ABSTRACT
Information on the occurrence of pharmaceuticals in the environment of Nigeria is limited, only a single publication previously on pharmaceutical occurrence in the environment of Nigeria, which measured general estrogen levels in Enugu, South-East Nigeria. In order to establish a first overview, surface water samples from six locations as well as ten sewage sludge samples from waste water treatment plants were analysed for a range of different pharmaceuticals, including antibiotics, estrogens, and lipid-lowering drugs. The results of this monitoring campaign were evaluated in comparison to published measured environmental concentrations in Africa and worldwide. In surface water samples, 12 of 37 pharmaceutical substances were detected at concentrations ranging from Limits of Detection (LOD) up to 8.84 µg/l. Four of these pharmaceuticals were found at concentrations exceeding ecotoxicological predicted no-effect concentrations (PNEC). In industrial, domestic, and hospital sewage sludge, nine different pharmaceutical substances were detected with the NSAID diclofenac present in all samples at concentrations of up to 1100 µg/kg dry weight, exceeding the highest measured concentration of 560 µg/ kg reported in sludge samples worldwide. This study proves the presence of several pharmaceuticals at relevant concentrations in the environmental matrices studied. Further, more comprehensive monitoring campaigns, especially in locations with high population density and low dilution of treated or untreated waste water in receiving streams are recommended.
Key words: Nigeria, pharmaceuticals, liquid chromatography-tandem mass spectrometry (LC-MS/MS), water, sludge.
The global presence of pharmaceuticals in the environment has been validated as a real and ongoing situation of concern by several studies. This has in part led to the current initiative to have pharmaceuticals included in the list of environmentally and ecologically active chemical entities, under the global SUNEP (UBA, 2014). Pharmaceuticals enter subterranean and surface water environments after human/veterinary use in trace amounts regardless of attenuating bio-physical processes, which include degradation, dilution, and sorption (Scheytt et al., 2001; WHO, 2012), primarily through excretion and sewage release as completely non-metabolised parent drugs, partly metabolized bio-active metabolites, and re-activatable pro-drugs (Bound and Voulvoulis, 2005; Jjemba, 2008). Pharmaceutically Active Compounds [PhACs] have also been demonstrated to be incompletely removed from waste waters by current sewage treatment regimes, with som entirely unchanged by such processes (Nakada et al., 2007; Miege et al., 2009).
Since human waste streams have been implicated as the most significant sources of environmental pharmaceutical loading (Bound and Voulvoulis, 2005) the degree of human waste impaction on surface water bodies conceivably should determine the concentrations of detectable PhAC residues for individual locations. In developing country urban situations as studied here, waste water treatment is inadequate and sanitation poor. PhACs could potentially enter surface waters without any preliminary treatment, and the effects consequent on this fact, especially on non-target species such as fish and other aquatic fauna, could potentially be even more profound than conjectured at present.
Sewage Treatment Facility (STF) sludge could be a significant catchment matrix and source for environmental, especially water loading of pharmaceutical residues (Carballa et al., 2004; Ternes et al., 2004), especially where such post-treatment sludge is used as manure for crop production. Lagos, the commercial capital of Nigeria, with an estimated population of about 12,614000 million people (UN, 2014), is one of the world’s fastest growing metropolises, already gaining megacity status. High population density clusters in largely unplanned urban and peri-urban locations, poverty, and the overstretched public infrastructure and works, are largely descriptive of living conditions in Lagos.
The generally poor sanitary conditions in most of the city and suburbs, aggravated by the tropical climate characterized by seasonal heavy rains, and inadequate primary sewage treatment, make wastewater impaction on surface waters a stark and ongoing reality. This study seeks to identify and quantify the levels of pharmaceutical residues in some surface waters and sewage sludge from locations within Lagos municipality, and a nearby state, and to compare detected concentrations with existing information from other studies for perspective.
Sampling locations
Selection criteria for the locations surveyed included population (numbers, density, commercial activities, etc.), proximity to hospitals, pharmaceutical manufacturing concerns, and waste disposal sites. The chosen locations were Amuwo-Odofin, Ajido via Badagry, Isolo, Liverpool/Apapa, Ojo, and from the river Owo, a tributary of River Ogun which receives treated and untreated effluents from the Agbara Industrial Estate in Lagos and municipalities in Ogun States, south west Nigeria which it flows down through (Figure 1).
Sewage sludge
Sewage sludge was sampled in four domestic wastewater treatment plants, five industrial effluent treatment plants and one hospital wastewater treatment plant from January to April 2012 at different locations in Lagos, Oyo and Ogun State, all in South West Nigeria (Sindiku et al., 2013). Table 1 gives details of the sampled plants.
Water collection, processing, and analysis
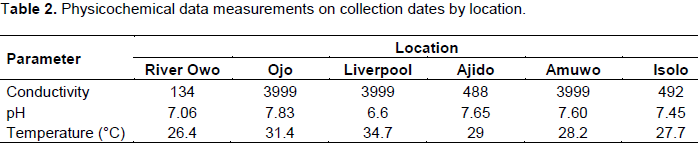
Water samples were collected (between August and September 2013) in duplicate, in the morning from each of the six locations into 500 mL amber coloured PET capped bottles, ensuring that contamination of the collection equipment, and samples before, during and after collection was minimal. The “clean hands, dirty hands” protocol (USGS, 2013) for ensuring the latter was employed in all cases. All locations were flowing water sites. Water was collected using the dip sampling method, which involved de-capping the bottles and dipping them below the surface till full. A little of the contents were routinely poured away to ensure enough room for expansion during cold storage. Field measurements including temperature, DO, conductivity and pH were done immediately after collection using a handheld Henna instruments® model HI-98129 meter (Table 2). Following collection, water bottles were placed in Styrofoam coolers with ice packs and transferred to the laboratory where kept at -20°C until analysed.
Analyses of water samples were carried out by IWW Water Centre in Muelheim, Germany according to published guidelines or validated in-house methods.
Polar pharmaceutical substances
Sample aliquots were spiked with an internal standard (4-chlorobenzoic acid), and adjusted to pH 2 by HCl addition. SPE was done using a pre-conditioned SPE column (200 mg ENV+ adsorber material). The analytes were eluted in 9 mL of ethyl acetate, concentrated to a volume of 200 µL under a gentle stream of nitrogen, and derivatized with diazomethane following DIN EN ISO 15913 guidelines prior to GC-MS analysis (column: Phenomenex ZB 50 or J&W DB 5).
Non-polar pharmaceutical substances
Sample aliquots spiked with an internal standard (Fluazifo-butyl) and adjusted to pH 7 by NaOH addition were extracted using pre-conditioned SPE columns (200 mg ENV+) as before. Desired analytes were eluted in 6 mL of acetone and concentrated to a volume of 200 µL prior to GC-MS analysis.
All GC-MS analyses was done using an Agilent GC-MS system (6890 GC and 5973 MSD single quadrupole mass analyzer) equipped with an automatic sampler (MPS 2) and Cooled Injection System (CIS 4, Gerstel, Mülheim, Germany).
Antibiotics
Typical sample aliquots were adjusted to pH 3 by HCl addition and extracted on preconditioned SPE columns (500 mg ENV+). The analytes were eluted in 10 mL of methanol, concentrated to dryness, and transferred into 1 mL of acetonitrile/water (v/v 60:40) solvent prior to analysis by LC‑MS/MS (column: Nucleosil C18 HD solvent prior to analysis by LC MS/MS (column: Nucleosil C18 HD 250 × 3 mm, 5 µm).
Tetracyclines
The analysis of the tetracyclines was conducted without pre-concentration using SPE by LC-MS/MS (column: Discovery HS C18150 x 2.1 mm, 3 µm).
Hormones
SPE was done on sample aliquots adjusted to pH 2 by HCl addition using preconditioned SPE columns (1.0 g RP-C18-Polar-Plus-Material). The analytes were eluted four times in 3 mL of methanol, concentrated to a volume of 100 µL, and diluted to a final volume of 500 µL by addition of water/methanol solvent prior to analysis with LC-MS/MS (column: Synergie Polar 4 µm, 250 mm × 2 mm ID). Waters Acquity ultra performance liquid chromatography (UPLC-TQD/PDA and UPLC-TQS) coupled with an electro-spray ionization tandem mass spectrometric system (Milford, USA) was used to carry out LC-MS/MS analyses.
Data acquisition, processing, and evaluation were carried out using Agilent ChemStation software combined with Gerstel Maestro software package. The limit of detection (LOD) was defined by a signal to noise ratio of 3:1 for all applied methods.
Sewage sludge collection, processing, and analysis
Sludge samples were collected from wastewater treatment plants and the respective sewage stabilization ponds in amber glass flasks. Samples were transported to the laboratory on ice for further treatment and processing. Sludge samples had a water content of approximately 95%.
The samples were air-dried, ground, and homogenized by sieving through a stainless steel 2-mm sieve, and consequently stored in a freezer (Sindiku et al., 2013). Analysis of the freeze-dried sewage sludge samples were carried out by the Institute of Energy and Environmental Technology (IUTA), Duisburg, Germany. Samples were extracted by accelerated solvent extraction (ASE 200, Dionex, Idstein, Germany) during a single extraction cycle of 15 min at temperature and pressure settings of 100°C and 100 bar respectively, using methanol as solvent. After evaporation, resolving and filtering, pharmaceuticals were analysed using an Agilent 1100 HPLC (Agilent Technologies, Böblingen, Germany), which was coupled to an API 3000 mass spectrometer (AB Sciex, Darmstadt, Germany).
The separation of the analytes was performed with a Synergi 4u Polar –RP 80A column (Phenomenex). Confirmation measurements were carried out on a SymbiosisTM Pico LC system (Spark Holland, Emmen, Netherlands) connected to a QTRAPTM 6500 mass spectrometer (AB SCIEX, Darmstadt, Germany). Analyst 1.6 software was used for data acquisition and processing. Limits of detection were defined as a signal to noise ratio of 3:1 for the applied method. Samples were quantified with the method of standard addition according to DIN 32633. Hormone samples were analysed by GC-MS.
Therefore, extracted samples were filtered, dried until dryness, derivatized, and analysed using a Thermo Trace GC and a Thermo ISQ mass analyser (Thermo Fisher Scientific GmbH, Dreieich, Germany). Data acquisition, processing, and evaluation were carried out using Excalibur software.
Water
Preliminary results from analysis of water samples for the presence or absence of a wide variety of pharmaceuticals and their degradates yielded information about the presence of twelve pharmaceutical entities out of Thirty seven checked for, which comprised of six antibiotics, four non-steroidal anti-inflammatory analgesics (NSAIDs), one antilipidemic and, phytoestrogen each (Table 3).
.png)
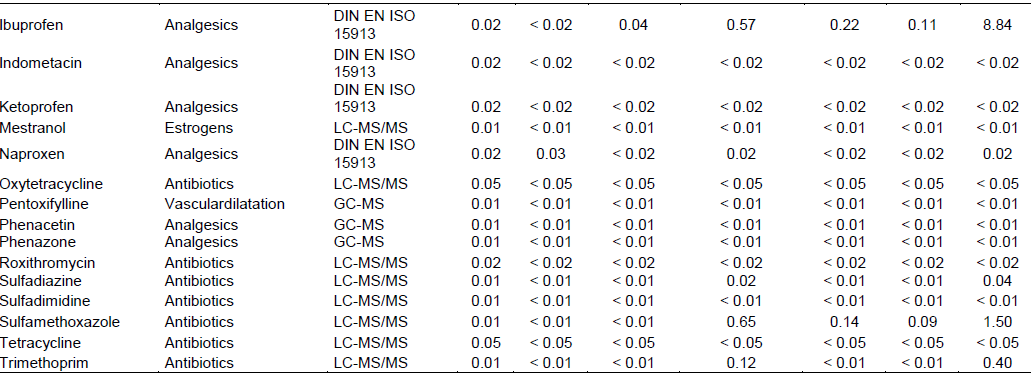
The detected agents were found at concentrations exceeding the detection limit of used analytical methods. The highest individual pharmaceutical concentrations measured were detected in the sample taken from the Isolo sampling location for ibuprofen (8.84 µg/l), sulfamethoxazole (1.5 µg/l) and erythromycin (1.0 µg/l). Total locational concentrations for determined pharmaceuticals were highest for the Isolo location at 13.55 µg/l followed by Amuwo Odofin at which total concentrations were 2.13 µg/l. All compounds detected were found in the water sample from Amuwo Odofin, followed by Isolo where all but acetylsalicylic acid were detected (Table 2).
Betasitosterol was detected in all locations including Isolo where detected levels were highest (0.67 µg/l). Ajido recorded the fewest pharmaceutically active compounds with just two entities detected, betasitosterol and naproxen. Detection frequency of detected residues were in the order betasitosterol (100%) > ibuprofen (45.5%) > chloramphenicol, diclofenac and sulfamethoxazole (33. 37%) > naproxen (27.27%) > erythromycin–A--dihydrate, erythromycin, sulfadiazine and trimetoprim (18%) >clofibrate (9%).
Table 4 displays maximum concentrations measured in the six water samples from Nigeria in comparison to measured concentrations in surface water or groundwater in other African countries, and in comparisonto ecotoxicological effect concentrations (PNEC predicted no effect concentrations).
Sewage sludge
Nine of nineteen pharmaceutical substances were detected in at least one of the studied ten sludge samples (Table 4). Diclofenac was detected in each of the ten sludge samples in a concentration range of < 10 to 1140 µg/kg dry weight, followed by Carbamazepine with positive detection in nine samples (< 10 to 70 µg/kg) and Erythromycin in five samples (< 10 to 147 µg/kg). Bezafibrate, Clarithromycin, Ibuprofen, Metoprolol, Propranolol, Sulfamethoxazole, and Trimethoprim were positively detected in one or two sludge samples only (concentration range < 10 to 360 µg/kg). Two substances (Fenofibric acid and Naproxen) could not been evaluated due to high matrix interferences. Tables 5 and 6 contain details of maximum concentrations measured in the ten sewage sludge samples from Nigeria in comparison to maximum concentrations in sewage sludge samples measured in Africa and worldwide respectively
There are several routes of entry of human and veterinary pharmaceuticals into environmental matrices including water. Earlier work has firmly established the presence of pharmaceutically active compounds, their derivatives and other forms of the active moieties in all environmental water compartments (Servos et al., 2007; Sanderson, 2011; Jongh et al., 2012). Potential hazards due to pharmaceutical release into the environment have become increasingly important to government regulators, the pharmaceutical industry and the lay public which has increasingly more access to information about the issue, sometimes not from the most factual sources e.g. the popular media. It however cannot be said that the public’s renewed interest in this matter is misplaced.
The increased detection capabilities using bio-analytical approaches are increasingly being enhanced as a result of improved analytical capabilities and thecommissioning of focused field surveys (Daughton, 2001; Focazio et al., 2004).
There are arguments that the levels at which these are found are well below those deemed inimical to human health. However, other pertinent perspectives look farther than the latter and try to establish the effects of long term/chronic, sublethal effects on the environment and denizen flora and fauna, a perspective called pharmacoenvironmentology, a term still to catch on in the regular ecotoxicological lexicon, but which encapsulates the new thinking about the relevance of continual pharmaceutical loading of the environment and the need for constant surveillance of impacted environments.
Most work on Nigeria has focused on heavy metal pollution, and several papers exist documenting this phenomenon while others are being churned out on a regular basis. To our knowledge, there has been only a single publication previously on pharmaceutical occurrence in the environment of Nigeria, which measured general estrogen levels in Enugu, South-East Nigeria (Maduka et al., 2010).
There thus exists a need to deepen the focus of research into water, especially, micro-pollutant water pollution, as the probable effects on resident humans, denizen fisheries, and potable water quality could be inimical to the interests of surrounding populations, the generality of whom are poor and for who fish remains the most affordable source of protein. As noticed in other countries where pharmaceutical pollution of ground, surface and drinking water have been well documented, and household and healthcare facility wastes and effluents incriminated in such pharmaceutical loading, it is conceivable that these sources could also be significant contributors in Nigeria.
In Nigeria, as is common elsewhere, purpose built STFs are of the activated sludge type. For the most part, wastewater treatment is ineffective or frankly lacking in Lagos, thus the attempts at improved water treatment to reduce quantities of active pharmaceutical ingredients by STF treatment before release and reuse are not applicable. In continuation, management and use practices in various parts of the world are incongruent as exposure pathways in one may be less important in another, and thus not directly extrapolatable between countries (Boxall et al., 2012).
The choice of water collection locations for pharmaceutical presence estimations in this study was deliberately done on the basis of human populations in these areas, relative to the total population of the city itself.
Since the major route for pharmaceuticals’ entry to surface water is primarily through discharge of human wastewater into surface waters such as streams, rivers, and lakes, water samples from the selected locations (generally sewage impacted) were more likely than others to show detectable levels of these substances and measured concentrations conceivably should follow a trend along impaction severity and population. The exception in this deliberate choosing of sample locations was Ajido, a location with a relatively smaller population as compared to other locations. It was thus chosen as the reference location.
In the present study twelve pharmaceutical entities were determined and discovered at environmentally significant concentrations in water samples. Higher concentrations have been previously recorded for ten of eleven pharmaceuticals in other African countries than in the present study.
However, erythromycin has been found in higher concentrations in the Nigerian water samples than in any other African surface water investigation. Notably, four substances (Acetylsalicylic acid, Beta-Sitosterol, Clofibric acid, and Sulfadiazine) detected in the present study have not previously been measured/not been reported in studies from other African countries. Four of the pharmaceuticals measured in Nigerian surface waters were found at concentrations exceeding ecotoxicological effect concentrations (Chloramphenicol, Diclofenac, Erythromycin, and Sulfamethoxazole). It therefore has to be expected that these pharmaceutical substances have adverse effects on ecosystem health in Nigerian surface waters at these locations though as yet undocumented. The frequency of detection of antibiotic and NSAID residues in samples from studied locations is an indication of their widespread use, as observed in other locations in countries worldwide. The especially high concentrations of erythromycin, sulfamethoxazole and Ibuprofen in the sample ISOLO could be attributable to the proximity of the water collection location to a midsized government (referral) hospital, and a large municipal landfill in which a significant quantity of solid wastes from the hospital ends up.
It is conceivable that the common denominator explaining the general presence of beta-sitosterol in study locations would be faecal contamination with coprosterols. No endogenous or synthetic animal estrogens were detected in this study. Considering the dense population clusters in most of the studied locations and the virtually uncontrolled entrees of wastewaters and domestic sewage effluents into the surface waters same, the opposite would have been expected.
This situation could be explained in part considering that untreated wastes which impact the studied waters are often heavily laden with sewage microorganisms. In sewage treatment procedures, heterotrophic bacteria are routinely utilised for the controlled biodegradation of pharmaceuticals in sewage, and though the removal of estrogens is not total following such treatment, the levels are significantly reduced. Benka-Coker and Ojior (1995) reported high levels of heterotrophic bacteria in a sewage impacted river in Nigeria, corroborating results from a similar study by Olayemi (1994) which also established higher heterotrophic bacterial counts during the rainy season, probably due to increased sewage overflows into surrounding water bodies. Incidentally, our water collection was carried out during the rains. There are at the present time no functional STFs in all the studied locations, thus it is possible that the high levels of heterotrophs could be responsible for the increased mobilization and breakdown of endogenous human estrogens found in domestic sewage, and the dilution effect of higher water levels and flow during the rainy season could account for the null returns from analysis of sampled waters for these compounds.
At present, there are no verifiable figures for clofibrate prescription quantities in Nigeria. It is thus difficult to link concentrations detected and frequencies of detection to usage figures and statistics, information which could help explain the limited occurrence of clofibric acid in the current study. Considering the well documented persistence of clofibric acid in the environment, and specifically its resistance to photodegradation (most important here where concerted sewage treatment is poor), it would have been expected that clofibric acid would be detectable at more locations than found, due to continuing use and the aforementioned inherent environmental persistence. In the absence of more comprehensive usage data, the low levels of detection and concentrations of clofibric acid could attributable to low prescription rates in the studied locales. It is also conceivable that later studies which would include more locations, some highbrow (as obesity and consequent hypercholesteremia in Nigeria, though increasing across socioeconomic divides, is still more of a condition linked to affluence), would yield more representative results.
Pharmaceuticals have been demonstrated to exist in sewage sludge at concentrations much higher than in STF influents and effluents. Generally, study data on pharmaceutical occurrences in sewage sludge are few compared to surface water and STF data. None exist for Nigeria, and indeed Africa except those reported here. The NSAIDs, diclofenac and ibuprofen were found the highest concentrations in sludge with diclofenac occurring in all samples. Carbamezapine, an anticonvulsant and mood stabilising agent, was also generally discovered in all locations in significant quantities.
The well documented biorecalcitrance and environmental persistence coupled with poor removal rates by treatment may account for the near ubiquitous presence and high concentrations of diclofenac and carbamezapine recorded in the current study.
In addition the especially high diclofenac concentrations in the locations Agbara and Ikeja, could be due to the presence of many pharmaceutical production facilities in both locations which are both industrial/residential layouts. In Nigeria the use of STF sludge as fertiliser is not widespread, but studies into the presence of pharmaceuticals in soil are needed to give a more balanced-overall picture of pharmaceutical micropollution of the environment and associated ecological sequelae.
In summary, this study has confirmed the presence of pharmaceutically active compounds in waste water impacted surface water bodies and sewage sludge in Lagos, Nigeria. The findings from this investigation has also established that the concentrations of detected PHACs, and the locational frequency of detection are comparable to reports from other countries, especially from developing countries from where unfortunately, such reports are few, limiting further comparisons.
Based on the results, it was concluded that several pharmaceuticals occur at relevant concentrations in the environment of Nigeria. It is recommended that more comprehensive water monitoring campaigns be conducted, especially in locations with high population density and low dilution of treated or untreated wastewater in receiving streams. Furthermore, investigations of groundwater, tap water/drinking water, manure, soil, and sediments as additional matrices of concern needs to be done in the nearest future to help generate wholistic pictures of the spatial environmental presences and concentrations of PhACs in them. In doing this, representative sampling strategies, improved sample conservation and pre-treatment should be used.
This work was funded by the German Federal Environmental Agency (UBA), Project No. 31667. The authors acknowledge sampling of sludge samples by Professor Osibanjo, and Omotayo Sindiku (both of the University of Ibadan). Thanks to Dr. Roland Weber (POPs Environmental Consulting). Authors also especially thank Umweltbundesamt (Dr. Arne Hein, Dr. Anette Küster, and Ina Ebert) for financing, support, and valuable discussion.
The authors have not declared any conflict of interest.
REFERENCES
|
Andaluri G, Suri RS, Kumar K (2012). Occurrence of estrogen hormones in biosolids, animal manure and mushroom compost. Environ. Monit. Assess. 184(2):1197-1205.
Crossref
|
|
|
|
Andersson J, Woldegiorgis A, Remberger M, Kaj L, Ekheden Y, Dusan B, Svenson A, Brorström-Lundén E, Dye C, Schlabach M (2006). Results from the Swedish National Screening Programme 2005. Stockholm, Sweden.
|
|
|
|
|
Aneck-Hahn NH, Bornman MS, de Jager C (2009). Oestrogenic activity in drinking waters from a rural area in the Waterberg District, Limpopo Province, South Africa. Water SA 35(3):245-251.
|
|
|
|
|
Benka-Coker MO, Ojior, OO (1995). Effect of slaughterhouse wastes on the water quality of Ikpoba River, Nigeria. Bioresour Technol. 52(1):5-12.
Crossref
|
|
|
|
|
Bergmann A, Fohrmann R, Weber FA (2011). Zusammenstellung von Monitoringdaten zu Umweltkonzentrationen von Arzneimitteln (in German). UBA-Texte 66/2011,Umweltbundesamt,Dessau-Roßlau.
|
|
|
|
|
Bound JP, Voulvoulis N (2005). Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environ. Health Perspect. 113(12):1705-1711.
Crossref
|
|
|
|
|
Boxall A, Rudd M, Brooks B, Caldwell D, Choi K, Hickmann S, Innes E, Ostapyk K, Staveley J, Verslycke T, Ankley G, Beazley K, Belanger S, Berninger J, Carriquiriborde P, Coors A, DeLeo P, Dyer S, Ericson J, Gagné F, Giesy J, Gouin T, Hallstrom L, Karlsson M, Larsson D, Lazorchak J, Mastrocco F, McLaughlin A, McMaster M, Meyerhoff R, Moore R, Parrott J, Snape J, Murray-Smith R, Servos M, Sibley P, Straub J, Szabo N, Topp E, Tetreault G, Vance LT (2012). Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. pp. 120-129.
Crossref
|
|
|
|
|
Carballa M, Omila F, Lema JM, Llompart M, García-Jares C, Rodríguez I, Gómez M, Ternes T (2004). Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 38(12):2918-2926.
Crossref
|
|
|
|
|
Daughton C (2001). Pharmaceuticals in the environment: Overarching issues and overview. In: Daughton CG, Jones-Lepp TL (eds.). Pharmaceuticals and Personal Care Products in the Environment: Scientific and Regulatory Issues. Symposium Series 791; American Chemical Society. Washington DC USA.
|
|
|
|
|
de Jager C, Swemmer A, Aneck-Hahn NH, van Zijl C, van Wyk S, Bornman MS, Barnhoorn I, Jonker M, van Vuren JHJ, Burger AEC (2011). Endocrine Disrupting Chemical (EDC) Activity and Health Effects of Identified Veterinary Growth Stimulants in Surface and Groundwater; WRC Report No. 1686/1/11; Water Research Commission South Africa.
|
|
|
|
|
Federal Environment Agency (UBA) (2014). Pharmaceuticals in the environment, global occurrence, effects, and options for action. Report of an International Workshop held April 8th and 9th 2014, International Environment House II, Geneva, Switzerland.
|
|
|
|
|
Fick J, Lindberg RH, Kaj L, Brorström-Lundén E (2011). Results from the Swedish National Screening Programme 2010. Stockholm, Sweden, P 56.
|
|
|
|
|
Focazio MJ, Kolpin DW, Furlong ET (2004). Occurrence of human pharmaceuticals in water resources of the United States: A review. In: Kummerer, K. (ed.) Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks. 2nd ed. Berlin: Springer.
Crossref
|
|
|
|
|
Gardner M, Jones V, Comber S, Scrimshaw MD, Coelloâ€Garcia T, Cartmell E, Lester J, Ellor B (2013). Performance of UK wastewater treatment works with respect to trace contaminants. Sci. Total Environ. pp. 359-369, 456-457.
Crossref
|
|
|
|
|
Hendricks R (2011). Assessment of the biological quality of raw and treated effluents from three sewage treatment plants in the Western Cape, South Africa. Doctoral dissertation. Retrieved from University of the Western Cape electronic thesis and dissertations repository.
|
|
|
|
|
Ivashechkin P (2005). Literaturaus ertung zum Vorkommenge fährlicher Stoffeim Abwasser und in Gewässern. Berichtzum Vorhabenim Auftrag des Ministeriums für Umwelt und Naturschutz, Landwirtschaft und Verbraucherschutz Nordrhein-Westfalen.
|
|
|
|
|
Jjemba P (2008). Pharmaecology: The occurrence and fate of pharmaceuticals and personal care products. Hoboken NJ: John Wiley and Sons.
|
|
|
|
|
Jongh CD, Kooij P, Voogt PD, Laak TT (2012). Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water. Sci. Total Environ. pp. 70-77, 427-428.
Crossref
|
|
|
|
|
K'Oreje KO, Demeestere K, De Wispelaere P, Vergeynst L, Dewulf J, Van Langenhove H (2012). From multi-residue screening to target analysis of pharmaceuticals in water: Development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River Basin, Kenya. Sci. Total Environ. 437:153-164.
Crossref
|
|
|
|
|
Kouadio LD, Traore SK, Bekro YA, Véronique M, Dembele A, Mamadou K, Mazellier P, Legube B, Houenou P (2009). Contamination des Eaux de Surface par les Produits Pharmaceutiquesen Zones Urbaines de Côte D'ivoire: Cas du District D'abidjan. Eur. J. Scient. Res. 27(1):140-151.
|
|
|
|
|
Miege C, Choubert JM, Ribeiro L, Eusebe M, Coquery M (2009). Fate of pharmaceuticals and personal care products in wastewater treatment plants - Conception of a database and first results. Environ. Pollut. 157:1721-1726.
Crossref
|
|
|
|
|
Nakada N, Shinohara H, Murata A, Kiri K, Managakia S, Sato N, Takada H (2007). Removal of selected pharmaceuticals and personal care products [PPCPS] and endocrine-disrupting chemicals [edcs] during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 41:4373-4382.
Crossref
|
|
|
|
|
Nieto A, Borrull F, Marcé RM, Pocurull E (2007). Selective extraction of sulfonamides, macrolides and other pharmaceuticals from sewage sludge by pressurized liquid extraction. J. Chromatogr. A. 1174(1-2):125-131.
Crossref
|
|
|
|
|
Okuda T, Yamashita N, Tanaka H, Matsukawa H, Tanabe K (2009). Development of extraction method of pharmaceuticals and their occurrences found in Japanese wastewater treatment plants. Environ. Int. 35(5):815-820.
Crossref
|
|
|
|
|
Olayemi AB (1994). Bacteriological water assessment of an urban river in Nigeria. Int. J. Environ. Health Res. 4(3):b156-164.
Crossref
|
|
|
|
|
Sanderson H (2011). Presence and risk assessment of pharmaceuticals in surface water and drinking water. Water Sci. Technol. 63:2143-2148.
Crossref
|
|
|
|
|
Scheytt T, Mersmann P, Heberer T, Reddersen K (2001). Natural attenuation of pharmaceuticals. In Proceedings of the 2nd International Conference on Pharmaceuticals and Endocrine Disrupting Chemicals in Water, October pp. 9-11.
|
|
|
|
|
Senta I, Terzic S, Ahel M (2013). Occurrence and fate of dissolved and particulate antimicrobials in municipal wastewater treatment. Water Res. 47(2):705-714.
Crossref
|
|
|
|
|
Servos M, Smith M, McInnis R, Burnison B, Lee B, Seto P, Backus S (2007). The presence of selected pharmaceuticals and the antimicrobial triclosan in drinking water in Ontario, Canada. Water Qual. Res. J. Can. 42:130-137.
|
|
|
|
|
Sindiku O, Orata F, Weber R, Osibanjo O (2013). Per- and polyfluoroalkyl substances in selected sewage sludge in Nigeria. Chemosphere 92:329-335.
Crossref
|
|
|
|
|
Takada H, Shimizu A, Koike T, Takeshita A, Nakada N, Suzuki S (2012). Ubiquitous distribution of sulfamethoxazole in tropical Asian and African waters. SETAC 6th World Congress/SETAC Europe 22nd Annual Meeting, Berlin, Berlin.
|
|
|
|
|
Ternes TA, Herrmann N, Bonerz M, Knacker T, Siegrist H, Joss A (2004). A rapid method to measure the solid-water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res. 38(9):4075-4084.
Crossref
|
|
|
|
|
Tredoux G, Genthe B, Steyn M, Germanis J (2012). Managed aquifer recharge for potable reuse in Atlantis, South Africa. In: Water reclamation technologies for safe managed aquifer recharge. Eds: Christian Kazner TW, TMelin T, Dillon pp. 121-140.
|
|
|
|
|
United Nations Department of Economic and Social Affairs, Population
|
|
|
|
|
USGS (2013). Organic W astewater Compounds in W ater and Sediment in and near Restored Wetlands, Great Marsh, Indiana Dunes National Lakeshore, 2009 - 11. pdf. Available at:
View
|
|
|
|
|
WHO (2012). Pharmaceuticals in drinking-water. W orld Health Organization, Geneva 35pp.
|
|