ABSTRACT
Swayne’s Hartebeest (SHB) is an endangered endemic animal to Ethiopia. However, its activity pattern and social behavior are not well documented. Hence, we investigated the diurnal activity pattern and social behavior of SHB in the Maze National Park. Data were collected by direct observation of focal-animal from October 2018 to April 2019 and analyzed using descriptive statistics and X2-test. A total of 1004 observations were made for activity pattern study. The SHB were performing four major activities (remain standing: 37.6%, grazing: 32.9%, walking: 15.7%, lying: 11.2%) showing significant differences in the total observation frequencies (X2 = 205.69, P < 0.05). Standing was the dominant activity followed by grazing in the wet season and vice versa in the dry season. Observation frequency for standing showed significant difference between seasons (X2 = 6.614, P < 0.05). Observation frequency for the activities within season (wet season: X2 = 120.6, P < 0.05; dry season: X2 = 100.38, P<0.05) showed variation. A total of 951 observations were made for social behavior study. We found significant differences in the total observation between types of social groups (X2 = 109.52, P<0.05) and between seasons (X2 = 22.722, P<0.05). Female SHB with young calf showed the dominant vigilant behavior. The findings revealed a decrease in the rate of occurrence for vigilance behavior with increasing social group size. We suggest the management plan of the park shall consider the findings of this study as a useful input for sustainable conservation of this endangered endemic species.
Key words: Social organization, Swayne’s hartebeest, time budget, vigilance.
The Swayne’s Hartebeest (SHB) is a large antelope, endemic to the southern Rift Valley of Ethiopia (East, 1999). It was distributed throughout the Rift Valley of the country in the past and extended eastward into northwestern Somalia (IUCN, 2013). Today, its distribution is limited to two protected areas in Ethiopia that is, Maze National Park and Senkelle Swayne’s Hartebeest Sanctuary (Abiot, 2013; Simon, 2016; Shibru et al., 2020). Therefore, for the conservation of this species knowledge of its diurnal activity pattern and behavior is one of the essential prerequisites.
The change of activity patterns in ungulates, including SHB, serves as an evolutionary adaptation to optimize fitness in frequently fluctuating environments (Yerushalmi and Green, 2009; Kurauwone et al., 2013). Thus, the seasonality of activity patterns might be highly flexible in response to seasonal fluctuation in food supply, habitat, predator avoidance and corresponding temperature (Vasey, 2005; Ruckstuhi and Neuhaus, 2009). Seasonal variation in the activity patterns of SHB and other ungulates (Wondimagegnehu and Afework, 2015) were influenced by the quality and quantity of food, human disturbance, accidental fire, habitat fragmentation, tourism service, hunting and livestock grazing (Norris et al., 2010). Livestock pressure on habitats of wild animals in Maze National Park was reported by Wondimagegnehu and Afework (2013). Based on the information obtained from Maze National Park, about 50,000 heads of livestock were grazing in the park per day during the dry season. The human activities and overgrazing on forage resources adversely affect the daily activity patterns of SHB in the dry season (Shibru et al., 2020) in Nech Sar National Park. These findings were in line with a number of studies on the other ungulates in different protected areas (Liu et al., 2008;; Chu et al., 2009; Lin et al., 2012).
The event of non-prescribed fire has disturbed the diurnal activity pattern of SHB during the dry season in Maze National Park (Almaz, 2009; Wondimagegnehu and Afework, 2011; Abiot, 2013). Disturbances from livestock and human activities caused the highest vigilance of Swayne’s hartebeest, which reduces and confuses their usual diurnal activity pattern during the dry season. Similar studies were reported for ungulates of Goiter gazelles (Xia et al., 2011).
Although detailed information on the diurnal activity pattern and social behavior of wild ungulates is essential for their effective conservation, yet such data are lacking for most of the Ethiopian endemic species (Lewis and Wilson, 1979; Yosef et al., 2015). Likewise, though the Swayne’s Hartebeest was endemic and critically endangered, no previous research was conducted on its activity patterns and social behavior in Maze National Park. Therefore, this study investigated diurnal activity patterns and social behavior of SHB in Maze National Park.
Study area
Maze National Park is located at 460km south of Addis Ababa in the Southern Nations, Nationalities and People’s Regional State (Figure 1). It lies between 06°3' to 06°30'N latitude 37°25' to 37°40'E longitude. Its altitude ranges from 900 to 1200 masl and covers total area of 202 km2 (Befekadu and Afework, 2006). The area was known for its bimodal rainfall pattern and is one of the semi-arid agro-ecological zones in Ethiopia. The annual rainfall ranges between 843 and 1321 mm (Befekadu, 2005). The rainy season runs from March to October, while the dry season is from November to February (Befekadu, 2005; Yosef et al., 2012). The minimal temperature in the wet season is 15.3°C in June and the maximal (33.5°C) is in February in the dry season (Wondimagegnehu and Afework, 2011; Yosef et al., 2012). The Park is home of 39 species of large and medium mammals and 196 bird species (EWCA, 2012). It is also known for hosting a critically endangered endemic Swayne’s Hartebeest. Most of the plains of the Park are covered by open Combretum-Terminalia wooded grasslands (Siraj et al., 2016).
Diurnal activity pattern
Data were collected using scan sampling method (Altman, 1974; Jarman, 1974). Direct observations were made on focal animals. The wet season data were collected from October 2018 and March to April 2019. The dry season data were collected from November to December 2018 and January 2019. A total of 1004 observations were made (Wet season: 50.8%; Dry season: 49.2%). Observations were recorded on grazing, standing, walking, lying, grooming, defecating, watering, nursing and playing. The activities between the age and sex structures were also recorded. Individual animal was randomly selected for age and sex category. However, when the focal animals were in group, the dominant activity of the group was recorded at the beginning of the observation. Each observation was carried out for 5 min at 15 min interval from early morning (6:00 h) to late afternoon (18:00 h) across seasons. Time of the day is recorded for each activity. The durations of each activity were recorded using stopwatch. Pictures were taken for further confirmation using digital camera. The activity pattern was observed two times per month for a total of 12 observation days. Animals were observed using unaided eye and/or binocular from appropriate place for their clear visibility. The observed individuals were designated as adult males, adult females, sub-adult males, sub-adult females and young ones (Kingdon, 1997; Wondimagegnehu and Afework, 2015). Age and sex were determined based on body size, size and shape of the horn and body color. Individuals small in body size were recorded as young ones, individuals medium in body size were recorded as sub adult males and sub adult females, individuals large in their body size were recorded as adult males and adult females (Kingdon, 2015).
Social behavior
Data were collected using focal-animal sampling method (Altman, 1974; Jarman, 1974). Observations were done on focal individual at a time. The wet season data were collected from October 2018 and March to April 2019. The dry season data were collected from November to December 2018 and January 2019. Two or more individuals were taken as a focal sampling when those animals were continuously visible throughout the sampling period. A total of 951 observations were made for social behavior study of the species. The predominant social behavior was recorded. Each observation was carried out for 5 min at 15 min interval from early morning (6:00 h) to late afternoon (18:00 h) across seasons. The durations of each displayed social behavior were recorded using stopwatch. The social behavior of SHB was observed two times per month for a total of 12 observation days. After the social groups were identified and defined; the observation for their social behavior such as agonistic, vigilance, territoriality, and intraspecific competition or competition for resources within and between their social groups were recorded.
Data analysis
Data were checked and organized into excel sheet before analysis. Descriptive statistics and Chi-square test were used to analyze the data in SPSS version 20 and r-program (α = 95% level of significance, P < 0.05). Observation frequencies were compared using Chi-square test across season and between activities. The occurrence frequencies in each social behavior for each age/sex group during dry and wet seasons were computed by Chi-square test.
Diurnal activity pattern
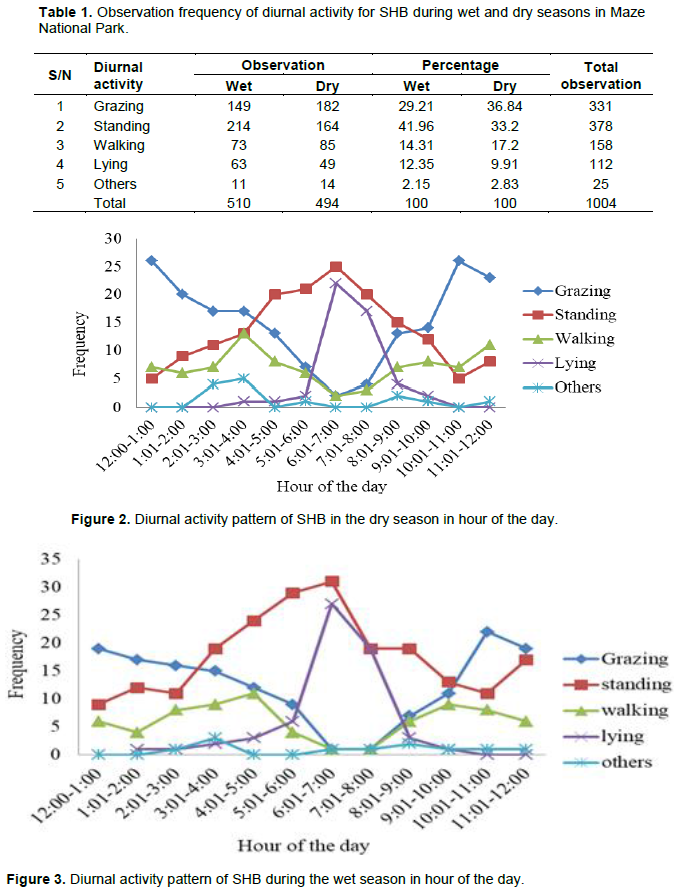
The diurnal activity pattern for SHB in the study area is presented in Table 1. The diurnal activity of the species in the park for the hours of the day is presented in Figures 2 and 3 for the dry and wet seasons, respectively. The diurnal activity pattern of the species among age/sex group is depicted in Figure 4. The observational frequency of SHB was 37.6% for remain standing, 33.0% grazing, 15.8% walking and 11.1% lying. Significant differences were revealed in the total observation frequencies between the diurnal activities (X2 = 205.69, df =3, P < 0.05). Standing and grazing were the major activities during the wet and dry season, respectively. Observation frequency for standing showed significant difference between the dry and wet seasons (X2 = 6.614, P<0.05). However, we did not reveal significant differences for grazing (X2 = 3.29, df = 1, P>0.05), walking (X2 = 0.911, df = 1, P>0.05) and lying (X2 = 1.75, df = 1, P>0.05) between the dry and wet seasons. Observation frequency for diurnal activity within each season showed significant differences (X2 = 120.6, df = 3, P<0.05). There were significant differences in the frequency of the hour of the day for various daily activity patterns of SHB within a season (Wet: χ2 = 153.91; Dry: χ2 = 163.82, P< 0.05).
Significant differences were also revealed among age/sex groups (χ2= 17.0, = P< 0.05) for feeding activities (χ2= 13.091, df = 3, P < 0.05) between seasons and among age/sex groups (χ2= 259.440, df = 3, P < 0.05) for other daily activities (Figure 4).

Social behavior
Among a total of 951 observations made for social behavior study of SHB, 43.5% were for mixed type group, 38.9% were for adult male-adult female and 17.5% were for adult female with young social group type. We found significant differences in the total observation between types of social groups (X2 = 109.52, df = 2, P < 0.05). The frequency of occurrence between seasons also showed significant differences (X2 = 22.722, df = 1, P < 0.05). Comparison of the frequency of occurrence for each type of social group showed no significant difference between seasons for mixed type group (X2 = 1.1691, df = 1, P > 0.05), adult male-adult female (X2 = 0.0108, df = 1, P>0.05) Table 2.
Table 3 presents comparison of the occurrence frequencies of the social interactions within mixed type social group. Comparison of occurrence frequencies of social behavior between mixed type social group did not
show significant differences (X2 = 6.566, df = 3, P > 0.05). Comparison of occurrence frequencies of social groups within each mixed type social group showed significant differences for territorial male (X2 = 13.8, df = 3, P < 0.05), sub adult male (X2 = 33.426, df = 3, P < 0.05), female with young (X2 = 39.3, df = 3, P < 0.05), adult female (X2 = 15.2, df = 3, P < 0.05), and sub adult female (X2 = 33.1, df = 3, P < 0.05), but not for Bachelor male (X2= 5.86, df = 3, P > 0.05). Comparison of each social behavior between mixed type social groups depicted significant difference for agnostic behavior (X2 = 78.89, df = 5, P < 0.05), vigilance behavior (X2 = 54.17, df = 5, P < 0.05), and territoriality (X2 = 66.11, df = 5, P < 0.05), but did not depict significant variations for intraspecific competition (X2 = 9.38, df = 5, P > 0.05).
Table 4 shows the occurrence frequency of social behavior between adult-male to adult-female group size pair. Comparison of the total occurrence frequency of social behavior between adult-male to adult-female group size pair did not reveal significant differences (X2 = 4.30, df = 3, P > 0.05). On the other hand, comparison of occurrence frequency of each social behavior between the different group size pair revealed significant variation for agonistic behavior (X2 = 35.09, df = 3, P< = 0.05), Vigilance behavior (X2 = 7.817, df = 3, P < 0.05), intraspecific competition (X2 = 28.18, df = 3, P < 0.05) and territoriality (X2 = 15.79, df = 3, P < 0.05). Likewise, comparison of the occurrence frequency of social behaviors within each pair showed significance variation for one to one (X2 = 44.35, df = 3, P < 0.05), three to five (X2 = 13.26, df = 3, P < 0.05) and five to eight (X2 = 30.97, df = 3, P < 0.05), but did not show significant variation for two to three group size pair (X2 = 1.03, df = 3, P > 0.05).

This study revealed significant difference in the observed frequencies for standing between wet and dry seasons. This could be due to sufficient amount and availability of quality forage during the wet season that results in spending more time in standing than in dry season when grazing took much time while searching for quality forage through day time (Vymyslicka et al., 2010).
In this study SHB showed two feeding peak hours for both wet and dry season in the day time (Figures 2 and 3). These were early morning (12:00-2:00 h) and late afternoon (10:00-12:00 h). Whereas standing and lying were recorded for one peak time of the day (6:00h-8:00 h) in both seasons. Similar patterns were reported for Nech Sar National Park (Vymyslicka et al., 2010) and Senkelle SHB Sanctuary (Lewis and Wilson, 1979; Berhanu and Yirga, 2004). High feeding peaks in the early morning and late afternoon for Swayne’s hartebeest might be due to low disturbances by human activities and low ambient temperatures (Cain et al., 2006). The same results were stated by Aberham et al. (2016) for ungulates of African buffalo in Chebera Churchura National Park.
Among age/sex groups of Swayne’s hartebeest, adult females were seen more frequent while feeding than adult male. On the other hand, adult males were observed more frequently in standing than other age/sex groups. This was in line with other studies for mammals that females need to forage more time to satisfy their nutritional requirement, makes differed activity patterns between males and females (Neuhaus and Ruckstuhl, 2004). It was stated that foraging time is positively correlated with body size in African herbivores (du Toit and Yetman, 2005). Long time spent in standing of male Swayne’s hartebeest might be due to its involvement in territoriality and vigilance next to females with young. Activities those are categorized under other activities are mostly revealed for adult females in dry season. These might be due to calving of Swayne’s Hartebeest in dry season that involves nursing, licking and grooming.
Different studies proposed and suggested four factors that influence ungulate’s activity patterns. These are seasonal variation in forage quantity and quality (Moncorps et al., 1997); time of the day and seasonality in temperature variations (Shi et al., 2003); livestock movements and human activity (Schaller, 1998); and biological process occurring in 24 hour intervals (Maher, 1991).
In this study, females with young showed highest vigilant behavior of occurrence with frequency rate of 36.78% and territorial male was the second vigilant member with average occurrence rate of 27.58% in which long time invested looking for predation or any disturbing events happening around (human and non-human disturbances). Hunter and Skinner (1998) reported the same trend for other antelopes of Wildebeest and Impala in South Africa. Territories were maintained by territorial males in mixed social group type and defend against occupation by other adult males. The result was consistent with Berger and Hilton-Barber (2004). Researchers reported that, animals on the edge of herds or out of their social group spent more time to vigilance than those in the central location of the group in South Africa (Bednekoff and Ritter, 1994; Burger and Gochfield, 1994). From this finding, it can be said that the vigilance behavior of the Swayne’s Hartebeest was negatively correlated with the size of its social group. This showed that as the social group size increase, the rate of occurrence for vigilance behavior decrease. This was in line with studies by Sonja and James (2009) on ungulates of Impala. On the other hand, as the size of social group increased, the intraspecific behavior also increased. For instance; for already defined social group of male to female pair type the occurrence rate for intraspecific behavior, for smallest group size two was 3.6% and in large group size of 13 was 42.19%. In addition, when comparing the social behavior of SHB within the same group, the territorial male to all others, there were statistically significant variation except for adult male and sub adult female, while female with young has no significance variation except with adult male. Adult male showed significance variation only with bachelor male and sub adult male while sub adult male has no significance difference with bachelor male and sub adult female but no variation with others and sub adult female has no significant variation with all members in group.
Standing in the wet and grazing in the dry season were the major activities of SHB in the study area. Observation frequency for standing showed significant difference across the seasons. The observation frequency for diurnal activity within each season showed significant differences. Significant differences were revealed among age/sex groups for activities between seasons. Mixed type group, adult male-adult female, adult female with young social group types were identified in the present study. We found significant differences in the total observation frequency between types of social groups. Within each mixed type social group, we found significant differences for territorial male, sub adult male, female with young, adult female, and sub adult female. Each social behavior between mixed type social groups depicted significant difference for agnostic behavior, vigilance behavior, and territoriality. Occurrence frequency of each social behavior between the different group size pair revealed significant variation for agonistic behavior, vigilance behavior, intraspecific competition and territoriality. Occurrence frequency of social behaviors within each pair showed significance variation for one to one, three to five and five to eight group size pair. The findings of this study could provide useful information for effective conservation of Swayne’s Hartebeest in particularly in the studied area and can also be applied to other protected areas and other large herbivores as the SHB’s general behavioral patterns overlap with those of other large herbivores.
The authors have not declared any conflict of interests.
The authors are grateful to Arba Minch University for granting fund for this research and our gratitude also goes to the staff members of Maze National Park for their cooperation and support.
REFERENCES
|
Aberham M, Mundanthra B, Gurja B (2016). Diurnal activity budget of African buffalo (Syncerus caffer (Sparrman, 1779) in Chebera Churchura National Park, Ethiopia. African Journal of Ecology 56:436-444.
Crossref
|
|
|
|
Abiot H (2013). Effect of habitat disturbance on the population of Swayne's Hartebeest (Alcelaphus buselaphus Swaynei) in Senkelle Swayne's Hartebeest Sanctuary. MSc. Thesis School of Wildlife Management and Ecotourism Studies, Wondo Genet College of Forestry and Natural Resources, Hawassa, Ethiopia.
|
|
|
|
|
Almaz T (2009). Sustaining the Allideghi grassland of Ethiopia: Influences of Pastoralism and Vegetation change. PhD Dissertation, Utah State University, Utah, USA.
|
|
|
|
|
Altman J (1974). Observational study of Behavior. Sampling methods. Behavior 43:227-269.
Crossref
|
|
|
|
|
Bednekoff PA and Ritter R (1994). Vigilance in the Nxai Pan Springbok. Antidorcas marsupialis. Behaviour 129: 1-11.
Crossref
|
|
|
|
|
Befekadu R (2005). Population status of Swayne's Hartebeest in Ethiopia. Fifth Annual Sahleo-Saharan interest group Meeting report, Souss, Tunisia, Pp.10-15.
|
|
|
|
|
Befekadu R, Afework B (2006). Population status and structure of Swayne's Hartebeest (Alcelaphus buselaphus swaynei) in Maze National Park, Ethiopia. International Journal of Ecology and Environmental Science 32:259-264.
|
|
|
|
|
Berger LR, Hilton-Barber B (2004). Field Guide to the Cradle of Human kind Sterkfontein, Swartkrans, Kromdraai and Environs World Heritage Site (2nd eds.). Struik Publishers, South Africa, Cape Town, Pp.163.
|
|
|
|
|
Berhanu G, Yirga S (2004). Seasonal Home Range of Swayne's Hartebeest (Alcelaphus buselaphus Swaynei) in Senkele Swayne's Hartebeest Sanctuary. SINET: Ethiopian Journal of Science 27(2):121-126.
Crossref
|
|
|
|
|
Burger J, Gochfield M (1994). Vigilance in African mammals: differences among mothers, other females and males. Behaviour 131:153-169.
Crossref
|
|
|
|
|
Cain JW Iii Krausman PR, Rosenstock SS, Turne, JC (2006). Mechanisms of thermoregulation and water balance in desert ungulates. Wildlife Society Bulletin 34:570-581.
Crossref
|
|
|
|
|
Chu HJ, Jiang ZG, Ge Y, Jiang F, Tao YS, Wang C (2009). Population densities and number of khulan & goitred gazelle in Mt. Kalamaili Ungulate Nature Reserve. Biodiversity Science 17:414-422.
Crossref
|
|
|
|
|
Du Toit JT, Yetman AC (2005). Effects of body size on the diurnal activity budgets of African browsing ruminants. Oecologia 143:317-325.
Crossref
|
|
|
|
|
East R (1999). African Antelope Database 1998. IUCN/SSC Antelope specialist Group. IUCN, Gland, Switzerland, P.434.
|
|
|
|
|
EWCA (2012). Maze National Park (MzNP).
|
|
|
|
|
IUCN (2013). International Union for Conservation of Nature and Natural resources; IUCN SSC Antelope Specialist Group Alcelaphus buselaphus swaynei. IUCN Red List of Threatened Species.
View. Accessed on November, 2018.
|
|
|
|
|
Jarman PJ (1974). The social organisation of antelope in relation to their ecology. Behaviour 48:215-267.
Crossref
|
|
|
|
|
Kingdon J (2015). The Kingdon Field Guide to African Mammals. London: Academic Press.
|
|
|
|
|
Kingdon J (1997). The Kingdon Field Guide to African Mammals. London: Academic Press, pp. 1-464.
|
|
|
|
|
Kurauwone MV, Justice M, Beven U, Olga K, Simon C, Tawanda T (2013). Activity Budgets of Impala (Aepyceros melampus) in closed environments: the Mukuvisi Woodland experience, Zimbabwe. International Journal of Biodiversity, pp. 1-8.
Crossref
|
|
|
|
|
Lewis J, Wilson R (1979). The ecology of Swayne's hartebeest. Biological Conservation 15(1):1-12.
Crossref
|
|
|
|
|
Lin J, XU, WX, Yang WK, Xia CJ, Liu W (2012). Habitat suitability assessment of Equus hemionus hemionus in Kalamaili Mountain Nature Reserve. Biodiversity Science 20:411-419.
Crossref
|
|
|
|
|
Liu W, Yang WK, Xu WX (2008). Food habits of the Khulan (Equus hemionus) in autumn. Acta theriologica Sinica 28:33-36.
|
|
|
|
|
Maher CR (1991). Activity budgets and mating system of male pronghorn antelope at Sheldon National Wildlife Refuge, Nevada. Journal of Mammalogy 72:739-744.
Crossref
|
|
|
|
|
Moncorps S, Bousses P, Reales D, Chapuis J (1997). Diurnal time budget of the moulfon (Ovis musimon) on the Kerguelen archipelago: influence of food resources, age, and sex. Canadian Journal of Zoology 75:1828-1834.
Crossref
|
|
|
|
|
Neuhaus P, Ruckstuhl KE (2004). Can the activity budget hypothesis explain sexual segregation in desert bighorn sheep? Behavior 141:513-520.
Crossref
|
|
|
|
|
Norris D, Michalski F, Peres CA (2010). Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. Journal of Mammalogy 91(3):551-560.
Crossref
|
|
|
|
|
Ruckstuhi KE, Neuhaus P (2009). Activity budgets and sociality in a monomorphic ungulate: the African Oryx (Oryx gazelle). Canadian Journal of Zoology 87:165-174.
Crossref
|
|
|
|
|
Schaller GB (1998). Wildlife of the Tibetan Steppe. University of Chicago Press, Chicago.
|
|
|
|
|
Shibru S, Vancampenhout K, Deckers J, Leirs H (2020). Human Pressure Threaten Swayne's Hartebeest to Point of Local Extinction from the Savannah Plains of Nech Sar National Park, South Rift Valley, Ethiopia. Journal of Biodiversity and Endangered Species 8(1):8.
|
|
|
|
|
Shi JB, Dunber RIM, Buckland D, Miller D (2003). Daytime activity budgets of feral goats (Capra hircus) on the Isle of Rum: Influence of season, age, and sex. Canadian Journal of Zoology 81:803-815.
Crossref
|
|
|
|
|
Simon S (2016). Responses of Vegetation, Small mammals and Large herbivores to human induced pressures in the Savannah Plains of Nech Sar National Park, South Ethiopia Rift Valley. PhD Dissertation, University of Antwerp, Belgium.
|
|
|
|
|
Siraj M, Zhang K, Sebsebe D, Zerihun W (2016). Floristic composition and plant community types in Maze National Park, Southwest Ethiopia. Applied Ecology and Environmental Research 15(1):245-262.
Crossref
|
|
|
|
|
Sonja MS, James WC (2009). Foraging efï¬ciency and vigilance behaviour of impala: the influence of herd size and neighbor density. African Journal of Ecology 47(1):109-118.
Crossref
|
|
|
|
|
Vasey N (2005). Activity budgets and activity rhythms in red ruffed lemurs (Vareci arubra) on the Masoala Peninsula, Madagascar: seasonality and reproductive energetic. American Journal of Primatology 66:23-44.
Crossref
|
|
|
|
|
Vymyslicka P, Hejcmanova P, Antoninova M, Stejskalova M, Svitalek, J (2010). Daily activity pattern of the endangered Swayne's Hartebeest (Alcelaphus buselaphus swaynei Sclater, 1892) in the Nechisar National Park, Ethiopia. African Journal of Ecology 49:246-249.
Crossref
|
|
|
|
|
Wondimagegnehu T, Afework B (2011). Current Population Status of the Endangered Endemic Subspecies of Swayne's Hartebeest (Alcelaphus buselaphus swaynei) in Maze National Park, Ethiopia. SINET: Ethiopian Journal of Science 34(1):39-48.
|
|
|
|
|
Wondimagegnehu T, Afework B (2015). Diurnal activity pattern of Oribi (Ourebia ourebi) in Maze National Park, Ethiopia. International Journal of Ecology and Eco solution 2(3):31-35.
|
|
|
|
|
Wondimagegnehu T, Afework B (2013). Population status of Oribi (Ourebia Ourebi Zimmermann, 1783) in Maze National Park, Southern Ethiopia. Bangladesh. Journal of Zoology 41(2):145-151.
Crossref
|
|
|
|
|
Xia CJ, Yang WK, Blank D, Qiao JF, Liu W (2011). Diurnal time budget of goitred gazelles (Gazella subgutturosa) in Xinjiang, China. Acta theirologica Sinica 30:144 -150.
Crossref
|
|
|
|
|
Yerushalmi S, Green RM (2009). Evidence for the adaptive significance of circadian rhythms. Ecological Letter 12:970-981.
Crossref
|
|
|
|
|
Yosef M, Addisu A, Girma M (2015). Social organization in the mountain nyala (Tragelaphus buxtoni) population in the Bale Mountains National Park, Ethiopia. International Journal of Biodiversity and Conservation 7(2):103-111.
Crossref
|
|
|
|
|
Yosef M, Girma M, Aramede F, Kefyalew S, Mezemir G (2012). Status of the Swayne's Hartebeest (Alcelaphus buselaphus swaynei) meta-population under land cover changes in Ethiopian Protected Areas. International Journal of Biodiversity and Conservation 4(12):416-426.
|
|