ABSTRACT
Tropical forests generally host high biodiversity and are important sources of ecological, socio-cultural and economic services. There are also many sacred forests in many parts of the world, including burial sites in Gobeya rural administrative, having a significant ecological and socio-economic value. This study aims to quantify species diversity, abundance and vegetation structure, and also to determine the ecological importance of woody plant species in burial forest sites. The study was carried out between February and April, 2015 in Gobeya rural administrative which is located approximately at 430 km North of Addis Ababa, Ethiopia. Three largest burial sites (Sideni, Merma and Gubahil) were inventoried where three quadrats of 20 × 20 m at 100 m interval around the edge, and other three quadrats of 20 × 20 m at 50 m interval around the interior were randomly laid. A total of 28 woody plant species belonging to 19 families and 26 genera were identified. Analysis of species rarefaction curve at 95% confidence level reveals that Sideni cemetery site tends to have the highest species richness (α-diversity, n=22) with a possibility of finding new species. Estimation of total species richness using Chao-1 also shows that Sideni site to have the highest richness (n=25). Similarly, non-metric multi-dimensional scaling (MDS) ordination shows that Sideni burial site appears to have the highest species turnover (β-diversity). This is probably due to the presence of an intermediate disturbance in Sideni burial forest site. On the other hand, Acokanthera schimperi (A. DC.) Benth. & Hook. f. (Apocynaceae) is the most abundant tree (56.32%) in Sideni site while Carissa edulis (Forssk.) Vahl (Apocynaceae) is the most abundant shrub in Merma site (29.34%) and Gubahil site (38.13%). In this regard, religious teaching takes the highest social motive behind the tradition of protecting burial sites in the study area. Burial sites provide many socio-economic services including firewood, construction products, livestock forage, medicinal plants and other household products. They also play as important refugia for wild animals such as hyena, fox, porcupine, monkey and many other bird species. So, protection of burial sites should be ensured over long terms, and the indigenous practices of protecting burial forest sites should be preserved. Therefore, burial sites can be delineated as an important landscape to protect and preserve these socio-economically important plant species.
Key words: Burial forest, diversity, abundance, species richness, species composition, multi-dimensional
Tropical forests generally are the centers of biodiversity and high endemism, and are the greatest carbon sink and so regulating global warming. Like many tropical countries, Ethiopia is rich in biodiversity emanated from variation in agro-climatic conditions and altitudinal variability (Erenso et al., 2014). However, the country’s forest resource has been degraded over several decades due to habitats change, deforestation and climate change (Aynekulu et al., 2011; Kacholi, 2014). Up to the end of 1992, studies revealed that Ethiopian forest coverage has declined from the original 35% to an estimated 3 to 4% (Hundera, 2007). Such prolonged habitat reduction has remarkably changed the structure and species composition of forest landscapes (Echeverria et al., 2006).
In Ethiopia, there are several forms of forest conservation practices by local and religious communities (Mulat, 2013). These are traditionally managed small patches of remnant forest yet having a great potential for conservation of many species (Bhagwat and Rutte, 2006; Wassie et al., 2010). Similarly, the local peoples in Gobeya rural administrative of Tehuledere district have a long tradition of planting trees and conserving forests in burial sites. However, appropriate recognition of this management practice is not put in place so far by the government and other stakeholders. Management of such forest ecosystems at the local community level has numerous economic, social and cultural benefits as well as ecological services (Feyissa, 2001). Hence, species diversity should be inventoried to determine the importance of a particular landscape for conservation purposes. Alpha diversity is a measure of the number of species counted in a sample; Beta diversity is the rate of change in species composition along gradients, while Gamma diversity is the diversity of a region or a landscape (Whittaker, 1969; Colwell, 2009; Gotelli and Colwell, 2011).
To the knowledge of the researcher, there have not been any formal researches conducted on these sacred forest sites in the area, though the sites are important habitats for native and endemic plant species. In this regard, most burial sites in Gobeya rural administrative are covered with remnant of potentially native and socio-economically important plant species. So, this particular study is designed to quantify the species diversity, abundance and vegetation structure, and to document the ecological importance of woody plant in burial forest sites in Gobeya rural administrative of Tehuledere district, South-Wollo, Ethiopia. The study is expected to fill the gap of limited researches in the subject area. The survey also aims to promote conservation of burial forest sites across the region. The outputs of this study are expected to have a contribution for policy legislation and management of sacred forest resources in the district.
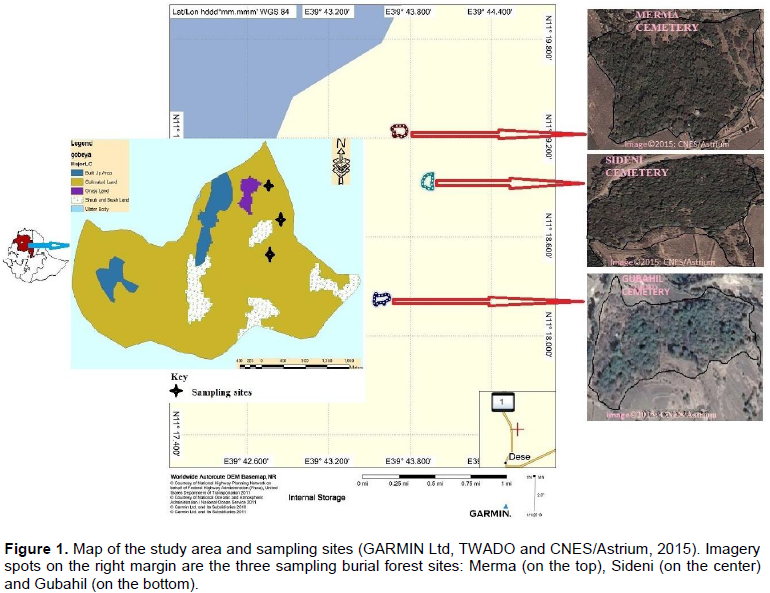
Gobeya rural administrative is one of the 19th rural administration units in Tehuledere district. According to ‘Tehuledere Woreda Agriculture and Development Office (TWADO)’, the study area is reported to cover a total area of 1293.75 ha. Information from TWADO shows that the area is divided into five land-use land-cover types (Figure 1). These include built-up area (86.85 ha), cultivated land (1070.6 ha), grassland (16.9 ha), shrub and bushland (118.8 ha) and water body (0.6 ha). This survey was conducted in three larger burial forest sites that have more than 1.5 ha each. The livelihood of the surrounding community is mainly agriculture. Most important crops harvested in the area include teff (Eragrostis tef), sorghum (Sorghum bicolor), maize (Zea mays), wheat (Triticum aestivum), barely (Hordeum vulgare), common oat (Triticum dicoccon), chickpea (Cicer arietinum), field pea (Pisum sativum) and many other horticultural crop species.
Measuring species richness, diversity and abundance of woody plants
The study area has about seven burial forest sites with variable sizes. For this study, however, only three larger burial forest sites (Sideni, Merma and Gubahil), having a size of more than 1.5 ha, were selected as representatives to carry out the survey. Generally, 20 × 20 m quadrats were randomly laid across the edge and the interior-habitat. In particular, 3 quadrats of 20 × 20 m at each 100 footsteps interval around the edge-habitat and 3 more quadrats of 20 × 20 m at each 50 footsteps interval around the interior-habitat of each forest site. Areas within 30 m range from the edge line were considered as edge-habitats, and areas beyond 30 m from the edge line were regarded as the interior-habitats. Then, species richness, diversity and vegetation structure of woody plants in each site were surveyed according to methods adapted from Bonham (2013) and Smith and Smith (2001). The total numbers of individual shrubs/trees counted in each quadrat were regarded as abundance while the number individual trees and shrubs of each species in a hectare represented a density.
Identification of woody plant species
Locally recognizable or native plants were identified while in the field and their names were verified using Flora Books of Ethiopia and Eritrea (Hedberg and Edwards, 1989; Edwards et al., 1995; Hedberg et al., 2003). On the other hand, nearly eight unidentified plant specimens were transported to Addis Ababa University National Herbarium for processing and identification. Categorizing woody plant species into threatened and unthreatened was made based on personal observation on their local/regional abundance in the surrounding ecosystems to the burial forest sites.
Data analysis
Multivariate data analyses including species rarefaction curve (to see the trend of species richness with sample size) and Chao-1 estimator (to extrapolate missing woody plant species in each sampling sites), and hierarchical clustering (to compare the level of species turnover and similarity in species composition between and within sampling sites) and non-metric multi-dimensional scaling (to clearly identify changes in species composition along the habitat gradients) were computed using ‘PAST’ version 3.06 (Hammer et al., 2001).
Diversity and abundance of woody plants
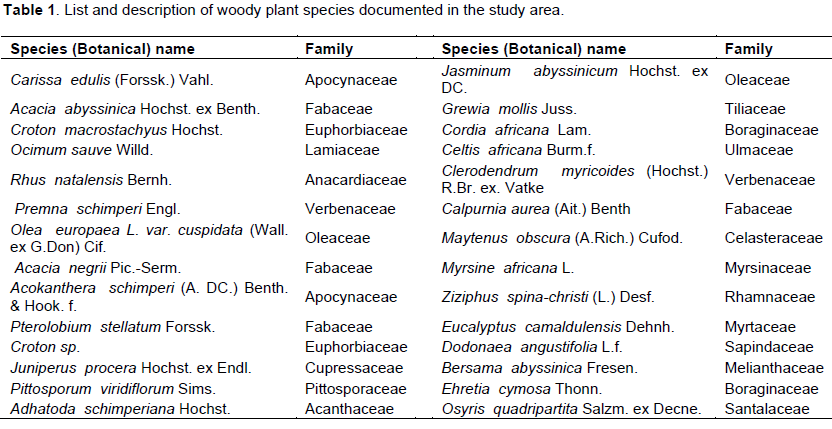
A total of twenty eight (N=28) woody plant species were recorded from all sampling sites combined. Of these, twenty seven species are found to be angiosperms (flowering plants) while only one species (Juniperus procera Hochst. ex Endl.) is a gymnosperm (non-flowering plant). Similarly, almost all species (n=27) are proven to be native to Ethiopia while only one species (Eucalyptus camaldulensis Dehnh.) is found to be an exotic one (Table 1). The species are belonging to 19 families and 26 genera. Family Fabaceae is found to have 4 species, and Apocynaceae, Boraginaceae, Euphorbiaceae, Oleaceae, Santalaceae and Verbenaceae are represented by 2 species each, and the remaining families have only one species each.
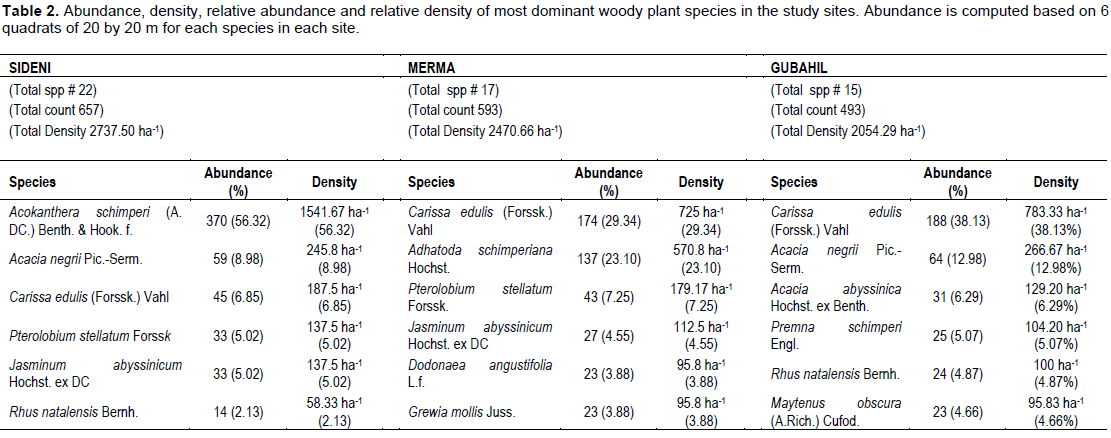
The study sites are located somewhat in the same ecological zones that it was expected to come across with similar species compositions in the study sites. Apparently, some shared woody plant species were discovered among the sampling sites. So, only nine woody plant species including Carissa edulis, Acacia abyssinica, Ocimum sauve, Olea europaea, Acacia negrii, Pterolobium stellatum, Jasminum abyssinicum, Grewia mollis and Calpurnia aurea are found in all the three sites. Similarly, each forest site is found to support different number of woody plant species where a total of twenty two, seventeen and fifteen woody plant species are recorded in Sideni, Merma and Gubahil sites, respectively. A study by Denu and Belude (2012) reported an equivalent number of species in average in their study on ‘Floristic Composition of Traditional Sacred Landscapes in Bedelle Woreda, Illubabor Zone, Oromia Regional State, Ethiopia’.
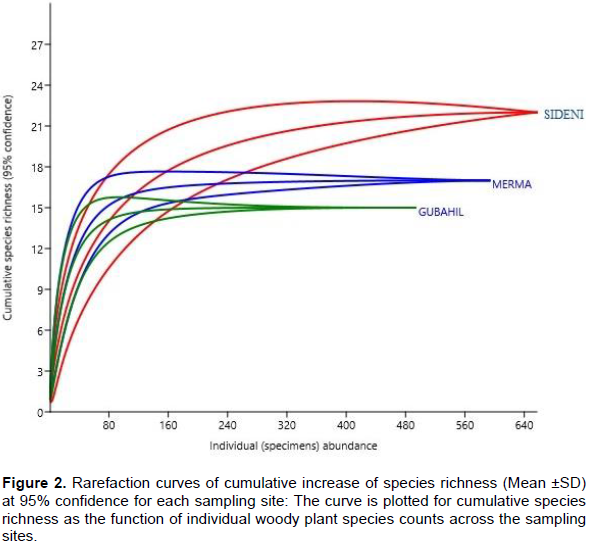
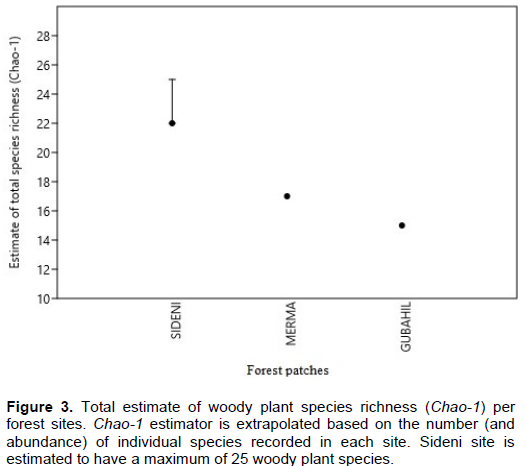
Species rarefaction curves at 95% confidence level also shows that Sideni burial site is characterized by the highest species richness (α-diversity) where even new species are expected to be found if more sampling plots were included (Figure 2). The increasing trend of rarefaction curve indicates the possibility of discovering more plant species than what is actually found if more plots were included. This implies that Sideni cemetery could have more than 22 woody plant species if the whole population was surveyed. On the contrary, the rarefaction curves for Merma and Gubahil sites become flat as the number of individual counts (and so sampling sites) increase. An estimate of species richness using Chao-1 estimator (Figure 3) based on abundance of individual species also suggests that Sideni site could have three more missed species undocumented. This makes the site to possibly have 25 woody plant species. On the other hand, the other two sites (Merma and Gubahil) would only have 17 and 15 woody plant species, respectively. This suggests that the probability of finding new (rare) woody plant species is unlikely even more sampling site were incorporated during the survey. The lower plant species records in Merma and Gubahil burial forest might be related to the presence of very low disturbance regime. In both sites, it was observed that grazing and product extraction are very limited. In this regard, Graham and Duda (2011) reported as a low and high disturbance could reduce species spatial diversity in a given area.
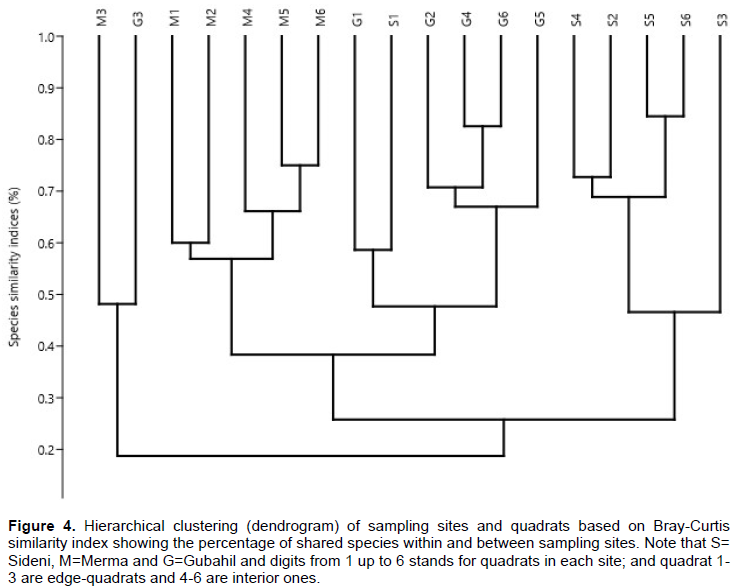
The hierarchical clustering (Figure 4) shows that for each site, 5 out of 6 quadrats cluster together indicating that burial sites tend to have different species composition. With few exceptions, however, there seems to be limited variation of species compositions between quadrats of the same site. The hierarchical clustering also generally indicates the average percentage of species similarity between quadrats in each sampling site. Overall, Sideni site is characterized by high species turnover and species richness. As it was stated earlier, this might be due to the fact that the degree of disturbance is observed to be intermediate (and controlled) in the site so that it might have promoted species richness and diversity in the site.
The apparent changes in species composition were clearly observed on a non-metric MDS ordination (Figure 5), which clearly shows that species composition actually changes while moving along sampling sites, but not while moving along edge and interior-habitats. This indirectly implies that the edge-effect has no systematic effect everywhere for the changing species composition across the sampling sites. It is, however, worth to note that important changes in species composition of a quadrats compared to the other quadrats of the same site were observed only from edge-quadrats, that is, in the 1st quadrat of Sideni site and in the 3rd quadrat of Merma site.
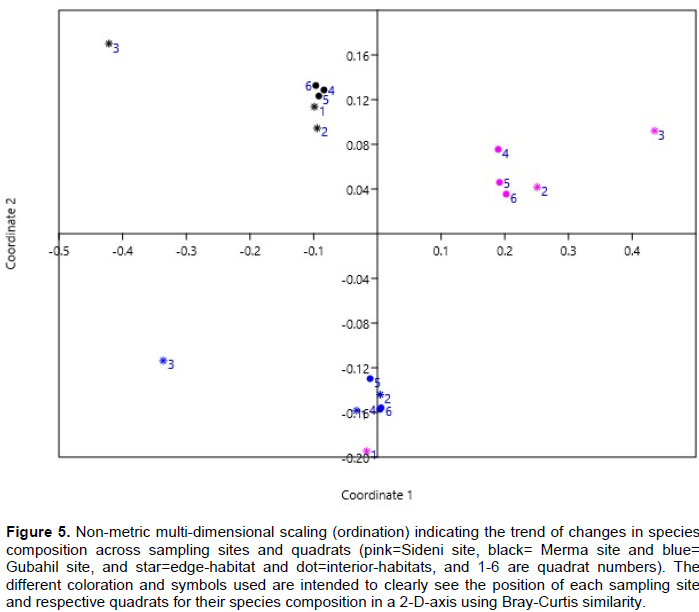
Therefore, it can be suggested that the edge and interior-habitats appeared to have almost the same species richness and species turnover in all sampling sites. As it was observed that the edge-habitats always have no lower (or higher) species richness, it also become clear that edge-habitats showed no significant differences in species spatial assemblage as compared to the interior-habitats in each sites. However, Kacholi (2014) has reported as the edge-habitats showed lower species heterogeneity in his study at Kilengwe Forest in Morogoro Region, Tanzania. The higher species richness and species turnover (Figures 4 and 5) observed at Sideni site could be related to the presence of controlled and intermediate disturbance regime either in the form of grazing and/or product extractions. The researcher also suggests that the pedological differences could also have contributed for the variation in species composition among sites. According to Intermediate Disturbance Hypothesis (IDH), controlled and intermediate disturbances are reported to increase spatial heterogeneity of species provided that other environmental conditions such as nutrients, water, sun light, temperature and other physical and biological factors are favorable (Graham and Duda, 2011). Disturbance has both negative and positive effects on species richness and composition based on its intensity and duration (Pausas and Austin, 2011). A report by Laloo et al. (2006) on medicinal plants in the sacred forests of Meghalaya, Northeast India also shows that species richness varies in disturbed and undisturbed
sacred forests.
Each forest site was also found to have different plant community type. For instance, Sideni graveyard is characterized by a community type over-dominated by Acokanthera schimperi (1541.6 per ha, 56.32%) followed by A. negrii (245.8 per ha, 8.98%). On the other hand, Merma and Gubahil graveyards are largely dominated by C. edulis which accounts 725 and 783.33 individual plants per hectare, respectively (Table 2). Overall, it can be concluded that Sideni burial site is apparently dominated by trees, while Merma and Gubahil graveyards are mainly dominated by shrubs. Aerts et al. (2006) also reported that the most abundant and widespread species in Afromontane church forests in the northern highlands of Ethiopia include A. schimperi, Acacia etbaica, Euclea racemosa, Justicia schimperiana, Leucas abyssinica, and Pavetta gardeniifolia. Other study report by Wassie et al. (2010) on church forests in a fragmented Ethiopian
Highland landscape shows that J. procera, O.europaea and Maytenus arbutifolia to be the dominant woody plant species in terms of their importance value in all forests they sampled.
Ecological importance of burial forest sites
The local community has solid awareness and understanding on the local and regional climatic changes and so the importance of ecosystem conservation and management. In this regard, the local government has played an important role in educating the locals about the need of soil conservation and ecosystem restoration. The local community, meanwhile, has witnessed that the vegetation cover in their vicinity had temporally and spatially decreased as the result of deforestation and unwise utilization of forest resources. The local community has a deep-rooted custom of protecting and conserving burial sites for many socio-cultural purposes. Apart from their socio-cultural importance, burial sites in the study area have several ecological values such as refugia (sources and sink) for certain wild animals including Hyena, Fox, Monkey, Porcupine, Gazelle, Partridge, and many other bird, bat and reptile species. Mgumia and Oba (2003) also reported as sacred natural sites serve as key refugia for plants and animal species, and other ecosystem services as well.
A study in west Kalimantan, Indonesia, also shows that sacred forests also serve as burial sites and fruit gardens (Marjokorpi and Ruokolainen, 2003). The socio-ecological and conservation values of sacred forests are also reported by Aerts et al. (2006) in their study on Afromontane church forests in northern highlands of Ethiopia.
Implication of burial forests for conservation
Forests are important sources of many life forms including plants, animals and other micro and meso-faunas. But, forests are under high pressure of deforestation and fragmentation as well as encroachment. Ethiopian forest cover is currently estimated to be 4 to 5%, most of which is confined in South West of the country. Most natural forest in northern highland part of the country are found in religious sites including monasteries, churches, mosques and graveyards predominantly located in rural areas. Burial sites in the study area are home for many plants that are missing in the larger landscapes of the country.
All burial sites in the study area are naturally regenerated forest patches protected by the local people for many socio-cultural and economic reasons. These burial forest sites provide many services such as livestock forage, medicinal plants, and firewood as well as ecological services in the form of habitat (refugia) for some wild animals: hyena, fox, gazelle, monkey and many birds and reptiles. So, it is vital to put efforts for sustainable conservation and management on burial forest sites as they are important sources of valuable plant species. In the study, the great proportions of woody plant species recorded are noticed to be locally threatened and are extremely less abundant across the surrounding ecosystems.
So, to avoid local extinction of these economically and socially important plant species, burial sites can be delineated as an important landscape to protect and preserve these locally threatened plant species. Therefore, conserving plant species in burial sites would help the local community to maintain the socio-cultural and economic services they obtain from the sites as most of the woody plant species recorded are not adequately found outside these burial forest patches.
Due to the fact that burial forest sites provide many ecological, socio-cultural and economic importances, the following recommendation are suggested for sustainable protection and reservation of plants resources in burial forest sites in the study area: (1) protection and conservation of burial forest sites and conservation of plants should be continued, sustainable and promoted, (2) any forms of colonization of burial sites by alien and invasive plant species should be routinely managed and protected, (3) the local government body and all other potential stakeholders should financially and technologically support the local people for their commitment of protecting burial plant resources, and (4) the local government should acknowledge the culture and the effort made by the local people towards protection burial forests and the resources within it.
The authors have not declared any conflict of interest.
REFERENCES
|
Aerts R, Van Overtveld K, Haile M, Hermy M, Dacker J, Muys B (2006). Species composition and diversity of small Afromontane forest fragments in northern Ethiopia. Plant Ecol. 187:127-142.
Crossref
|
|
|
|
Aynekulu E, Denich M, Tsegaye D, Aerts R, Neuwirth B, Boehmer HJ (2011). Dieback affects forest structure in a dry Afromontane forest in northern Ethiopia: Short communication. J. Arid Environ. 75:499-503.
Crossref
|
|
|
|
Bhagwat S, Rutte C (2006). Sacred groves: Potential for biodiversity management. Frontiers Ecol. Environ. 4:519-524.
Crossref
|
|
|
|
Bonham CD (2013). Measurements for terrestrial vegetation, 2nd ed. Chichester, West Sussex; Hoboken, NJ: Wiley-Blackwell, New York.
Crossref
|
|
|
|
Colwell RK (2009). Biodiversity: concepts, patterns, and measurement. Pages 257-263 in S. A. Levin, editor. The Princeton Guide to Ecology. Princeton Univ. Press, Princeton, NJ.
|
|
|
|
Denu D, Belude T (2012). Floristic Composition of Traditional Sacred Landscapes in Bedelle Woreda, Illubabor Zone, Oromia Regional State, Ethiopia. Ethiop. J. Educ. Sci. 8(1):75-91.
|
|
|
|
Echeverria C, Coomes D, Salas J, Rey-Benayas JM, Lara A, Newton A (2006). Rapid deforestation and fragmentation of Chilean temperate forests. Biol. Conserv. 130:481-494.
Crossref
|
|
|
|
Edwards S, Mesfin Tadesse, Hedberg I (1995). Flora of Ethiopia and Eritrea, Canellaceae to Euphorbiaceae. The National Herbarium, AAU, Addis Ababa & Uppsala. 2(2).
|
|
|
|
Erenso F, Maryo M, Abebe W (2014). Floristic composition, diversity and vegetation structure of woody plant communities in Boda dry evergreen montane forest, west Showa, Ethiopia. Int. J. Biodivers. Conserv. 6(5):382-391.
Crossref
|
|
|
|
Feyissa R (2001). Forest resource ownership and use rights and the role of communities in forest management: In imperative problems associated with forestry in Ethiopia proceedings of a workshop organized by biological society of Ethiopia, Feb, 1, 2001.
|
|
|
|
Gotelli NJ, Colwell RK (2011). Estimating species richness. Pages 39-54 in A. E. Magurran and B. J. McGill, editors. Frontiers in measuring biodiversity. Oxford University Press, New York.
|
|
|
|
Graham JH, Duda JJ (2011). The Humpbacked Species Richness-Curve: A Contingent Rule for Community Ecology. Int. J. Ecol. pp. 1-15.
Crossref
|
|
|
|
Hammer Ø, Harper DAT, Ryan PD (2001). PAST. Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1):9.
|
|
|
|
Hedberg I, Edwards S (Eds.) (1989). Flora of Ethiopia, Vol. 3, Pittosporaceae to Araliacae. The National Herbarium, Addis Ababa University, Addis Ababa & Uppsala.
|
|
|
|
Hedberg I, Edwards S, Nemomissa S (2003). Flora of Ethiopia and Eritrea, Apiaceae to Dipsaceae. The National Herbarium, AAU, Addis Ababa & Uppsala. 4(2).
|
|
|
|
Hundera K (2007). Traditional forest management practices in Jimma zone, South West Ethiopia. Ethiop. J. Educ. Sci. 2(2):1-10.
|
|
|
|
Kacholi DS (2014). Edge-Interior disparities in tree species and structural composition of the Kilengwe forest in Morongoro region, Tanzania. Hindawi Publication Corporation 24:1-9.
|
|
|
|
Laloo RC, Kharlukh, L, Jeeva S, Mishra BP (2006). Status of medicinal plants in the disturbed and undisturbed sacred forests of Meghalaya, northeast India. Population structure and regeneration efficacy of some important species. Curr. Sci. 90 (2):221-232.
|
|
|
|
Marjokorpi A, Ruokolainen K (2003). The role of traditional forest gardens in the conservation of tree species in west Kalimantan, Indonesia. Biodivers. Conserv. 12:799-822.
Crossref
|
|
|
|
Mgumia F, Oba G (2003). Potential role of sacred groves in biodiversity conservation in Tanzania. Environ. Conserv. 30:259-265.
Crossref
|
|
|
|
Mulat Y (2013). The role of Ethiopian Orthodox Church and Monasteries in forest management practices in Chilga and Mettema Woredas (Districts), North Gondar zone. Inter. J. Innov. Res. Dev. 2(7):437-446.
|
|
|
|
Pausas JG, Austin MP (2011). Patterns of plant species richness in relation to different environments: An appraisal. J. Veg. Sci. 12:153-166.
Crossref
|
|
|
|
Smith RL, Smith TM (2001). Ecology and Field Biology, 6th ed. Addison Wesley Longman, San Francisco, pp. 1-771.
|
|
|
|
Wassie A, Sterck FJ, Bongers F (2010). Species and structural diversity of Church forests in a fragmented Ethiopian highland landscape. J. Veg. Sci. 21:938-948.
Crossref
|
|
|
|
Whittaker RH (1969). Evolution of diversity in plant communities. Brookhaven Symp. Biol. 22:178-95
|






