Full Length Research Paper
ABSTRACT
Municipal wastewater contains high levels of pollutants since it is a collection of wastes from various sources of human activities. Municipal treatment of such wastewater is usually aimed at lowering the levels of pollutants to permissible amounts before discharge into recipient environment. Several conventional methods are available for treatment of wastewater. However, most of them are costly and not economically feasible due to their secondary environmental impact. Wastewater treatment using natural plants has been considered the most environmentally friendly method. A study has been conducted to establish the metal removal rates from waste water using arrowroots. To establish if arrowroots can tolerate high levels of pollutants under natural conditions, raw arrowroots and raw wastewater were used. Pollutant removal efficiency levels was carried out by setting up a model constructed wetland where arrowroots were planted in raw influent of municipal wastewater and concentration levels of both planted and unplanted influent and effluent determined. Pollutant removal efficiency was compared using the relative treatment efficiency index technique. The results of the metal removal rates in wastewater cultivated with arrowroots were: Fe (68%), Mn (98%), Zn (54%) and Cu (50%) while pollutant removal in wastewater without arrowroots were: Fe (38%), Mn (48%), Zn (9%) and Cu (9%). The percent pollutant removal rate when arrowroots were planted in the effluent from unplanted maturation ponds was: Fe (97%), Mn (97), Zn (48%) and Cu (50%) with relative treatment efficiency of 0.28, 0.34, 0.71, and 0.65, respectively and p < 0.002 at 5% confidence interval. The difference in percent removal showed that more metal ions could be removed both from the raw sewage and final effluent when cultivated with arrowroots than in uncultivated maturation ponds, suggesting that arrowroots can be of significant benefit as a tertiary wastewater treatment alternative.
Key words: Wastewater, treatment, efficiency, influent, effluent, pollutants
INTRODUCTION
Constructed wetlands of natural plants are proving to be a valid treatment option for hazardous wastewaters, petroleum refinery wastes, compost and landfill leachates, agricultural wastes and pre-treated industrial wastewaters, such as those from tannery, pulp and paper mills and textile mills, treatment of raw sewage among others (Igwe and Abia, 2007; Volesky, 2007; Karanthanasis and Johnson, 2003; Ramirez, 2002; Tonderski et al., 2005; Maine et al., 2006; Calheiros et al., 2007; Molle et al., 2005).
The most significant functions of wetland emergent plants in relation to water purification are the physical effects brought by the presence of the plants, which provide a huge surface area for attachment and growth of microbes. The physical components of plants stabilize the surface of the beds, slow down the water flow, thus assist in sediment settling and trapping process and finally increasing water transparency. Wetland plants play a vital role in the removal and retention of nutrients and help in preventing the eutrophication of wetlands. A range of wetland plants has shown their ability to assist in the breakdown of wastewater. These plants have a large biomass both above (leaves) and below (underground stem and roots), the surface of the substrate. Turnover of root mass in plants creates macropores in a constructed wetland soil system allowing for greater percolation of water, thus increasing effluent/plant interactions (Marchand et al., 2010). The sub-surface plant tissues grow horizontally and vertically, and create an extensive matrix, which binds the soil particles and creates a large surface area for the uptake of nutrients and ions (Reeb and Werckmann, 2005).
Contaminants are removed from wastewater through several mechanisms. Processes of sedimentation, microbial degradation, precipitation and plant uptake remove most contaminants. Heavy metals in a wetland system may be sorbed to wetland soil or sediment, or may be chelated or complexed with organic matter. Metals can precipitate out as sulfides and carbonates, or get taken up by plants. Compounds in sediment, such as iron oxides, show preference for certain metals. This behavior can affect how efficiently of a metal is adsorbed in a wetland. A system that has reached the limits of its adsorption capacity can exhibit a reduction in contaminant removal rates. After a system has reached its capacity for metal sorption, metal sulfide formation becomes the main method of metal removal. Sulfate-reducing bacteria oxidize organic matter and reduce sulfate to form hydrogen sulfide. Hydrogen sulfide reacts with metals to form metal sulfides, which precipitate. Compared to sediments, plants do not take up much metal, but they are involved in oxygenation and microbiological processes that contribute to the ability of the wetland to remove metals (Reeb and Werckmann, 2005).
EXPERIMENTAL DESIGN
Pollutant removal efficiency levels from wastewater using arrowroots was carried out by setting up a model constructed wetland where arrowroots were planted in raw influent of wastewater obtained at a waste water treatment plant at a quarry in Eldoret and concentration levels of both influent and effluent determined. Pollutant removal efficiency of both treated and untreated wastewater was compared by using the RTEI technique and RII index as suggested by Marchand et al. (2010). The efficiency of the metal removal with and without the arrowroot was investigated using the differences in metal removal between the influent and the effluent for the treatment (T%) with the arrowroot and compared with the control (C%) which was wastewater without the arrowroots. To obtain this, the simplified equation
was used. Values of RTEI approaching 1 indicated strong benefits of metal removal, values around 0 indicated no effect of the treatment when arrowroots were used, whereas values approaching -1 indicated strong inhibition of the metal removal (Marchand et al., 2010). The effluent of the planted and the unplanted treated wastewater were also compared. The findings were recorded in Tables 1 and 2 and Figure 1 and 2.
RESULTS AND DISCUSSION
Table 1 shows results of percent removal efficiency of metal ions by life arrowroots from wastewater compared to biological method.
From Table 1, the percent removal of metal ions from raw sewage without arrowroots after 7 days of allowing natural biological action in the maturation ponds was: Fe (38%), Mn (48%), Zn (9%) and Cu (9%). However, for the same raw sewage that was cultivated with arrowroots in a model constructed wetland environment, the percent reduction after 7 days was; Fe (68%), Mn (98%), Zn (54%) and Cu (50%) with order of preference of Mn > Fe > Zn > Cu. This trend could have partly been influenced by the initial concentration of the metal ions in the raw sewage and the ionic size. Mn, having higher concentration levels in raw sewage were preferentially adsorbed followed by Fe and the least was Cu probably due to its very low concentration levels. This trend was also similar to that established by Marchand et al. (2010). This percent removal rate using the arrowroots was also found to be comparable with the removal rates of other plant species (Maine et al., 2007). The difference in percent removal showed that more metal ions could be removed both from the raw sewage and final effluent when cultivated with arrowroots than in uncultivated maturation ponds. There was also higher percent removal of Mn and Fe by arrowroots in the final biologically treated effluent compared to raw sewage. This could be because of the low concentration in the final effluent after passing through the maturation ponds.
Figure 1 shows a significant difference in the removal of metal ions from waste water using unplanted maturation ponds with that cultivated with arrowroots. Further reduction of the metal ions in the final effluent after passing through natural unplanted ponds by arrowroots was also noted in the Figure 1, showing that arrowroots can either be planted in the raw sewage or final effluent passed through the planted arrowroots in order to reduce the level of metal ions to recommended levels before being released to rivers or of streams.
In order to test the significance contribution of using life arrowroots in removal of metal ions from municipal waste water, values obtained in Table 1 were subjected to relative treatment efficiency index (RTEI). V and null hypothesis tested using the paired t- test at 5 percent significant level (Kothari, 2011). Values obtained were recorded in Table 2.
In Table 2, C% represented percent removal for the wastewater without arrowroots and represented the control, while T% represented pollutant removal infot the treated wastewater with arrowroots. The RTEI in Table 2 was calculated using equation, RTEI=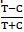 (Marchand et al., 2010). and null hypothesis worked out according to Kothari, (2011).
(Marchand et al., 2010). and null hypothesis worked out according to Kothari, (2011).
From the results in Table 2, the index which represented the net metal removal due to interaction of benefits and disadvantages of the treatment using arrowroots were high for Zn2+ and Cu2+ ions but slightly low for Fe2+ and Mn2+. All the values of RTEI were positive hence the positive benefit of arrowroots. RTEI were high for Zn and Cu hence indicated strong benefits for metal removal while RTEI values for Mn and Fe were less and hence indicating less effect on the treatment of the wastewater cultivated with arrowroots. This difference could have been again due to the difference in levels of the ions in raw sewage where high RTEI was high for Zn and Cu probably due to their low concentration levels.
This could possibly suggest that life arrowroots can be of more significant use if used to further treat recycled treated sewage by passing it through arrowroot wetlands and also as a tertiary wastewater treatment alternative. This could also imply that arrowroots can effectively be used to remove metal ions from dilute solutions containing relatively low levels of dissolved metals, thus supporting similar arguments that were made by Abdel and Elchaghaby (2007) and also Brisson and Chazarenc (2009).
Results in Figure 2 showed high percent removal of the four metal ions, when the arrowroots were cultivated in waste water. Low percent removal of the metal ion pollutant in wastewater without arrowroots was also observed. This showed that most metal ions were removed by the arrowroots. From the null hypothesis results; t(3) = 2.110, p < 0.002, the null hypothesis was rejected implying that arrowroots can significantly adsorb heavy metals from dilute solutions such as the river water and model solutions of wastewater containing higher concentrations of metal ions.
CONCLUSION
Life arrowroots effectively removed Zn, Cu, Fe and Mn from both raw untreated municipal wastewater and from already naturally treated wastewater effluents from maturation ponds. The following percent removal rates were established. From raw untreated waste water, the percent removal efficiency using arrowroots was; Fe (68%), Mn (98%), Zn (54%) and Cu (50%). These percentages were found to be higher compared to the percent removal efficiency for unplanted maturation ponds when left for 7 days where the same metal ions were removed by natural process. For unplanted ponds, the percent removal efficiency was; Fe (38%), Mn (48 %), Zn (9%) and Cu (9%). Arrowroots also effectively lowered the levels of the metal ions from the already treated waste water effluents from maturation ponds to minimal levels recommended for final discharge into surface waters such as rivers and streams by the following percent removal rates; Fe (97%), Mn (97%), Zn (48%) and Cu (49%). A paired t-test that was conducted to test the null hypothesis concerning the significance of life in removing metal ions from wastewater showed that the alpha level was below 0.05 at 5% confidence level (p < 0.002) and hence rejecting the null hypothesis at 5% significant level and concluded that arrowroots can significantly be used to remove metal ions from municipal waste waters.
RECOMMENDATIONS
Some treatment plants such as Eldoret Water and Sanitation Company (Eldowas) that have large parcel of land should consider using arrowroots in tertiary ponds of wastewater in addition to natural unplanted biological ponds. Further research should be carried out to study the adsorption efficiency of other pollutants using arrowroots.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel GN, Elchaghaby GA (2007). Influence of operating conditions on the removal of Cu, Zn, Cd and Pb ion from wastewater by adsorption. International Journal of Environmental Science and Technology 4(4):451-456. |
|
|
Brisson J, Chazarenc F (2009). Maximizing pollutant removal in constructed wetlands. Should we pay more attention to macrophytes species selection? Science of Total Environment 407:3923-393. |
|
|
Calheiros CSC, Rangel AOS, Castro PKL (2007). Constructed wetland systems vegetated with different plants applied to the treatment of tannery wastewater. Water Research 41:1790-1798. |
|
|
Igwe JC, Abia AA (2007). A bioseparation process for removing heavy metal from wastewater using biosorbents. African Journal of Biotechnology 5(12):1167-1179. |
|
|
Karanthanasis AD, Johnson CM (2003). Metal removal potential by three aquatic plants in an acid mine drainage wetland. Mine Water and the Environment 22:22-30. |
|
|
Maine MA, Sune N, Hadad H, Sánchez G, Bonetto C (2006). Nutrient and metal removal in a constructed wetland for waste-water treatment from a metallurgic industry. Ecological Engineering 26:341-347. |
|
|
Maine MA, Sune N, Hadad H, Sánchez G, Bonetto C (2007). Removal efficiency of a constructed wetland for wastewater treatment according to vegetation dominance. Chemosphere, 68:1105-1113. |
|
|
Marchand L, Mench M, Jacob D, Otte M (2010). Metal and metalloid removal in constructed wetlands, with emphasis on importance of plants and standardized measurements: A review. Environmental pollution 158(12):3447-3461. |
|
|
Molle P, Liénard A, Boutin C, Merlin G, Iwema A (2005). How to treat raw sewage with Constructed wetlands: an overview of French systems. Water Scencie and Technology 51:11-21. |
|
|
Ramírez JA (2002). Passive treatment of acid mine drainage at the La Extranjera Mine (Puertollano, Spain). Mine Water and the Environment 21:111-113. |
|
|
Reeb G, Werckmann M (2005). First Performance Data on the Use of Two Pilot-constructed Wetlands for Highly Loaded Non-domestic Sewage. In Natural and Constructed Wetlands: Nutrients, Metals and Management; Vymazal, J., Ed.; Backhuys Publishers: Leiden, Netherlands pp. 43-51. |
|
|
Tonderski KS, Greenland E, Billgren C (2005). Management of sugar effluent in the Lake Victoria region. In Proceedings of the workshop wastewater treatment in wetlands. Theoretical and Practical Aspects Toczyłowska, I., Guzowska, G., Eds.; Gdansk University of Technology Printing Office: Gdansk, Poland pp. 177-184. |
|
|
Volesky B (2007). Biosorption and me; Water Research 41:4017-4029. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0