in newly diagnosed type 2 diabetic patients in Jos Nigeria. African Journal of Medicine and Medical Sciences 33:19-22. |
|
|
Akinkugbe OO, Ikeme AC, Johnson TO (1997). Non-communicable diseases in Nigeria.Final Report of National Survey, Federal Ministry of Health and Social Services, Lagos,pp.64-90. |
|
|
Alebiosu CO, Ayodele OE (2005). The global burden of chronic kidney disease and the way forward. Ethnicity and Disease 15:418-423. |
|
|
Alwan A (2011). Global status report on noncommunicable diseases 2010.World Health Organization. |
|
|
An JH, Cho YM, Yu HG, Jang HC, Park KS, Kim SY, Lee HK (2009). The clinical characteristics of normoalbuminuric renal insufficiency in Korean type 2 diabetic patients: a possible early stage renal complication. Journal of Korean Medical Science, 24:S75-S81. |
|
|
Andermann A, Blancquaert I, Beauchamp S, Déry V (2008). Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bulletin of World Health Organization 86:317-319. |
|
|
Araoye MO (2004). Research Methodology with Statistics for Health and Social Sciences, first edition. Nathadex publishers, Ilorin: pp. 25-120 |
|
|
Brentnall M, Weir DB, Rongvaux A, Marcus AI, Boise LH (2014). Procaspase-3 regulates fibronectin secretion and influences adhesion, migration and survival independently of catalytic function. Journal of Cell Science 127:2217-2226. |
|
|
De Zeeuw D, Ramjit D, Zhang Z, Ribeiro AB, Kurokawa K, Lash JP, Chan J, Remuzzi G, Brenner BM, Shahinfar S (2006). Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney international 69:1675-1682. |
|
|
Dods R (2010). Diabetes Mellitus In: Clinical Chemistry: Theory, Analysis, Correlation , St. Louis, Missouri, Mosby. pp 729-754. |
|
|
Elving LD, Bakkeren JA, Jansen MJ, de Kat Angelino CM, de Nobel E, van Munster PJ (1989). Screening for microalbuminuria in patients with diabetes mellitus: frozen storage of urine samples decreases their albumin content. Clinical Chemistry 35:308-310. |
|
|
Fagerudd JA, Groop PH, Honkanen E, Teppo AM, Grönhagen-Riska C (1997).Urinary excretion of TGF-β1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patients. Kidney International Supplement 63:195-197. |
|
|
Hellemons ME, Kerschbaum J, Bakker SJL, Neuwirt H, Mayer B, Mayer G, de Zeeuw D, LambersHeerspink HJ, Rudnicki M (2012). Validity of biomarkers predicting onset or progression of nephropathy in patients with Type 2 diabetes: a systematic review. Medication Diabetes 29:567-577. |
|
|
Hong CY, Chia KS (1998).Markers of diabetic nephropathy.Journal of Diabetes and its Complications 12:43-60. |
|
|
Hosseinpoor AR, Stewart Williams J, Amin A, Araujo de Carvalho I, Beard J, Boerma T, Kowal P, Naidoo N, Chatterji S (2012). Social determinants of self-reported health in women and men: understanding the role of gender in population health. PLoS ONE 7:e34799. |
|
|
Kanakamani J, Ammini AC, Gupta N, Dwivedi SN (2010). Prevalence of microalbuminuria among patients with type 2 diabetes mellitus--a hospital-based study from north India.Diabetes Technology and Therapeutics 12:161-166. |
|
|
Kleinman HK, Cannon FB, Laurie GW, Hassell JR, Aumailley M, Terranova VP, Martin GR, DuBois-Dalcq M (1985). Biological activities of laminin. Journal of Cellular Biochemistry 27:317-325. |
|
|
Lehmann R, Schleicher ED (2000). Molecular mechanism of diabetic nephropathy. Clinica Chimica Acta, 297:135-144. |
|
|
Lott JA, Mitchell LC, Moeschberger ML, Sutherland DE (1992). Estimation of reference ranges: how many subjects are needed? Clinical Chemistry 38:648-650. |
|
|
Lynch KL, Wu AHB (2010). Renal Function In: Clinical Chemistry: Principles, Techniques, and Correlations, New York, Lippincott Williams & Wilkins. pp. 557-597. |
|
|
Mario UD, Morano S, Cancelli A, Bacci S, Frontoni S, Pietravalle P, Gambardella S, Andreani D (1989). New Parameters to Monitor the Progression of Diabetic Nephropathy. American Journal of Kidney Diseases 13:45-48. |
|
|
Matheson A, Willcox MDP, Flanagan J, Walsh BJ (2010). Urinary biomarkers involved in type 2 diabetes: a review. Diabetes-Metabolism Research and Reviews, 26:150-171. |
|
|
Miyake H, Nagashima K, Yagi H, Onigata K (1993). Urinary laminin P1 as an index of glycemic control in children with insulin-dependent diabetes mellitus. Diabetes research (Edinburgh, Scotland) 23:131-138. |
|
|
Naresh VVS, Reddy ALK, Sivaramakrishna G, Sharma PVGK, Vardhan RV, Kumar VS (2009).Angiotensin converting enzyme gene polymorphism in type II diabetics with nephropathy.Indian Journal of Nephrology 19:145-148. |
|
|
Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen C-E, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J, ADVANCE Collaborative Group (2009). Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. Journal of the American Society of Nephrology 20:1813-1821. |
|
|
Nyenwe EA, Odia OJ, Ihekwaba AE, Ojule A, Babatunde S (2003). Type 2 diabetes in adult Nigerians: a study of its prevalence and risk factors in Port Harcourt, Nigeria. Diabetes Research and Clinical Practice 62:177-185. |
|
|
Ogbu ISL, Iheanacho CP, Ofoegbu EN (2009). The Prevalence of Microalbuminuria in Type 2 Diabetic Patients in Enugu Metropolis.Journal of College of Medicine, 14(2).unpaginated. |
|
|
Oputa RN, Chinenye S (2015). Diabetes in Nigeria-A translational medicine approach. African Journal of Diabetes Medicine,23:7-10. |
|
|
Orluwene CG, Mommoh MO (2008).Screening for microalbuminuria in newly diagnosed type 2 diabetics at a staff clinic in Port Harcourt. Port Harcourt Medical Journal 3:10-14. |
|
|
Parchwani DN, Upadhyah AA (2012). Diabetic nephropathy: Progression and pathophysiology. International Journal of Medicine and Public Health 1:59-70. |
|
|
Remuzzi G, Schieppati A, Ruggenenti P (2002). Clinical practice.Nephropathy in patients with type 2 diabetes. New England Journal of Medicine 346:1145-1151. |
|
|
Takahashi M (1995) Increased urinary fibronectin excretion in type II diabetic patients with microalbuminuria. Nihon JinzoGakkai Shi 37:336-342. |
|
|
Vasiliades J (1976). Reaction of alkaline sodium picrate with creatinine: I. Kinetics and mechanism of formation of the mono-creatinine picric acid complex. Clinical Chemistry 22:1664-1671. |
|
|
Weissman M, Klein B (1958). Evaluation of glucose determinations in untreated serum samples.Clinical Chemistry 4:420-422. |
|
|
Whiting DR, Guariguata L, Weil C, Shaw J (2011). IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice 94:311-321. |
|
|
Yamazaki M, Tani N, Igarashi K, Nakamura H, Momotsu T, Ito S, Shibata A (1991). Changes in the glomerular pore size selectivity in patients with type II diabetes mellitus. Journal of Diabetic Complications, Proceedings of the International Symposium on Diabetic Nephropathy 5:138-139. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0