ABSTRACT
Chromium(III), copper(II) and lead(II) are among the heavy metals produced and released in large amounts by anthropogenic sources worldwide, including Burkina Faso. Previous studies have demonstrated the successful application of domestic natural mixed clays for the removal of these metal ions as a cheap and environmentally friendly method. Qualitative mineralogical characterization of the clays revealed that they consist of kaolinite, illite, orthrose and quartz, and minor quantities of albite and montmorillonite. pHPZC for the clays, as determined by potentiometric titrations, are in the range 6.8 to 7.3. In this study, the interactions of chromium(III), copper(II) and lead(II) ions with these clay minerals were examined by the use of extended X-ray absorption fine structure (EXAFS) spectroscopy. Cr3+ forms tetrameric hydrolysis complexes on the mineral surface with a Cr–O bond distance of 1.98 Å, and two Cr···Cr distances at 3.02 and 3.62 Å. This is indicative of a tetrameric [Cr4(OH)6(H2O)12]6+ entity bound to the clay mineral surface. A distance of 3.17 Å, regarded as a Cr···Fe distance, indicates that one Cr3+ ion in the hydrolyzed tetramer binds to two oxygens in the mineral surface which are bound to either one or two iron(III) ions in the surface. Pb2+ binds two oxygen atoms at an average bond distance of 2.31 Å, with a significant contribution of linear multiple scattering from the PbO2 entity. The EXAFS results of Cr3+ sorption are consistent with the presence of a hydrolysis product of polymeric Cu2+ species with a surface complex or precipitate.
Key words: Sorption, extended X-ray absorption fine structure (EXAFS) spectroscopy, clay, precipitation, Burkina Faso.
Water pollution, with high levels of particular heavy metals have been found in streams and soils mainly due to anthropogenic activities such as mining, agriculture, society’s effluents and discharge from industrial and sewage plants in Burkina Faso (Etienne et al., 1997; Inoussa et al., 2009). Compounds of chromium(III), copper(II) and lead(II) are among the most common heavy metal pollutants found in Burkina Faso. Efficient removal of toxic metal ions from drinking water as well as wastewater is an important research area and several technologies have been developed over the years (Deans and Dixon, 1992; Sajidu et al., 2008). The potential of natural or alkaline mixed clays to sorb heavy metals dissolved in aqueous systems and the possible sorption mechanism was investigated in order to find effective and inexpensive materials (Sajidu et al., 2006a, 2008; Sällström, 2008; Pare et al., 2012, 2013).
A large number of studies have investigated the adsorption of Cr3+, Cu2+ and Pb2+ with different kind of materials. Cr3+ was found to: coordinate with phosphate ions and substitute calcium ions on bone char surfaces in aqueous solution (Jose et al., 2016); be physically adsorbed on the surface of chorfa silt material (Ouadjenia-Marouf et al., 2013); be uptake on micaceous polymineral from Kenya (Attahirua et al., 2012); be adsorbed following pseudo-second-order kinetics on natural clay from Kono-Bowe in Nigeria (Ajemba, 2014). Pb2+ was adsorbed as an acetylacetonate complex by hydrophobic interaction on mesoporous silicate surface (Oshima et al., 2005) and with electrochemical ion-exchange between Pb2+ and clays surfaces (smectite and kaolin), and complexation reaction (Etoh et al., 2016).
It was found to adsorb through ion exchange between metal cations and protons at the surface of activated carbon cloths, including precipitation (Kadirvelu et al., 2010). Copper(II) adsorption onto magnetite surface was found to be a fast process following pseudo-second order kinetics (Adewuyi and Pereira, 2016). It was successfully adsorbed on amino-functionalized magnetic nanoparticle surfaces with ion exchange-surface complexation being the main adsorption mechanism (Li et al., 2013) and also found to be adsorbed on the upper layer of the crystalline structure of activated carbon prepared from Azadiracta indica bark by means of physisorption (Balakrishnan et al., 2010). An EXAFS study of chromium(III) sorbed on silica (Fendorf et al., 1994) indicated formation of monodentate Cr3+ surface complex on silica with a Cr···Si distance of 3.39 Å and formation of polynuclear chromium hydroxide octahedra. Sajidu et al. (2008) found that on a natural mixed alkaline clay from Malawi, Cr3+ forms polynuclear hydrolysis complexes on the mineral surface with Cr–O bond and Cr···Cr distances of 2.00 and 3.03 Å respectively.
Copper(II) was found to bind to phosphate groups on the surface at low pH and had a first shell of coordinated oxygen atoms with Jahn–Teller distortion with Cu–O bonds of 1.96 Å for the equatorial ones, at 2.30 and 2.65 Å for the axial oxygens, and a Cu–P distance at 3.29 Å was distinguished as well. Studies reported that Cu2+ coordinates with sulphur on the surface of pyrite with an average bond length of 2.27 Å, and precipitated as Cu(OH)2 with a Cu-O bond length of 2.00 Å at pH 8.5 (Weisener and Andreas, 2000). No precise chemical environment around the Pb2+ was observed by Sajidu et al. (2008). Previous works by the author highlighted the potential of natural mixed clays in removing heavy metal from aqueous solution. This study investigates the local structural environment of Cr3+, Cu2+, Pb2+ ions sorbed on natural clay surfaces KOR and SIT without any pre-treatment as function of pH using EXAFS to deduce the mechanism of the sorption process.
Samples
The clay samples were collected from two locations in Burkina Faso as indicated in previous works (Sorgho et al., 2011). Mineralogical characterization of the clays by XRD revealed that the KOR clay contains montmorillonite, quartz, albite, illite, kaolinite, goethite and orthose (Sorgho et al., 2011), and the SIT clay contains kaolinite, montmorillonite, quartz, illite/mica, albite and orthoclase (Kam et al., 2009). The pHpzc values of the clays were 7.3 and 6.8, for KOR and SIT, respectively, and the cation exchange capacity was 42.4 ±1.1 and 53.6±1.1 (cmolc/kg) for KOR and SIT, respectively (Sorgho et al., 2011).
Metal sorption
20 mmol·dm−3 aqueous solutions of Cr3+, Cu2+ and Pb2+ were prepared by dissolving weighed amounts of the nitrate salts Cr(NO3)3∙9H2O, Cu(NO3)2∙3H2O, and Pb(NO3)2 (analytical grade, and impurity of less than 0.5%), respectively, in deionized water. 25 mL of prepared metal solution was added to 1.0 g of the clay and the pH was adjusted using 1.0 mmol·dm−3 nitric acid or sodium hydroxide under vigorous stirring to maintain homogenous suspensions. After stirring at room temperature for 48 h, the clay suspensions were centrifuged to separate the clays from the aqueous phase. The remaining clays were dried in air before the EXAFS measurements. For each sample, pH was adjusted to slightly acidic and alkaline pH values as shown in Table 1.
EXAFS - Data collection
Chromium and copper K-edge and lead L3-edge X-ray absorption spectra were collected at the wiggler beam-line I811, MAX-lab, Lund University, Sweden, which was operated at 1.5 GeV and a maximum current of 200 mA. Data collection was performed in transmission and fluorescence mode simultaneously. The fluorescence detection was made with a passivated implanted planar silicon (PIPS) detector (Canberra, 2013). The EXAFS station was equipped with a Si[111] double crystal monochromator. In order to remove higher order harmonics, the beam intensity was detuned to 30, 50, and 50% for chromium, copper and lead, respectively, at the end of the scans. Internal energy calibration was made with corresponding metal foil with first inflection point on the absorption edge at 5989.0, 8980.3 and 13038 eV for chromium (K edge), copper (K) and lead(L3), respectively (Thompson et al., 2001). The treatment of the EXAFS data was carried out by means of the EXAFSPAK program package (George and Pickering, 1993), using standard procedures for pre-edge subtraction and spline removal.
EXAFS - Data analysis
The program package EXAFSPAK was used for data treatment and refinement of structure parameters as indicated elsewhere (Sajidu et al., 2008; George and Pickering, 1993). The EXAFS functions were obtained after performing standard procedures for pre-edge subtraction, and spline removal. The k3-weighted model functions were calculated using ab initio calculated phase and amplitude parameters using the FEFF7 program (Zabinsky et al., 1995). The standard deviations are obtained from k3-weighted least squares refinements of the EXAFS function χ(k), and do not include systematic errors of the measurements. These statistical error estimates provide a measure of the precision of the results and allow reasonable comparisons, e.g. of the significance of relative shifts in the distances. However, the variations in the refined parameters, including the shift in the E0 value (for which k = 0), using different models and data ranges, indicate that the absolute accuracy of the distances given for the separate complexes is within ±0.01-0.02 Å for well-defined interactions (Sajidu et al., 2008).
Chromium(III) treated clay samples
Fits of the EXAFS spectra and FT of chromium(III) treated clay samples are shown in Figure 2a and b, with quantitative fitting results listed in Table 2. The EXAFS spectra and Fourier transforms (FTs) of the samples studied are similar for the two pH values studied, acidic (pH 3.3 and 3.4) and basic (pH 11.0) for the KOR and SIT samples. The FTs show that Cr3+ binds to six oxygens at mean bond distance of 1.98 Å, in octahedral configuration, and the corresponding CrO6 core multiple scattering was observed at 3.93 Å (Table 2). In addition, three distances to relatively strong back-scatterers at 3.0, 3.2 and 3.6 Å are observed. The distances at 3.0 and 3.6 Å correspond most probably to the tetrameric [Cr4(OH)6(H2O)12]6+ complex, dominating in weakly acidic aqueous solution (Torapava et al., 2009), with Cr···Cr distances of 2.98 and 3.59 Å. This complex seems also to be the dominating one on the surfaces of both KOR and SIT clay with two oxygens binding to Fe(III) in the surface with a Cr···Fe distance of 3.2 Å.
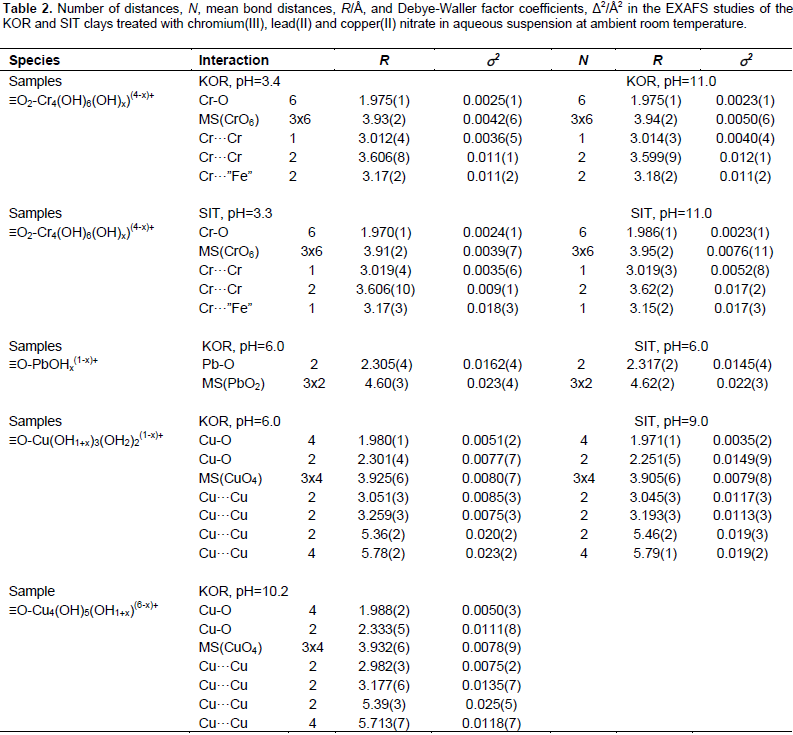
This Cr···Fe distance corresponds to a tetrahedral Cr-O-Fe angle. These results are in close agreement with the adsorption of Cr3+ to the surfaces of alkaline clays from Malawi (Sajidu, 2008), showing formation of hydrolyzed Cr3+ complexes on the surfaces of basic clays. Fendorf et al. (1994) discerned formation of polynuclear chromium hydroxide octahedral on silica. From our study, the following alternative reaction equilibria on the clays surface are expected:
2 ≡FeOH + [Cr4(OH)6(H2O)12]6+ (≡FeO)2Cr(H2O)2Cr3(OH)6(H2O)8]4+ + 2 H3O+
≡FeOH + [Cr4(OH)6(H2O)12]6+ ≡FeO2Cr(H2O)2Cr3(OH)6(H2O)8]5+ + H3O+ + 2 H2O
The Cr···Cr bond distances in the hydrolyzed tetrameric Cr3+ indicate that one chromium in the hydrolyzed tetramer binds to the surface through two oxygens to one or two iron(III) ions in the mineral surface. Iron, present on the clay surface plays key role in the removal of Cr3+.
Lead(II) treated clay samples
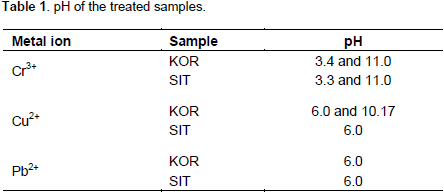
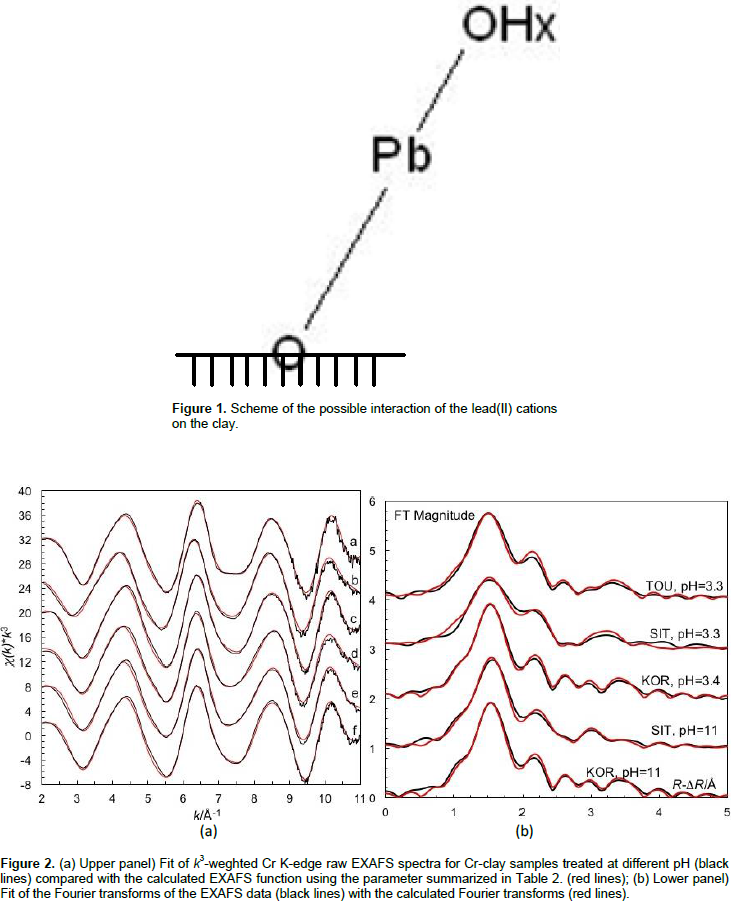
The EXAFS data of lead(II) adsorbed to the surfaces of the KOR and SIT clays shows a very short Pb-O bond distance, 2.31 Å, with a relative small Debye-Waller coefficient being a Pb2+ complex (Persson et al., 2011). Furthermore, a weak contribution of multiple scattering within the PbO2 entity at ca. 4.61 Å supports a linear complex. It has not been possible to detect any Pb···X distance making it impossible to estimate the angle this complex has versus the surface. The finding of linear O-Pb-O (possibility shown on Figure 1) with such a short Pb-O bond distance is unusual. The number of reported linear lead(II) complexes is very limited. Pb-O bond distance of 2.334 Å has been found in [Pb2(H2O)2(ClO4)2]n, (Persson et al., 2011), and a distance of 2.32 ± 0.03 Å has been reported in Pb2+ co-precipitated with Fe3+ oxyhydroxide (Kelly et al., 2008). Whether the formation of a surface complex with Pb(II) also results in hydrolysis of Pb2+ cannot be distinguished from the present study leaving both possibilities open.
≡MOH + [Pb(H2O)6]2+ ≡MO-Pb-OH2+ + H3O+ + 4 H2O
or
≡MOH + [Pb(H2O)6]2+ ≡MO-Pb-OH+ 2 H3O+ + 3 H2O
Experimental and fitted EXAFS data in k-space, and the corresponding Fourier transforms are given in Figure 3a and 3b, respectively.
Copper(II) treated clay samples
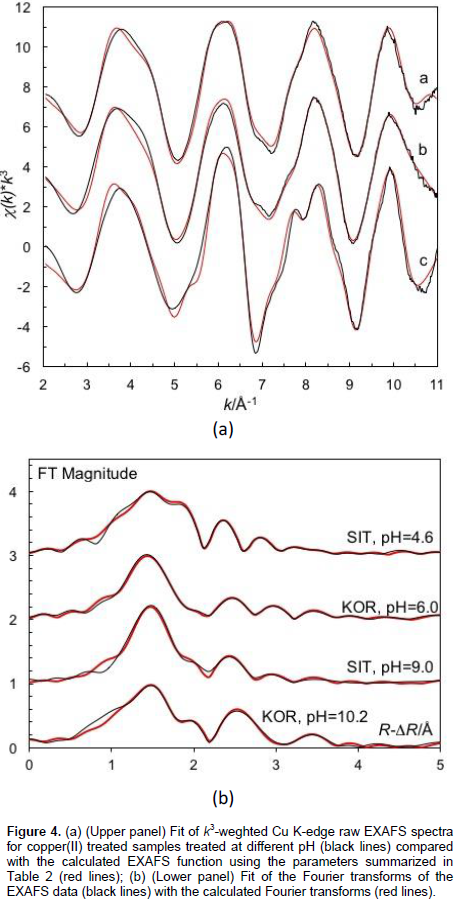
The EXAFS spectra recorded at acidic pH 6 for the KOR sample and in basic medium with pH 9.0 for SIT and pH 10.2 sample are almost identical (Figure 4). The FT spectra as well as the fittings result (Table 2) show specific Cu···Cu distances clearly indicating that copper(II) has hydrolyzed. The pattern of Cu···Cu distances is fairly similar to that of crystalline trihydroxidonitratodicopper(II), Cu2(OH)3NO3 (Effenberger, 1983). This is not surprising as Cu2+ nitrate was used as starting material, and Cu2+ is known to hydrolyze at the pH values applied (Paulson and Kester, 1980). It can furthermore be assumed that copper(II) adsorbed to a mineral or clay surface is more readily hydrolysed than the hydrated copper(II) ion in aqueous solution. This study can however not determine whether the copper(II) species on the surface is a polymeric surface complex or a crystalline or amorphous solid phase. The significantly longer Cu-O bond distances in these complexes on the clay surfaces, ca. 1.98 Å, than in the hydrated copper(II) ion, 1.95 Å, indicates that copper(II) is mainly binding hydroxide groups as the atomic radius of the hydroxide oxygen is ca. 0.03 Å larger than of water oxygen (Shannon, 1976).
Chromium(III), copper(II) and lead(II) sorbed on some natural mixed clays from Burkina Faso were studied using EXAFS spectroscopy. Chromium(III) is adsorbed as hydrolysed tetrameric complexes at both acidic and basic pH values, one chromium in the hydrolyzed tetramer binds to the surface through two oxygens to one or two Fe3+ ions in the mineral surface. Linear coordination of Pb2+ is rare, but especially Pb2+ complexes with hydroxide ion display low coordination number, e.g. the trishydroxidoplumbate complex, [Pb(OH)3]-, is the dominating species in hyper- alkaline aqueous solution (Bajnóczi et al., 2014). Copper(III) is hydrolyzed to a polymeric complex or precipitateadsorbed to the clay surface. Hydrolysis and precipitation are seen as main mechanism governing heavy metal uptake by the two study natural clays from Burkina Faso.
The authors have not declared any conflict of interests.
Financial support of the BUF:02/ISP/IPICS/ project from the International Science Program (ISP) Uppsala-Sweden, through the International Program in Chemical Sciences (IPICS) is gratefully appreciated. Authors are also grateful to MAX-lab, Lund University, for the allocation of beam time and laboratory facilities. MAX-labis supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation, with additional support from the Crafoord Foundation and the Faculty of Science and Technology, Norwegian University of Science and Technology.
REFERENCES
|
Adewuyi A, Pereira FV (2016). Nitrilotriacetic acid functionalized Adansonia digitata biosorbent: Preparation, characterization and sorption of Pb (II) and Cu (II) pollutants from aqueous solution. J. Adv. Res. 7: 947-959.
Crossref
|
|
|
|
Ajemba OR (2014). Kinetics and equilibrium modeling of lead(II) and chromium(III) ions' adsorption onto clay from Kono-bowe, Nigeria. Turk J. Eng. Environ. Sci. 38(3). ISSN 1303-6157.
View Date accessed: 28 aug. 2017.
|
|
|
|
|
Attahirua S, Shiundua PM, Wambu EW (2012). Removal of Cr(III) from aqueous solutions using a micaceous poly–mineral from Kenya. Int. J. Phys. Sci. 7(8):1198-1204.
|
|
|
|
|
Bajnóczi EG, Pálinkó I, Körtvélyesi T, Szabolcs T, Bálint S, Bakó I, Sipos P, Persson I (2014). Speciation and the structure of lead(II) in hyperalkaline aqueous solution. Dalton Trans. 43:17539-17543.
Crossref
|
|
|
|
|
Balakrishnan V, Arivoli S, Shajudha BA, Jafar AA (2010). Studies on the adsorption mechanism of Cu(II) ions by a new activated carbon. J. Chem. Pharm. Res. 2(6):176-190.
|
|
|
|
|
Canberra (2013).
View, access June 7, 2017.
|
|
|
|
|
Deans JR, Dixon BG (1992). Uptake of Pb2+ and Cu2+ by novel biopolymers. Water Res. 26(4):469-472.
Crossref
|
|
|
|
|
Effenberger H (1983). Verfeinerung der Kristallstruktur des monoklinen Dikupfer(II)tri¬hydroxi-nitrates, Cu2(NO3)(OH)3. Z. Kristallogr. 165:127-135.
Crossref
|
|
|
|
|
Etienne B, Bouda S, Ouedraogo L (1997). Physical, Chemical and biological characteristics of reservoirs in Burkina Faso. Tech. Center for Agric. Rural Co-operation, ACP-EU 28-42.
|
|
|
|
|
Etoh MA, Dina DJD, Ngomo HM, Ketcha JM (2016). Adsorption of Pb2+ Ions on two clays: Smectite and kaolin the role of their textural and some physicochemical properties. Int. J. App. Res. 1(13):793-803.
|
|
|
|
|
Fendorf SE, Lamble GM, Stapleton MG, Kelley MJ, Saparks DL (1994). Mechanisms of chromium(III) sorption on silica. 1. Chromium(III) surface structure derived by extended x-ray absorption fine structure spectroscopy. Environ. Sci. Technol. 28(2):284-289
Crossref
|
|
|
|
|
George GN, Pickering IJ (1993). EXAFSPAK – A Suite of Computer Programs for Analysis of X-ray Absorption Spectra. SSRL, Stanford, CA.
|
|
|
|
|
Inoussa Z, Jean-Pierre L, Hama AM, Joseph W, Francois L (2009). Removal of hexavalent chromium from industrial wastewater by electro-coagulation: a comprehensive comparison of aluminium and iron electrodes. Sep. Purif. Technol. 66:159-166.
Crossref
|
|
|
|
|
Jose VFC, Roberto LR, Francisco CM, Antonio AP, Jacob JSR, Socorro LR (2016). Adsorption mechanism of Chromium(III) from water solution on bone char: Effect of operating conditions. Adsorption 22:297-308.
Crossref
|
|
|
|
|
Kadirvelu K, Faur-Brasquet C, Le Cloirec P (2010). Removal of Cu(II), Pb(II), and Ni(II) by Adsorption onto Activated Carbon Cloths. Langmuir 16(22):8404-8409.
Crossref
|
|
|
|
|
Kam S, Zerbo L, Bathiebo J, Soro J, Naba S, Wenmenga U, Traoré K, Gomina M, Blanchart P (2009). Permeability to water of sintered clay ceramics. Appl. Clay Sci. 46:351-357.
Crossref
|
|
|
|
|
Li H, Xiao De-li, He H, Lin R, Zuo Peng-li (2013). Adsorption behavior and adsorption mechanism of Cu(II) ions on amino-functionalized magnetic nanoparticles, Trans. Nonferrous Met. Soc. China 23:657-2665.
Crossref
|
|
|
|
|
Oshima S, Jilska MP, Kathy AN, Hisao K, Geoffrey WS, Yu K (2005). Adsorption Behavior of Cadmium(II) and Lead(II) on Mesoporous Silicate MCM-41. Sep. Sci. Technol. 41:1635-1643.
Crossref
|
|
|
|
|
Ouadjenia-Marouf F, Marouf R, Schott J, Yahiaoui A (2013). Removal of Cu(II), Cd(II) and Cr(III) ions from aqueous solution by dam silt. Arab. J. Chem. 6:401-406.
Crossref
|
|
|
|
|
Pare S, Persson I, Guel B, Lundberg D (2013). Trivalent chromium removal from aqueous solution using raw natural mixed clay from Burkina Faso. Int. Res. J. Environ. Sci. 2:30-37.
|
|
|
|
|
Pare S, Persson I, Guel B, Lundberg D, Zerbo L, Kam S, Traoré K (2012). Heavy metal removal from aqueous solutions by sorption using natural clays from Burkina Faso, Afr. J. Biotechnol. 11:10395-10406.
Crossref
|
|
|
|
|
Paulson AJ, Kester DRJ (1980). Copper(II) ion hydrolysis in aqueous solution. J. Solution Chem. 9:269-277.
Crossref
|
|
|
|
|
Persson I, Lyczko K, Lundberg D, Eriksson L, Placzek A (2011). Coordination chemistry study of hydrated and solvated lead(II) ions in solution and solid state. Inorg. Chem. 50:1058-1072.
Crossref
|
|
|
|
|
Sajidu SMI, Persson I, Masamba WRL, Henry EMT, Kayambazinthu D (2006a). Removal of Cd2+, Cr3+, Cu2+, Hg2+, Pb2+ and Zn2+ cations and AsO43−anions from aqueous solutions by mixed clay from Tundulu in Malawi and characterisation of the clay. Water SA. 32:519-526.
|
|
|
|
|
Sajidu SMI, Persson I, Masamba WRL, Henry EMT (2008). Mechanisms of heavy metal sorption on alkaline clays from Tundulu in Malawi as determined by EXAFS. J. Hazard. Mat. 158:401-408.
Crossref
|
|
|
|
|
Sällström M (2008). Physico-chemical characteristics of some soils from Mali and their potential in heavy metal removal, Master Thesis, Swedish University of Agricultural Sciences, Uppsala.
|
|
|
|
|
Shannon RD (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr. Sect. A 32:751-767.
Crossref
|
|
|
|
|
Sorgho B, Paré S, Guel B, Zerbo L, Traoré K, Persson I (2011). Study of locale mixed clay from Burkina Faso for the removal of Cu2+, Pb2+ and Cr3+. J. Soc. Ouest-Afr. Chim. 031:49-59.
|
|
|
|
|
Thompson A, Attwood D, Gullikson E, Howells M, Kim K-J, Kirz J, Kortright J, Lindau I, Pianatta P, Robinson A, Scofield J, Underwood J, Vaughan D, Williams G, Winick H (2001). Xray Data Booklet; LBNL/PUB-490 Rev. 2, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA.
|
|
|
|
|
Torapava N, Radkevich A, Davydov D, Titov A, Persson (2009). Composition and structure of polynuclear chromium(III) hydroxo complexes. Inorg. Chem. 48: 10383-10388.
Crossref
|
|
|
|
|
Weisener CG, Andrea RG (2000). Cu(II) adsorption mechanism on pyrite: an XAFS and XPS study. Surf. Interface Anal. 30(1):454-458.
Crossref
|
|
|
|
|
Zabinsky SI, Rehr JJ, Ankudinov AL, Albers RC, Eller MJ (1995). Multiple scattering calculations of X-ray absorption Spectra. Phys. Rev. B52:2995-3009.
Crossref
|
|