A study on reproductive and feeding ecology of rodents was conducted in agricultural areas of some selected kebeles (the smallest administrative unit in Ethiopia) of Sekoru district from February 2014 to December 2014. Rodents were captured by snap trapping using rat traps. During the whole study period, four rodent species, namely, Rattus rattus, Mastomys natalensis, Arvicanthis dembeensis and Lemniscomys barbarus were identified from a total of 326 rodents captured in 1320 trap nights. The proportion of male and female individuals captured was not different from a 1:1 ratio. Scrotal males and perforate females were captured throughout the study period though reproduction was seasonal. Breeding started in the later part of the rainy season and declined at the beginning of the dry season. The average number of embryo counted per pregnant females was 5.57±1.09, 8.65±1.80, 6.38.±1.70 and 4.00 ± 1.41 for R. rattus, M. natalensis, L. barbarus and A. dembeensis, respectively. The food items identified during the stomach content analysis were grouped into leaves, seeds, animal matter and unrecognized food item. There was no variation in the type of food item identified among the four rodent species. Some variations were observed in the proportion of the different food items consumed by the four rodent species in different trapping sessions. Awareness creation and the need of rodent pest control in all growth stage of crops and after harvest were recommended.
Pest animals cause a considerable yield loss throughout the world. Among the crop pests, vertebrate pests especially rodents are responsible for much of the loss and farmers often list rodents as one of the most significant pests to their crops (Singleton et al., 1999; Makundi et al., 2005; Sudarmaji et al., 2003; Tuan et al., 2003). Rodents are regarded as the number one group of mammals in terms of the problems they create in agriculture, horticulture, forestry and public health (Makundi et al., 1999; Brown et al., 1999).
In East Africa, pest rodents cause considerable loss of agricultural crops. In Tanzania, for example, they cause an estimated pre-harvest loss of 15% in maize per annum (Mulungu et al., 2003). Mwanjabe and Leirs (1997) reported the damage to be more than 80% in certain cropping seasons and locations during rodent outbreaks. Taylor (1968) reported 20% damage to maize plantation, 34-100% loss of young wheat in some fields and 34% loss of barely after outbreak of rodents in western Kenya in 1962. Earlier studies in Ethiopia have indicated crop loss of about 20% per annum (Bekele and Leirs, 1997). Later, Bekele et al. (2003) reported an annual yield loss of 26.4% of maize by pest rodents in experimental fields at Zeway (eastern Showa Zone, central Ethiopia) which was even higher than the earlier reports.
Designing and applying effective pest management strategy such as integrated pest management (IPM) can reduce the amount of yield loss caused by pest rodents. Experiences of rodent pest control practices in different countries and research findings to devise appropriate rodent management strategies have showed that successful management of rodent problems depends upon correct identification of the rodent species causing the problem, and obtaining sufficient information on the biology, ecology and behavior of species in the ecological setting where the problem occurs (Tubin and Fall, 2004). Though such ecological studies are essential to devise control strategies of pest species of a particular region, few of such studies were conducted in agricultural areas of Ethiopia (Bekele et al., 1993; Bekele and Leirs, 1997; Lavrenchenko et al., 1998; Gadisa and Bekele, 2006; Bekele et al., 2003; Gebresilassie et al., 2004, 2005, 2006). In spite of serious problems faced by the farmers and many reports on agricultural damages on crops by rodent pests (personal communication with the head of crop protection clinic of the zone), no serious attempts were made in the Jimma zone of southwest Ethiopia to devise rodent control strategy.
Study area
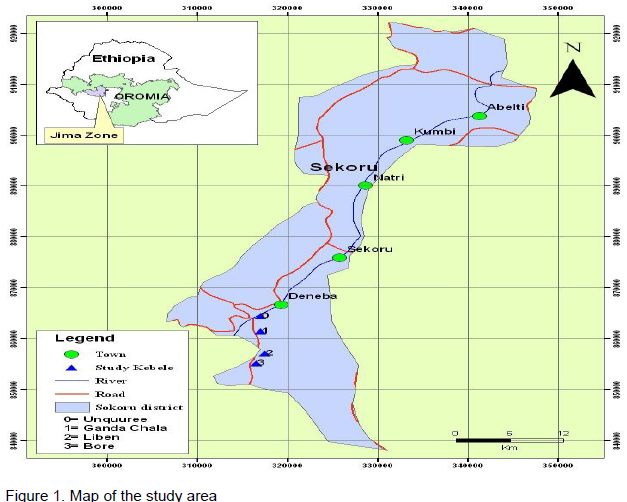
A study on reproductive and feeding ecology of rodent pests was conducted in some selected kebeles (the smallest administrative units in Ethiopia) of Sekoru district, Jimma Zone, southwest Ethiopia from February 2014 to December 2014 (Figure 1). The altitude of the district ranges from 900 - 2300 m a.s.l. The mean annual temperature of the district is 25°C and the mean annual rain fall is 1500 mm. The wet season is long and ranges from May to October and the dry season ranges from December to April.
Trapping and identification of rodents
Monthly snap trapping was carried out using rat traps for 11 consecutive months (February, 2014 to December, 2014). A total of 30 rat traps were used each day for four consecutive days every month throughout the study period. Traps were set in lines with 10-15 m trap space (Montgomery, 1985; Sutherland, 1996) and peanut butter was used as bait. To increase the trap success, the traps were set along the edges of cultivated fields or in cultivated fields where possible rodent refugia were available or where there were visible runways as rodents use specific paths (Kingdon, 1974;
Aplin et al., 2003). After collecting the captured animals, they were identified and standard external measurements were taken. Captured rodents were identified following taxonomic charac-teristics described by Aplin et al. (2003), Yalden et al. (1996) and Kingdon (1997).
Reproductive conditions
In males, sexual maturity or immaturity was assessed from the conditions of the testes and the scrotal sacs (Aplin et al., 2003; Bekele and Leirs, 1997; Gebresilassie et al., 2006). Captured rodents were considered juveniles if their testes are non-descended or have undeveloped scrotal sacs and sub-adults, if testes were partially descended. Rodents with fully descended testes were considered as adults. In females, the external signs of sexual maturity were assessed from the condition of the vagina and the teats (Aplin et al., 2003, Bekele and Leirs, 1997; Gebresilassie et al., 2006). Females with an open vagina and enlarged teats were considered as adults. A female rodent was considered as currently lactating if the teats are enlarged and the mammary glands are producing milk. Reproductive activities from internal characteristics were assessed after the animals were dissected. A male rodent was considered sexually mature when it possessed large testes, each with prominent blood supply and enlarged epididymis. In a female, features of the uterine horns that can be observed by naked eye or with dissecting microscope was used to determine sexual maturity. Slightly thicker and more elongated uterine horns with a more obvious blood supply but without embryos or placental scars characterize an individual that is entering its first breeding season. Thicker uterine horns with embryo in one or both uterine horns indicate pregnancy (Aplin et al., 2003). The number of embryo in each uterine horn was counted and recorded for pregnant females.
Diet analysis
Out of the snap trapped rodents, 150 were dissected and their stomachs were removed for identifying the diet of rodents in the laboratory following the methods of Leirs (1994). Stomachs were preserved in container containing 10% formalin after identification tag was attached to each stomach until microscopic examination of the stomach samples in the laboratory. In the laboratory, the samples were washed with distilled water to remove fine particles and sieved with sieve of mesh size 0.2 mm for proper identification and mixed thoroughly. Four slides were prepared for each stomach sample and observed under light microscope to identify the type as well as the proportion of the diet in the sample under study. To quantify the food items of the stomach samples, light microscope with 60x magnification was used. The proportion of different food fragments (leaves of monocots and dicots, seed coats and exoskeleton of arthropods) in the diet was determined by counting the fragments from the entire slide. The presence of food items was recorded in all the prepared slides and was grouped into different types: leaves, seeds, animal matter and unrecognized food item. Chi-square test (χ2) was used to test the 1:1 ratio of male and female rodents captured at 0.05 levels of significance.
Species composition
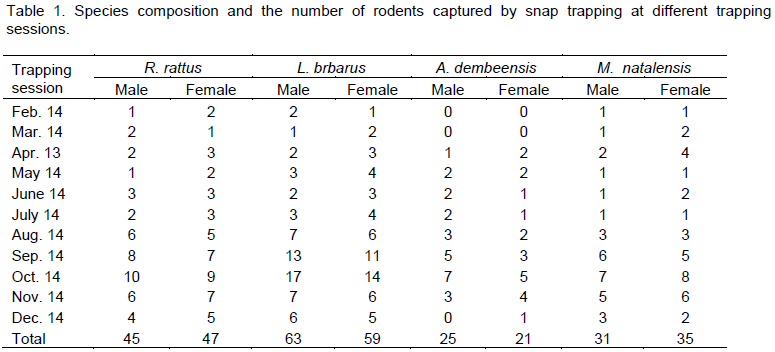
From a total of 326 rodents captured in 1320 trap nights, four rodent species were identified: namely, Rattus rattus, Lemniscomys barbarus, Mastomus natalensis and Arvicanthis dembeensis. Out of the total capture, Lemniscomys barbarus comprised the largest proportion of the capture with 123 individuals (37.73%) followed by R. rattus with 92 individuals (28.22%). M. natalensis and A. dembeensis comprised 20.25 and 17.69% of the total capture with 66 and 46 individuals, respectively (Table 1). Among snap trapped species, 164 (50.31%) were males and 162 (49. 69%) were females and this proportion was not significantly different from a 1:1 sex ratio (P = 0.856).
Reproduction
Reproductively active males (scrotal males) and females (perforate females) were captured throughout the whole study period. But, variations were observed in the proportion of scrotal males and perforate females captured in different trapping sessions. For most rodent species identified, the male individuals captured from February to August were scrotal and all females were perforate. From September to December, the proportion of scrotal males and perforate females was reduced due to introduction of abdominal males and imperforate females to the population for all the species identified (Table 2). The maximum proportion of abdominal males and imperforate females was recorded in October when more than 30% of the males and females were abdominal and imperforate males and females, respectively.
Among 164 males captured throughout the study period, 128 (78.05%) were scrotal and 133 (82.10%) of 162 females were perforate. In addition, all rodents captured from February to September were scrotal males and perforate females, but, pregnant females were not captured until June for most of the rodent species identified. The rate of pregnancy was high in August and September for almost all the four species and the rate of pregnancy declined after October (Table 3).
Litter size
Among the 39 perforate females of R. rattus, 14 were pregnant. The average number of embryo counted for this species was 5.57±1.09. The number of embryo counted per pregnant female ranged between four and eight. Embryo counts of five and six were more common while that of eight was the least frequent embryo count (Figure 2). Among 47 L. barbarus adult females captured during this study, 16 were pregnant. The maximum number of embryo counted for this species was 10
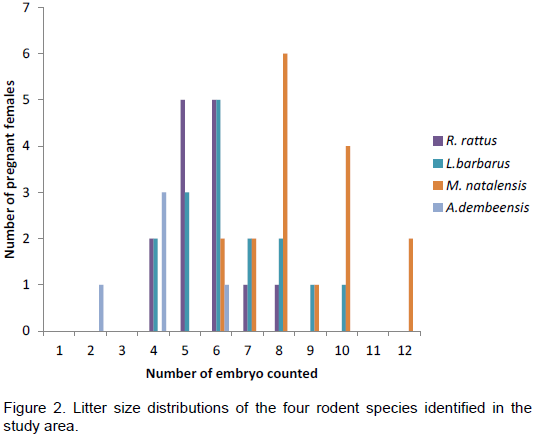
and the minimum count was four. The most frequently counted embryo number was six (Figure 2). The average litter size for the species was 6.38±1.70 For M. natalensis and A. dembeensis, 17 and 5 pregnant females were captured from 47 and 17 perforate females captured, respectively. The number of embryo counted ranged from 6 to 12 for M. natalensis and from two to six for A. dembeensis (Figure 2). The average litter size recorded for M. natalensis in this study was 8.65±1.80. For A. dembeensis, the mean number of embryo counted per pregnant female was 4.00±1.41.
Diet
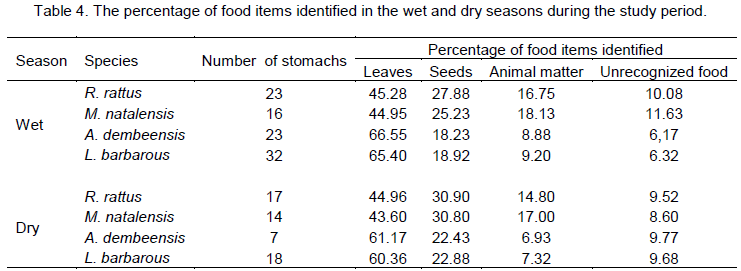
Stomach samples of 40 R. rattus (20 males and 20 females), 30 A. dembeensis (15 males and 15 females) and 30 M. natalensis (15 males and 15 females) and 50 L. barbarus (25 males and 25 females) were analyzed thought-out the study period. Some variations were observed in the proportion of food items the four different rodent species consumed during dry (November to April) and wet (May to October) seasons (Table 4). For R. rattus, the consumption of leaves was generally high in both seasons but it consumed relatively more leaves in the wet season than the dry seasons. Seed consumption of this species was high in the dry season and the consumption of animal matter was relatively more in the wet season. M. natalensis consumed more leaves in the wet season and its seed consumption was relatively high in the dry season. The consumption of animal matter was more in the wet season. The consumption of leaves by A. dembeensis and L. barbarous was more as compared to the consumption of leaves by the other two species. Leaves comprised more than 60% of the food items consumed by these species in both seasons.
Among the four rodent species identified in the present study area, M. natalensis and A. dembeensis were recognized as the major pest species in agricultural fields by different researchers in other parts of Ethiopia (Bekele et al., 1993; Bekele and Leirs, 1997; Bekele et al., 2003; Lavrenchenko et al., 1998). The presence of these important pest species in the study area suggests the potential of serious crop damages and losses by them and should be major concerns to the farmers in the area. In addition, all Mastomys species in Africa are reported to cause problems in agriculture and public health (Leirs et al., 1996; Fiedler, 1988a, b; Odhiambo and Oguge, 2003). This underlines the need of controlling these species in the study area though their number in the present investigation was low.
The other important species identified in this study are R. rattus and L. barbarous. In many literatures, R. rattus species is mentioned as one of the major pest species that causes a worldwide problem by damaging coconuts and food crops (Fiedler and Fall, 1994; Buckle, 1999). However, R. rattuswas not reported as a major pest problem in agricultural fields in Ethiopia in reports of earlier research works (Bekele and Leirs, 1997; Bekele et al., 2003).
Many individuals of this species were captured in farmlands with serious damages that were closer to human dwellings. Thus, this species could be a serious problem for farmers who have farmlands closer to their house. L. barbarus, though not reported as an important pest species in other parts of Ethiopia, was the dominant pest species in the present study area. Due to its diurnal behaviour, even farmers in the study area were able to see this species climbing the stem of maize and causing serious damage especially during the ripening stage.
Generally, scrotal males and perforate females were captured throughout the study period. But, the proportion of scrotal males and perforate females was lowered in some trapping sessions because of introduction of abdominal males and imperforate females to the population. From the variation in the proportion of scrotal males and perforate females observed, one could say that these species must have started reproduction before the months when reduction in proportion was observed and the new born individuals of both sexes have reached a trappable size at this month and the high proportion of scrotal males perforate females after December suggests that all the new born individuals that entered the population have reached the age of maturity (became adults) and reproduction have ceased to produce more young individuals of both sexes (Makundi et al., 2007). If breeding has continued, the addition of juveniles into the population continues but this did not happen. In general, it is possible to say that breeding for these four species is restricted to the wet season, especially to the later part of the rainy season and ceases in the dry season. This finding is in agreement with many research results obtained in many rodent pest species in other areas (Bekele and Leirs, 1997; Gadisa and Bekele, 2006; Odhiambo et al., 2005; Leirs, 1994; Lima et al., 2003).
Although the percentage of scrotal males and perforate females was very high in most of the months during the study period, pregnancy for the four snapped species was detected only from May to December. The first pregnant females were captured in May for M. natalensis and May and June for all the four species. Rate of pregnancy has reached peak in July and August and declined towards the dry season and the last pregnant females were captured in December. From this, it is possible to say that breeding commences from the middle of the rainy season and continues up to the early dry season. Such restrictions of breeding to the wet season for many rodent species have been observed by different workers in many different areas (Bekele and Leirs, 1997; Gadisa and Bekele, 2006; Odhiambo et al., 2005; Leirs, 1994; Lima et al., 2003).
The high percentage of scrotal males and perforate females before September and after December can be explained differently. The high percentages obtained before September were because breeding has started in June and the highest percentage of pregnant females was captured in August, and thus, the number of juveniles that could enter the population before September would be very small so that the percentage of scrotal males perforate females in the population would be very high. Although, the high percentage after December would have a different reason. In a month before December, the percentage of scrotal males and perforate females was relatively lower because of the presence of juveniles in the population. The high percentage after December could be because the juveniles that were present in the population have become adults and no juveniles could enter the population as breeding has ceased in the early dry season.
The average number of embryos counted (8.65± 1.80) in this study for M. natalensis was lower than the average number of embryos obtained (10.21 ± 4.32 for grass land M. erythroleucus and 12.84 ± 3.26 for maize field) by Bekele and Leirs (1997) at Koka (central Ethiopia). It was also lower than the average number of embryos obtained (11.80 ± 3.40) for the same species by Duplantier et al. (1996) in Senegal. On the other hand, the average number of embryos counted for A. dembeensis in this study (4.00 ±1.41) is in line with those (4.20± 2.20) obtained by Gadisa and Bekele (2006) at Bilalo (southeast Ethiopia) for the same species but lower than the ones obtained (5.74±2.65) by Bekele and Leirs (1997) for grassland at Koka. But in the same study, Bekele and Leirs (1997) obtained a higher number of embryos (7.42± 2.63) for maize field. The average litter size (5.85±0.34) obtained by Massawe et al. (2007) for A. neumanni (a related species) in central Tanzania is also higher than with the average litter of A. dembeensis in this study. The variations could be due to differences in environmental conditions such as availability of food.
Most species of rodents appears to be opportunistic feeders (Johnson, 1961). The result of the present study also confirms this. Regardless of the proportional difference, all rodents consumed plant and animal matters. For instance, the stomach contents of M. natalensis and A. Dembeensis included monocot seeds; monocot leaves and animal matters. However, the stomachs of A. dembeensis contained high percentage of grass in addition to other ingredients. This is in line with the finding by Gebresilassie et al. (2004), which have recorded higher percentage of grass and monocot seeds in the stomachs of A. dembeensis.
The food items identified in the stomach contents of the four rodent species were the same: all stomach contents consisted of leaves (monocot leaves, dicot leaves and grass), seeds and animal matter (Table 4). One probable reason for this could be less heterogeneity of vegetation in agricultural areas. However, the proportion of the various food items consumed by the different species showed some variations among the species and in the different trapping sessions. Compared the other two species (R. rattus and M. natalensis), the stomach contents of A. dembeensis and L. barbarous consisted of high percentage of leaves. In addition, the percentage of leaves was higher in the wet season as compared to the dry season. This finding is in agreement with the results obtained by Gebresilasie et al. (2004) in Myngus irrigation field, northern Ethiopia, who recorded more grass in the stomach content of A. dembeensis than the stomach content of M. erythroleucus. The percentage of the leaves consumed by the other two species was also higher during the wet season than the dry season. One probable reason for the high consumption of leaves in the wet season could be their relative high abundance than seeds and other plant matters.
Although, starting the latter part of the wet season (the time when crops start maturing) the relative consumption of leaves decreased and the consumption of seeds increased (Table 4). A similar shifting of diet composition was observed by Gebresilasie et al. (2004) by pest rodents of Myngus irrigation field, northern Ethiopia. Similarly, Oguge (1995) found M. natalensis to have eaten more grass and dicotyledonous shoots until the development of their preferred cereals and seeds. Leirs (1994) also found M. natalensis to consume more grass and arthropods at the beginning of the rainy season and more cereals during the late rainy season and during the early dry season. This shifting of diets of rodents observed in this study in different seasons indicates that the crops grown in the study area are under the attack of these pest rodents at their different stages of development.
In this study, variation in the consumption of animal matter was observed between the species and between trapping sessions for a species. The percentage of animal matter consumed by R. rattus and M. natalensis was relatively higher than the animal matter consumed by A. dembeensis.
In addition, all the four species consumed more percentage of animal matter during the wet season than the dry season. This variation may be related to the fulfillment of their protein requirement during the seasons of reproduction. The results of this study are comparable with the results of other workers. For example, Leirs (1994) reported that M. natalensis consume more grass and arthropods at the beginning of the rainy season and more cereals during the late rainy season and during the early dry season. Gebresilasie et al. (2004) also reported that reproductively active females consumed more arthropods, monocot shoots and wild herbs than other females. Similarly, Rabiu and Fisher (1989) have revealed that the scrotal males of Arvicanthis sp. have consumed more of monocotyledons and animal matter than the abdominal males.
Most rodents are small, secretive and nocturnal, so are rarely seen even when present in a given area in large numbers (Williams, 1993; Witmer, 2007). They also have a high reproductive potential and short period to reach sexual maturity, thus, can invade large area in a short period of time and cause serious damage (Aplin et al., 2003). Thus, farmers should be aware of the presence of these important pest species and advised to monitor their farmland regularly and carry out rodent control activities in all growth stages of their crops.
The authors thank Jimma University for funding the research. We are also grateful to the development agents (agricultural extension workers) for their help during data collection and the farmers who allowed as to carry out this study in their farm lands.