ABSTRACT
Globally heavy metals pollutants in aquatic systems are increasing and creating major ecological disturbance(s) and direct health problems. The study on accumulation of Copper (Cu), Cadmium (Cd), Lead (Pb) and Nickel (Ni) on the bottom surface sediments of Nairobi dam was conducted between November 2012 to April 2013. Seven sampling sites were selected within the dam and sediment samples were collected once in a month during the wet and dry season. Standard methods for sampling, storage and analysing according to APHA were used; statistical analysis in form of means, independent T-test and ANOVA were used to summarize the findings. (Mean±SE) (ppm) concentrations of Pb, Cd, Cu and Ni in the dam during the dry season are: 38.01±0.26, 30.86±0.21, 21.7±0.22, 8.44± 0.15, and in wet season are: 33.73±0.22, 26.60±0.19, 19.11±0.14 and 5.94±0.12, respectively. The levels of heavy metals in the sediments at the outlet are lower compared to the other sites within the dam. These metals accumulated in the order: Pb> Cd> Cu> Ni with higher concentration during the dry than wet season. It is concluded Nairobi dam sediments are contaminated with heavy metals that are significantly higher during the dry compared to wet season (P<0.0001). Therefore it is recommended that sediments of this dam are not suitable for cultivation and or any agricultural activities.
Key words: Accumulation, heavy metals, sediments, Nairobi dam.
Heavy metals such as copper, nickel, cadmium and lead are not found in abundant quantities in the earth’s crust and are considered to pose environmental hazards. Sediments are an important sink for a variety of pollutants, particularly heavy metals and may serve as an enriched source of such pollutants, especially in estuarine ecosystems. In some cases, sediments may hold over 99% of the total amount of heavy metals present in an ecosystem (Renfro, 1983). Sediments have high adsorptive capacities of metal pollutants. It has been noted from several studies that most metals entering the aquatic systems ultimately find their way into the sediments and inevitably the whole food chain.
Direct discharge or wet and dry depositions of contaminants increase the concentrations of trace elements of aquatic systems, thus resulting in their accumulation in sediments (Dunbabin and Bowmer, 1992; Sinicrope et al., 1992). Obodai et al. (2011) assessed the level of heavy metal pollution in water, sediments, and two species of fish in Benya and Nakwa lagoons, Ghana and it was concluded that the contamination was the highest in the sediments. Ogoyi et al. (2011) studied heavy metal in water, sediment and microalgae in Lake Victoria, Kenya and concluded that highest levels of trace metals were in the sediments of the lake
Nairobi dam
is contaminated with chemical pollutants. This results to bioaccumulations and biomagnifications which causes potential ecological disturbance(s) and direct health problems to local people who depend on the water and products from the resource; and particularly residents of Kibera informal settlement adjacent to the dam who are cultivating crops for domestic and commercial purposes within Nairobi dam.
The objective of the present study is to analyse, compare and contrast the accumulations of heavy metals on bottom surface sediments of Nairobi dam during the wet and dry seasons.
There is great deal of sedimentation taking place within Nairobi dam (UNEP, 2008). Very little work has been conducted on accumulation of heavy metals in sediments of the dam. Therefore, we took the initiative to study the contamination of heavy metals in sediments of Nairobi Dam. The findings of the present study will be useful for monitoring, auditing and assessing the status of any aquatic ecosystem(s) because heavy metals can pass to human beings through food chain.
Study area
The study was carried out at Nairobi
dam in Nairobi, Kenya. This dam is a source of water both for domestic and commercial purposes to the surrounding community. The dam is situated at a latitude of 1° 19' (1.3167°) south; longitude of 36° 48' (36.8°) east and average elevation of 1,686 meters. Its accumulating capacity was 98,422 cu. metres of water (3,477,800 cu ft) and surface area of 356,179 m
2 (approximately 86 acres) when it was designed (Figure 1).
Data collection
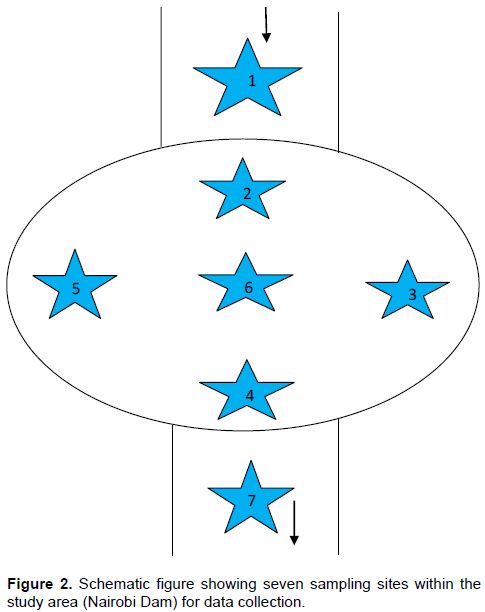
Riverbed sediment samples were collected 10 cm below the Nairobi dam bottom surface sediments using an auger on a monthly interval between November 2012 and April 2013, from each of the seven sampling stations
identified (Figure 2): the 1
st sample station was along the stream entering Nairobi dam 10 m before the discharge into the dam; 2
nd station was at a distance of 5 m after the discharge into the dam; 3
rd station was 5 m inwards of the west shore; 4
th station was 5 m inwards of the outlet, 5
th station was 5 m inwards of the east shore; 6
th station was 15 m inwards from the 3
rd station, and the 7
th station was 1 m from the outlet of Nairobi dam. The sediment samples were transferred into well labelled 250 g polypropylene jars, pre-cleaned with a metal-free non-ionic detergent solution, rinsed with tap water, soaked in 1 + 1 HNO
3 acid for 24 h at 70°C and then rinsed with metal-free water (APHA, 2005). These samples were then transported to
Kenyatta University laboratory and stored in the fridge freezer. About 20 g of each sediment sample was put into labelled 250 ml beakers and dried in an oven at 105°C overnight. The dried samples were ground using a motor and pestle after which they were stored in clean well labelled polypropylene bottles awaiting digestion (APHA, 2005).
Sample digestion and metal detection
1 g of each of the ground sediment samples was digested in Conc. HNO3: HCl in the ratio of 2:1 and three drops of 30% Hydrogen peroxide on a hot plate in a fume hood. After total digestion and subsequent cooling, the solution was diluted with distilled water to 100 ml volumetric flask and analyzed for Cu, Cd, Pb and Ni, using an Atomic Absorption spectrophotometer (AAS) (APHA, 2005), Shimadzu flame AAS model (AA-630)
Statistical analysis
From the data, mean heavy metals (Cu, Cd, Pb, Ni) values and standard errors were calculated; Independent T-test, analysis of variance (ANOVA) and Student-Newman-Keuls test at α=0.05 were performed on the metals mean values using SPSS version 18.
Concentration and relationships of heavy metals on Nairobi dam sediments
Copper
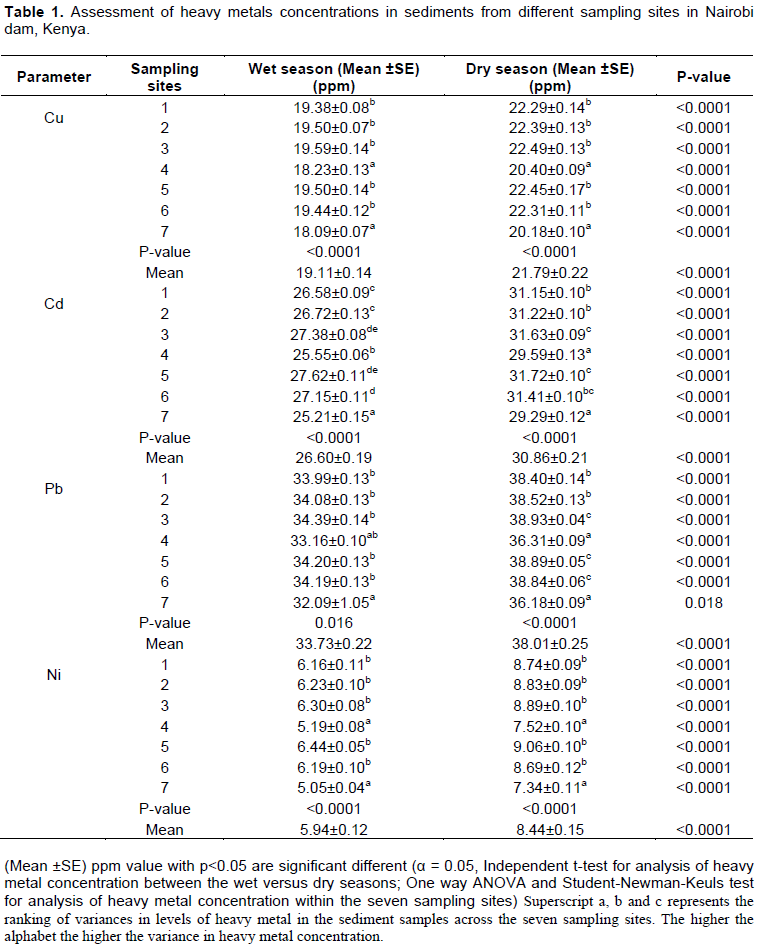
The mean copper concentrations of sediments in the different sampling sites (1 to 7) of Nairobi dam during the wet and dry season showed highly significant differences indicated by the P-value <0.001 on the columns. It can be seen that site 1, 2, 3, 5, and 6 had the same superscript letter (b) meaning that the values of copper are not significantly varied within these stations but site 4 and 7 denoted by alphabet (a) indicates that these two sites with concentrations of copper that are significantly different from those with letter (b) (Table 1). This can be explained by the fact that the purification mechanism within the wetland (Nairobi dam), resulted in removal of heavy metals from polluted sediments within the dam: Therefore the sediments at the outlet (site 4 and 7) of the dam had lower levels of copper. Copper levels are significantly higher in the dry than wet season (P<0.001) in all the sampling sites; this is attributed to concentration of metals on sediments due to higher temperatures in dry season.
Cooper et al. (1996) studied reed beds and constructed wetlands for waste water treatment in the U.K. and stated that the following pollution removal mechanisms exist in wetlands: Metal adsorption and cation exchange, complexation, precipitation in sediments, plant uptake and microbial oxidation /reduction processes, therefore resulting to pollutants purification.
Cadmium
The P-value (P<0.001) on the column for cadmium metal during the wet and dry season justifies that there is significant difference on cadmium concentrations in the sampling sites 1 to 7 (Table 1). In the dry season, the lower case superscript (c) on site 3 indicates there is a significantly higher level of the contaminant compared to site 4 which is denoted by superscript (a). This can be attributed to the fact that site 3 (adjacent to Kibera slums) received high levels of Cd contaminants from the area as well as from the dams inlet but site 4 which is closer to outlet of the dam contains purer sediments due to metals clean up within the wetland. Cadmium levels on the dams sediments are significantly higher in the dry compared to wet season (P<0.0001) in all the seven sampling sites. This can be explained by low water flow rate in the dam during the dry season resulting in sediment settlement and also higher temperatures in the dry season resulting in concentration of heavy metals.
Lead
There is a significant difference in lead concentration on Nairobi dam sediments from the seven sampling points during both the wet and dry season (P≤0.016). During the dry season it is shown that sediments at site 4 and 7 (Outlet) denoted by superscript (a) have significantly lower levels of Pb in comparison to site 1 and 2 having superscript (b); and even much lower Pb levels compared to site 3, 5 and 6 having (c). This can be justified by the presence of hydrophytes within the wetland that adsorbed the metals within the dam therefore lower levels of the metal in the bottom sediments at the outlet compared to inlet and the other sites.
Lead concentrations are significantly higher in the dry than wet season (P≤0.018) at all sampling sites. This is due to settlement of metals concentrated sediments and higher temperatures in the dry season resulting to increased concentration of the heavy metals.
Nickel
There is significant difference in nickel concentration within the seven sampling sites of the dam during the dry and wet season (P<0.0001). During the wet season at sampling sites 1, 2, 3, 5 and 6, Ni levels are significantly higher compared to sites 4 and 7 denoted by superscript (a). In all the seven sampling sites, nickel levels are significantly higher in dry compared to wet season (P<0.0001). This is majorly due to
higher temperatures in dry season resulting in concentration of heavy metals in the sediments.
A study by Schiffer (1989) on the effects of highway runoff on the quality of water and bed sediments of two wetlands in central Florida revealed that generally the levels of pH, nutrients, aluminum, lead and copper in water and sediments decreased with distance from the inlet. For example at the
Island Lake wetland , Lead was highest at site 5 (2,000 μg/g) and site 7 (also 2,000 μg/L), and decreased at far sites in the wetland, the lead concentration in the sediments at the outlet was only 40 μg/g. however for the sediments Zinc and chromium concentrations did not decrease as much with distance from the inlet as water. It was also noted that concentrations in sediments among the sites are highly variable because of the relation of constituents to particle size and organic carbon content, the uneven distribution of sediment sizes in many bottom samples, and varying conditions of deposition (variation in loads and hydraulic conditions with each storm).
The order of metals accumulation on Nairobi dam sediments is:
Pb> Cd> Cu> Ni. This is because during both the dry versus wet season, Pb had the highest average concentration at 38.01±0.25 versus 33.73±0.22, followed by Cd at 30.86±0.21 versus 26.60±0.19, then Cu at 21.79±0.22 versus 19.11±0.14 and the lowest was Ni at 8.44±0.15 versus 5.94±0.12 ppm respectively (Table 1). This indicates that the external environment to the sediment of the dam are polluted with heavy metals in this sequence due to socio economic, demographic and waste dumping activities including human waste, agriculture, car washing and industrial effluent observed. It has been reported that the differences in geography and geology, as well as activities in various rivers, affects the amount of metals present (Berner and Berner, 1987; Bricker and Jones, 1995).
A study on Deûle canal heavy metals sequential extraction by Nada et al. (2011) in France revealed that sediments were slightly contaminated with Cu but highly in Pb, Zn and Cd. Zerbe et al. (1999) studies of the bottom deposits from Goreckie Lake, Poland, showed accumulation of Fe to be highest at 9,150 mg/kg than Mn >Zn >Ni >Cu >Pb >Cd >Cr.
The mean Cu, Cd, Pb and Ni concentrations in the whole dam show significantly higher levels in the dry compared to wet season (P <0.0001) (Table 1). This is attributed to major precipitation of decomposed organic matter (aquatic biota) on bed sediments due to elevated temperatures during the dry season thereby higher metals deposition on dam’s bottom sediments. Higher flow rates observed during the wet season caused a great deal of erosion of sediments containing the heavy metals from the dam resulting to low metals on dams bottom sediments; however, during the dry season the low flow rate enhanced settlement of sediments
Similar findings were shown by Kikuchi et al. (2009) on a study of characterization of heavy metal pollution in river sediment of Hanoi City and its downstream area. That revealed concentrations of heavy metals (As, Cr, Cu, Mn, Ni and Zn) in the sediments of the Nhue River were higher in the dry season than in the rainy season. A study by Mondol et al. (2011) on seasonal variation of heavy metals concentrations in water and plant samples around Tejgaon industrial area of Bangladesh showed that in the rainy season the pollution was lower because heavy rainfall were flushed out through the canal into the adjoining vast flood zone. As the rainy season receded the soils and water were enriched with the pollution load (Chamon et al., 2009; Ullah et al., 1999).
Nairobi dam sediments are contaminated with Pb, Cd, Cu and Ni metals during both the wet and dry season within a range of 38.01±0.26 to 5.94±0.12 ppm. The sequence of metals accumulation on the sediments of Nairobi dam is: Pb > Cd > Cu > Ni. These results reaffirm that sediments act as a reservoir for contaminants in an ecosystem, therefore bed sediments from Nairobi dam are polluted with these heavy metals.
Heavy metals concentrations in the sediments significantly vary in the sampling sites (P <0.0001), with lower metals levels in the outlet sediments compared to those from other sites within the dam. This signifies the presence of pollution removal mechanism in the
wetland probably due to uptake by hydrophytes, precipitation in sediments, reduction/oxidation processes, complexation and the slower flow rate of water within the dam.
The concentration of Pb, Cd, Cu and Ni on the bed sediments of Nairobi dam are significantly higher during the dry in comparison to wet season (P<0.0001). This justifies that dry season gives the worst case scenario of pollution. The analysis suggests the frequent monitoring and assessment of the Nairobi dam sediments to check the activities responsible for metal contamination and the mechanisms involved into metal purification in the wetland.
The authors have not declared any conflict of interests.
REFERENCES
|
APHA (2005). Standard methods for the Examination of water and waste water. 20th Ed. America Public Health Association, Washington DC, USA.
|
|
|
|
Berner EK, Berner RA (1987). The global water cycle: Geochemistry and Environment: Englewood Cliffs, NJ: Prentice-Hall, Inc. pp. 142-155.
|
|
|
|
|
Bricker OP, Jones BF (1995). Main factors affecting the composition of natural waters, In: Trace Metals in Natural Waters: Ch. 1, B Salbu, E Steines. [Eds.], CRC Press. pp. 1-19.
|
|
|
|
|
Chamon AS, Mondol MN, Faiz B, Rahman MH, Elahi SF (2009). Speciation analysis of Ni in the soils of Tejgaon industrial area of Bangladesh. Bangladesh J. Sci. Ind. Res. 44(1):87-108.
Crossref
|
|
|
|
|
Cooper PF, Job GD, Green MB, Sholes RBE (1996). Reed Beds and Constructed Wetlands for Wastewater Treatment. Medmentam, Marlow U.K: WRC publications.
|
|
|
|
|
Dunbabin JS, Bowmer KH (1992). Potential use of constructed wetlands for treatment of industrial waste waters containing metals. Sci Total Environ. 111(2.3):151-168
|
|
|
|
|
Kikuchi T, Hai HT, Tanaka S (2009). Characterization of heavy metal pollution in river sediment of Hanoi City and its downstream area by multivariate analyses. Desalination Publications. 4(2009):240-247.
Crossref
|
|
|
|
|
Mondol N, Chamon AS, Faiz B, Elahi DSF (2011). Seasonal variation of heavy metal concentrations in water and plant samples around tejgaon industrial area of bangladesh. J. Bangladesh Acad. Sci. 35(1):19-41.
Crossref
|
|
|
|
|
Nada S, Dubourguier HC, Hamieh T (2011). Sequential Extraction and Particle Size Analysis of Heavy Metals in Sediments Dredged from the Deûle Canal France. The Open Environ. Eng. J. 4:11-17.
|
|
|
|
|
Obodai EA, Boamponsem LK, Adokoh CK, Essumang DK, Villawoe BO, Aheto DW, Debrah JS (2011). Concentrations of heavy metals in two Ghanaian Lagoons. Arch. Appl. Sci. Res. 3(3):177-187.
|
|
|
|
|
Ogoyi DO, Mwita CJ, Nguu EK, Shiundu PM (2011). Determination of Heavy Metal Content in Water, Sediment and Microalgae from Lake Victoria, East Africa, The Open Environ. Eng. J. 4:156-161.
|
|
|
|
|
Renfro WC (1983). Transfer of 65Zn from Sediments by Ma-rine Polycheate Worm. Mar. Biol. 21:305-316.
Crossref
|
|
|
|
|
Schiffer DM (1989). Effects of Highway Runoff on The Quality of Water
|
|
|
|
|
Sinicrope TL, Langis R, Gersberg RM, Busnardo MJ, Zedler JB (1992). Metal removal by wetland mesocosms subjected to different hydroperiods. Ecol. Eng. 1:309-322.
Crossref
|
|
|
|
|
Ullah SM, Gerzabek MH, Mondol MN, Rashid MM, Islam M (1999). Heavy metal pollution of soils and water and their transfer into plants in Bangladesh, Proc. of extended Abstracts: 5th International Conference on the Biogeochemistry of Trace Elements. WW Wenzel, DC Adriano, B Alloway, HE Doner, C Keller, NW Lepp, M Mench, R Naidu, GM Pierzynski, [Eds.], Vienna, Austria. 1:260-261.
|
|
|
|
|
UNEP (2008). NRBP Phase III: Survey and Situation Analysis of the Biological Characteristics of the Main Tributaries of the Nairobi Rivers, Reservoirs and Wetlands. Nairobi.
|
|
|
|
|
Zerbe J, Sobczyński T, Elbanowska H, Siepak J (1999). Speciation of Heavy Metals in Bottom Sediments of Lakes. J. Environ. Stud. 8(5):331-339.
|
|