ABSTRACT
The study was carried out in six Kenyan rift valley lakes, Nakuru, Magadi, Oloiden, Crater (Sonachi), Bogoria and Elementaita with the aim to determine the levels of heavy metals and other metal elements (Co, Mn, Zn, Cu, Cr, Cd, Pb, Ni, Hg and As) in water and sediment samples as well as assess its association with water bird distribution. High levels of Pb (42 ppm) above the Pb benchmark levels (36 ppm) as per EPA (2007) benchmarks were detected in Lake Oloiden sediments. Lakes Bogoria and Elementaita had high levels of Mn (3676.7 ± 6652.3 and 747.55 ± 510.95, respectively), also above the Mn benchmark levels (631 ppm), according to EPA (2007). The mean sediment concentrations for Zn, Pb, Ni, As and Hg varied significantly (P<0.05) among the six lakes. Apart from Zn, all other metals (Pb, Co, Mn, Cr, Cd, Fe and Cu) varied significantly in all water samples from the six selected lakes (P<0.05). A total of 15 water bird families were identified across the six lakes. The distribution of the families for lakes Nakuru, Magadi, Elementaita, Oloiden, Bogoria and Crater were 11, 9, 9, 7, 6 and 4, respectively. There was no association between metal elements concentration and water bird distribution in all the selected six lakes (P>0.05). It was concluded that metals concentration in Kenyan Rift Valley lakes has no significant influence on the distribution of water birds. High Mn levels in lakes Bogoria and Elementaita, and Pb in Oloiden may cause toxic effects to the aquatic life and humans as a result of bioaccumulation.
Key words: Ecotoxicology, heavy metals, water birds.
Ecology is the scientific study of abundance, distributions and relations of organisms and their interaction with the environment (Begon et al., 2006). Heavy metals such as common toxic chemicals in the environment since they are naturally occurring and they are resistant to biodegradation (Reena et al., 2011). Trace metals like lead, mercury, chromium and cadmium are among the copper, zinc and iron play a biochemical role in the life processes of all aquatic plants and animals. They are therefore essential in trace amounts (Jakimska et al. 2011). However, high concentrations of these essential elements are toxic to aquatic life (Jakimska et al., 2011). The sources of metal elements in water bodies include, natural weathering, industrial wastes, sewage, surface run-off and agricultural effluents (Paul et al., 2012). Lake sediments form the final pathway of both anthropogenic and natural contaminants in the environment. Sediment quality is therefore a good indicator of pollution in the environment (Gavin and Marco, 2008; Samir and Ibrahim, 2008; Hahladakis et al., 2013).
Water birds that rely on wetlands for food, nesting and breeding can be used as environmental indicators (Ogden et al., 2014) because they are high in trophic levels, able to move in response to both opportunity and adversity, and are easy to notice and quantify in both space and time (O’Doherty and Caro, 1999).
In recent years, there has been dynamic changes in water bird number in Kenyan Rift valley lakes and pollution levels due to heavy metals is among the incriminated factors suggested to be causing the change in water bird distribution (Motelin et al., 1995; Nelson et al., 1998; Motelin et al., 2000; Guynub, 2002; Ouko et al., 2016). A study on the association between water bird distribution and heavy metal concentration in the environment is needed. In this paper, baseline data on concentrations of Mn, Zn, Cu, Cd, Cr, Co, Ni, As, Pb and Hg in surface sediments and water samples from six Kenyan Rift Valley lakes (Nakuru, Magadi, Oloiden, Crater, Bogoria and Elementaita) is reported. Data on water bird families distribution across the six lakes and their association with heavy metal concentration is also given.
Study area and sampling
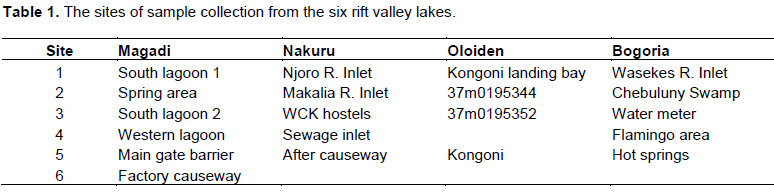
The study was carried out in six alkaline lakes in Kenya (Rift Valley): Lakes Nakuru, Magadi, Oloiden, Crater, Bogoria and Elementaita (Figure 1). The study sites were chosen based on convenience and sample size purposively determined depending on site characteristics. Water and surface sediment samples were taken from five sites (SS) in Lake Nakuru, five SS in Lake Bogoria, five SS in Lake Oloiden and six SS in Lake Magadi (Table 1). In Lakes Elementaita and Crater, five replicate subsamples were taken from one site. Surface sediment (0-2 cm layer) samples were collected in zip lock plastic bags, properly labelled and transported at 4°C in cool boxes to the laboratory awaiting analysis. Water samples were taken in plastic capped containers and transported in cool boxes at 4°C to the laboratory.
Sample preparation
Water samples were prepared in duplicates by measuring 50 ml of water into a beaker, adding 2 ml of concentrated nitric acid and heating the contents on a hot plate to reduce the volume to 10 ml. After that, the mixture was filtered into a 50 ml volumetric flask and topped to the mark with demineralized water ready for analysis. Each sediment sample was well mixed and 20 g were weighed in aluminum foil papers and dried in an oven at 60°C overnight. The sample was then ground in a pulverizer and 2.5 g weighed into a 250 ml beaker before adding 20 ml of water to make sludge. Concentrated nitric acid (20 ml) was then added to the sludge and the contents heated on a hot plate at 130°C for 1 hour without spurting to reduce the volume to 10 ml. This was then cooled and filtered into 50 ml volumetric flask, washed carefully with hot water and then topped to the mark ready for analysis. Known standards for each metal element were prepared from respective certified analytical standards at concentrations of 0.05, 0.1, 10 and 100 ppm. These known standards were read to generate calibration curves within the spectrophotometer before reading the samples. Standards were read after every reading of 5 samples to check the accuracy and precision of the machine and analytical process.
Instrumentation
Atomic absorption spectrophotometer (AAS) with an air/acetylene flame (Model SpectrAA-10) was used for analysis of Cd, Cr, Mn, Co, Zn and Cu in both water and sediment samples after preparation of appropriate calibration standards. Hg, Ni and As in sediments were analyzed using a X-ray Fluorescence (XRF) analyzer (s1 TITAN RS 200). Pb in water samples was analyzed by AAS and Pb in sediment samples analyzed by XRF after the AAS Pb lamp becoming faulty.
Reagents
Heavy metal analytical standards were of analytical grade from Sigma - Aldrich Limited, TraceCERT® products. Water used was deionised water from ReAgent chemical suppliers, Cheshire, England, UK.
Glassware cleaning
Glass was cleaned with tap water, soap and brush, rinsed three times with 0.5 M perchloric acid before rinsing with distilled water three times. They were then dried in the oven at 60°C.
Water bird identification
Water birds along drive ways, were observed in each SS using binoculars and identified using a bird guide book (Zimmerman et al., 1999) and identified with help of an expert ornithologist from the National Museums of Kenya (NMK).
Data obtained was entered into Microsoft Excel® spreadsheet, cleaned and then exported to Stata® for analysis. The means, standard deviations, maximum and minimum levels to determine toxicant levels within the six lakes were obtained.One way Anova test was used to analyze the variation between and within the lakes
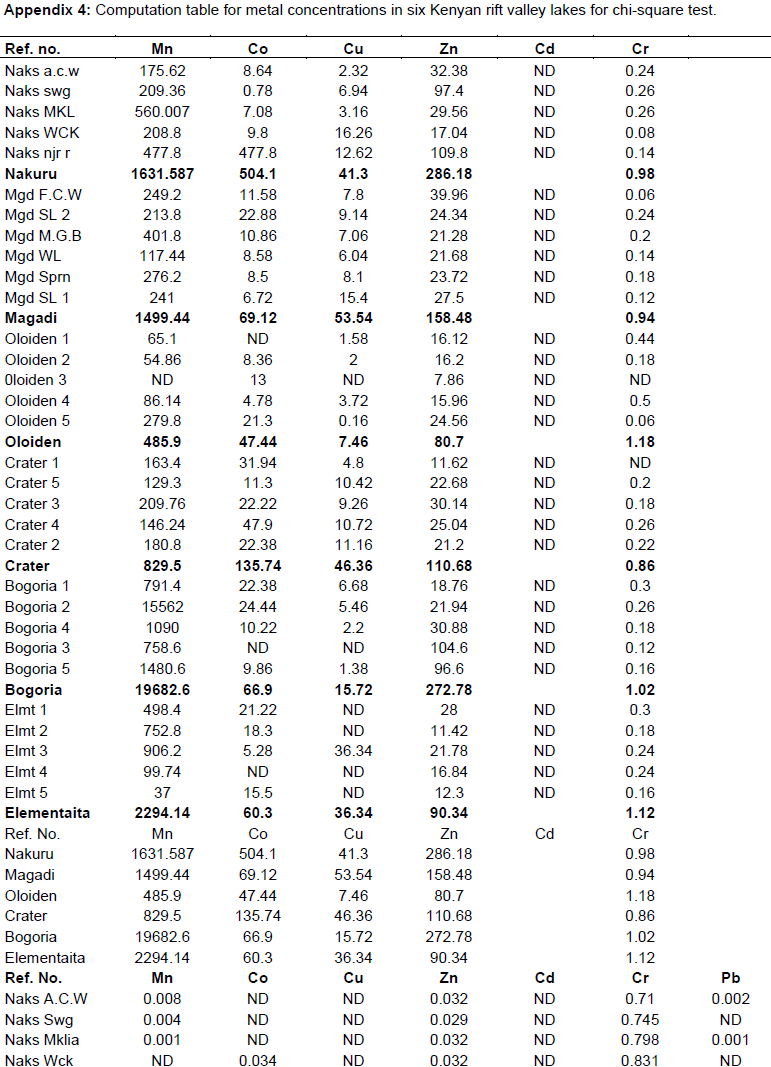
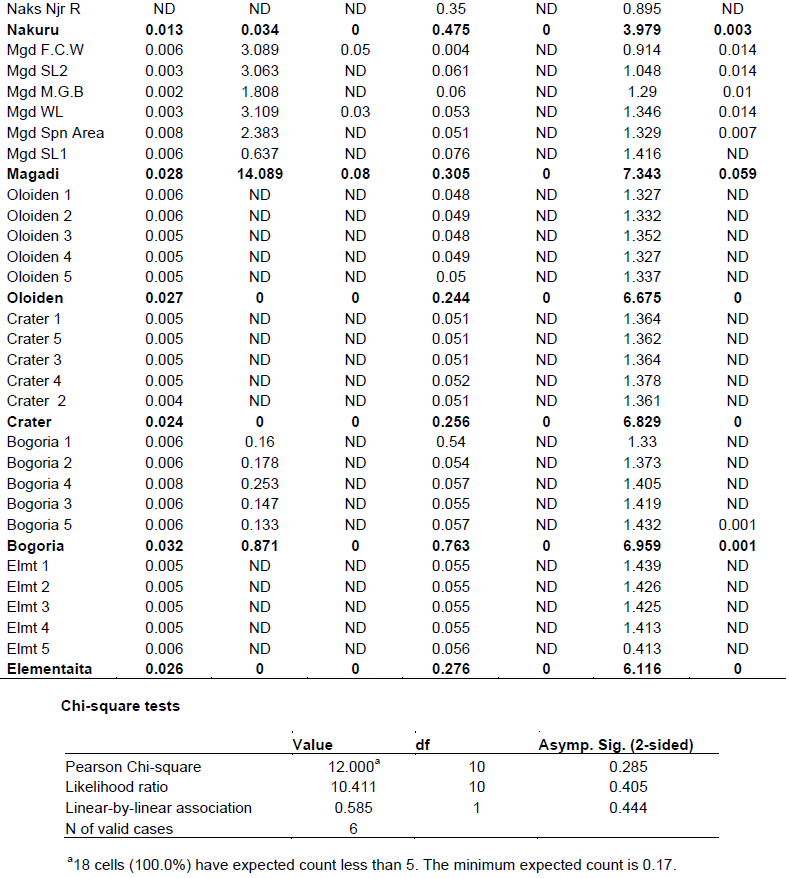
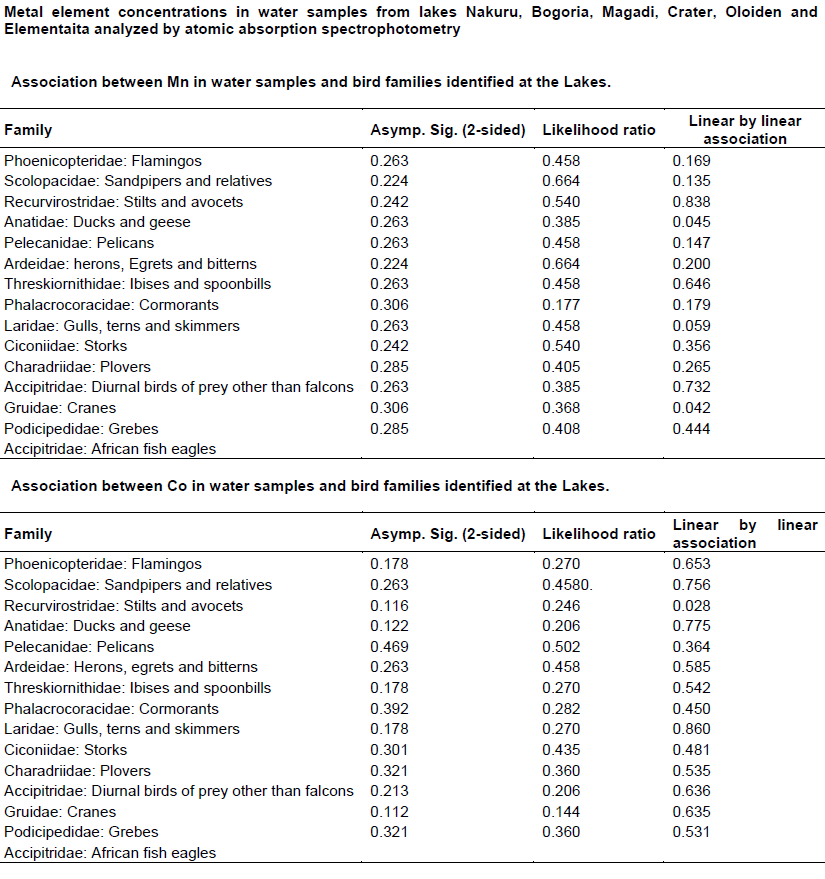
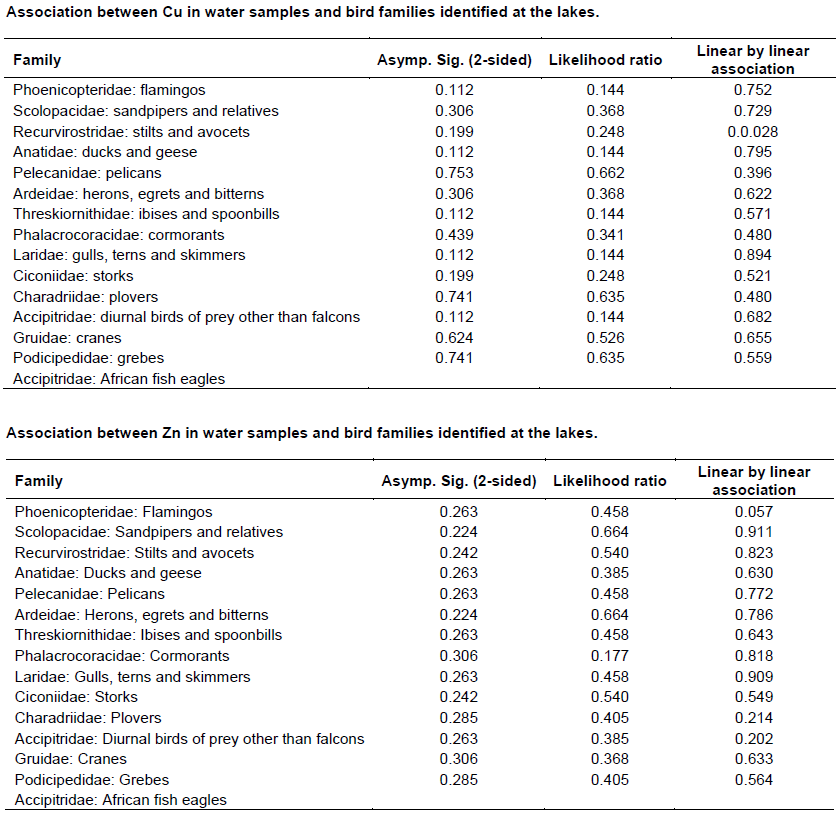
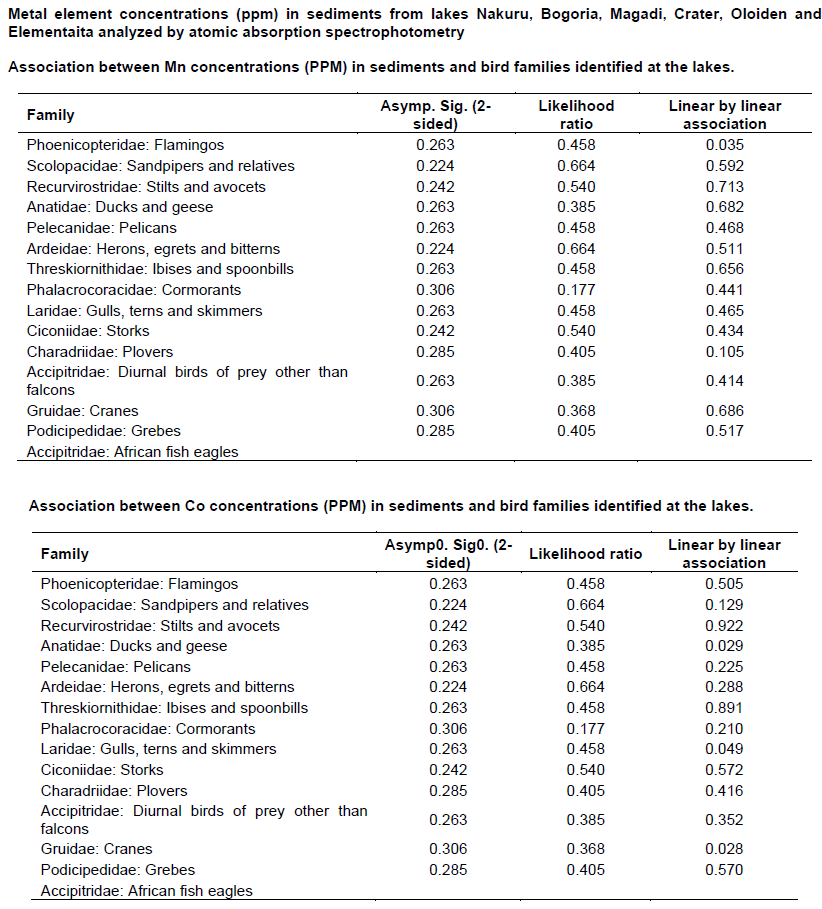
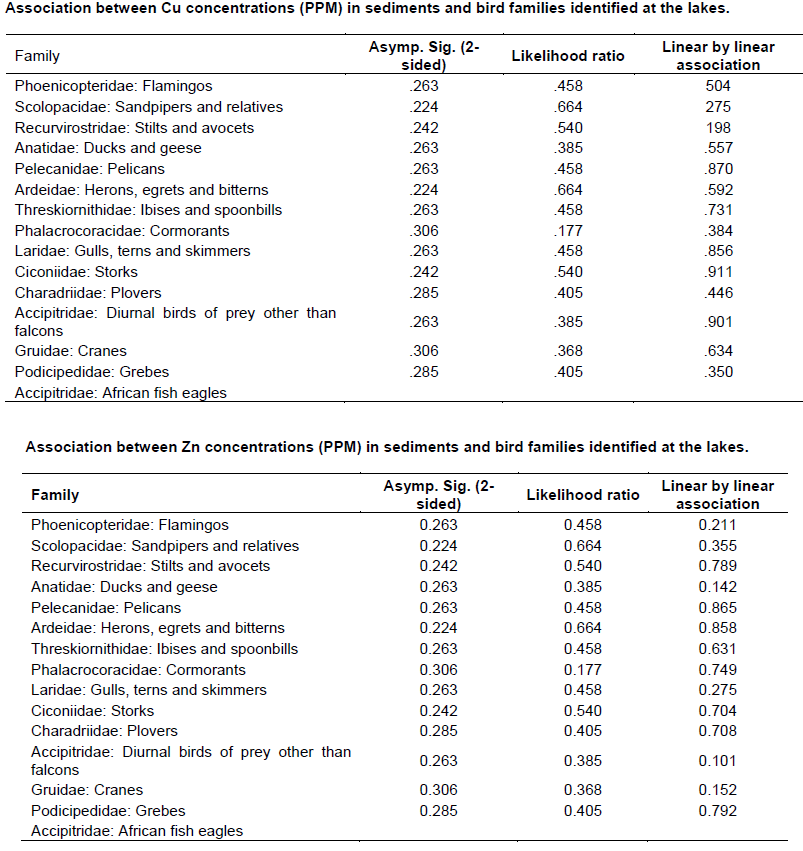
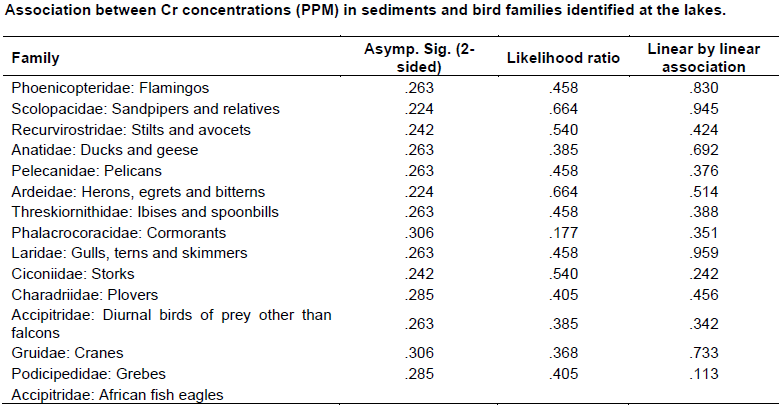
Chi - square test (Appendix 4) was used to test the association between metals concentration and water bird distribution among the lakes.
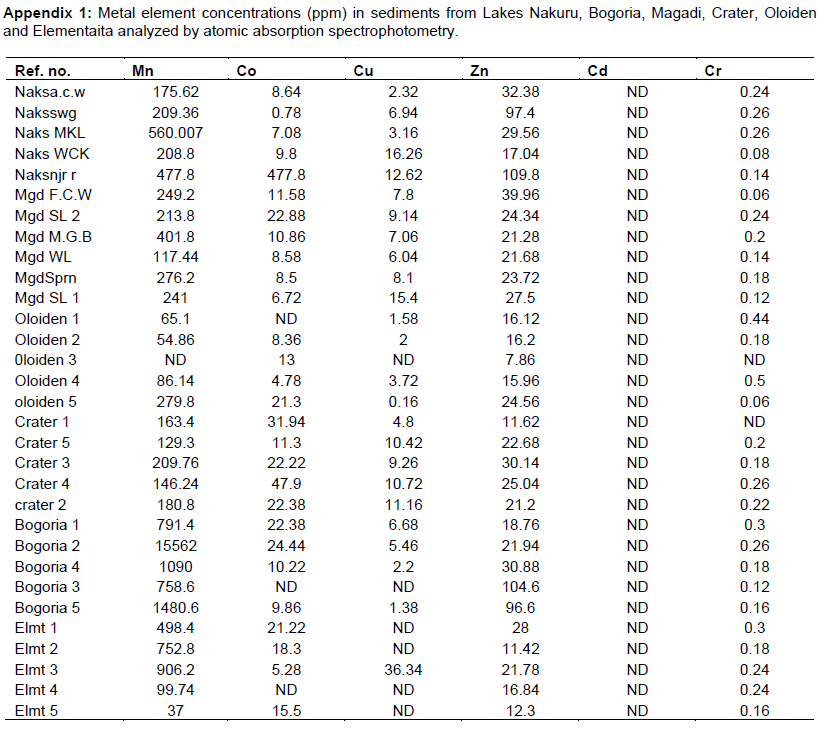
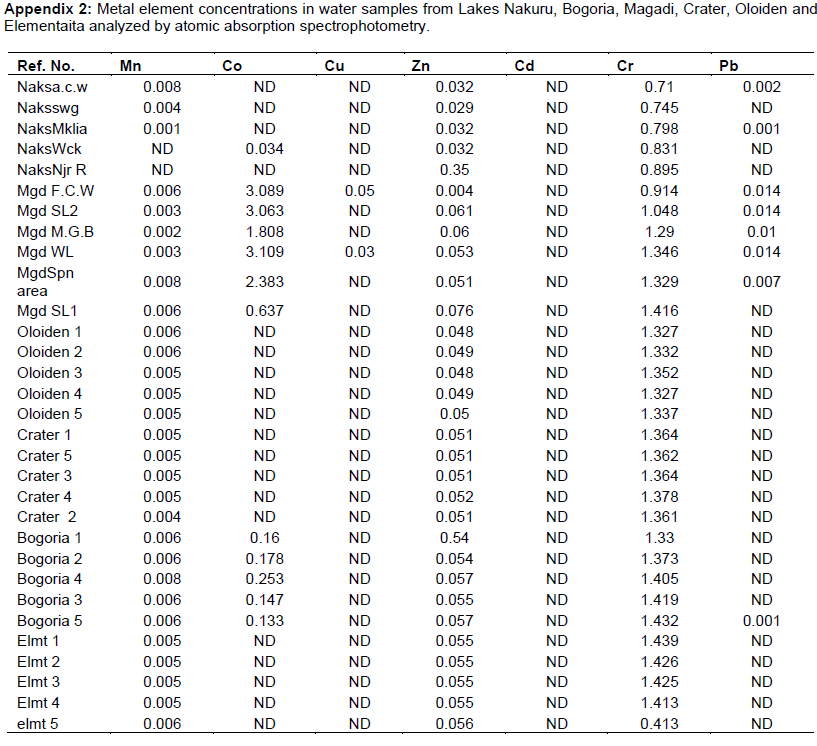
The mean concentrations of heavy metals Mn, Co, Cu, Zn, Cd, Cr, Pb, Hg, Ni and As analyzed in sediments and water samples from the six Rift valley lakes are showed in Tables 2, 3, 4, Appendix 1 and Appendix 2. Lake Bogoria recorded the highest Mn mean sediment concentrations of 3676 ppm; Lake Nakuru gave highest mean sediment concentrations of Co (100 ppm) and Lake Oloiden recorded highest mean concentrations of Pb (42 ppm) in sediments. The mean sediment levels of Mn in Lake Bogoria are within the ranges obtained by Ochieng et al. (2007) who obtained Mn mean sediment concentration of 3947 ± 121 ppm. The mean sediment concentrations for Cu, Zn, As and Cr were very low in all the six lakes (Table 2 to 4). Cd and Hg were not detectable in sediment samples from all the six lakes. The results on Cd concentrations in sediments differ from those obtained by Tenai (2015) who found traces of Cd (0.0004 to 0.076 ppm) in Lakes Crater, Elementaita, Nakuru and Oloiden. Ochieng et al. (2007) also obtained high levels of Cd (1.18 ppm) in sediment from Lake Elementaita. Cd was also not detectable in water samples from all the six lakes. This was in agreement with the results obtained by Tenai (2015) but contrary to those obtained by Ochieng et al. (2007) who recorded traces of Cd levels in water samples (2.0to5.0 Benchmark levels for sediment concentrations (EPA, 2007) bulk sediment toxicity benchmarks for benthic macroinvertebrates; ND, not detected; n, Number of samples. µg/L) in Lake Baringo, (5.0 to 41.0 µg/L) in Lake Bogoria Bogoria (1.3918 ppm), Crater (1.3658 ppm) and Oloiden (3.0 to 43.0 µg/L) in Lake Nakuru and (3.0 to 25.1 µg/L) in Lake Elementaita. Dilution as a result of rising water levels in Kenyan Rift Valley Lakes may be responsible for undetected Cd. The mean sediment concentrations for Zn, Pb, Ni, As and Hg varied significantly (P<0.05) among the six lakes. Concentrations of Mn in Lake Bogoria may be attributed to natural leaching and erosion from rocks, volcanic activity and soils (Stokes et al., 1988) since the Lake is remote from anthropogenic activities. These high levels is a threat to biological lives that depend on the lake especially water birds. Elevated levels of Mn affect fetal development, causes DNA damage and chromosomal aberrations thus toxic to embryo (ATSDR, 2000). The levels of Co in Lake Nakuru may be attributed to the increased industrial activities around the lake. Spillway from fresh water Lake Naivasha is the likely source of Pb levels in Lake Oloiden since a lot of anthropogenic activities especially flower farming occur around Lake Naivasha. Surface run-offs from these farms are a good source of Pb in the lake. Birds depending on the lake are also at risk of Pb toxicity.
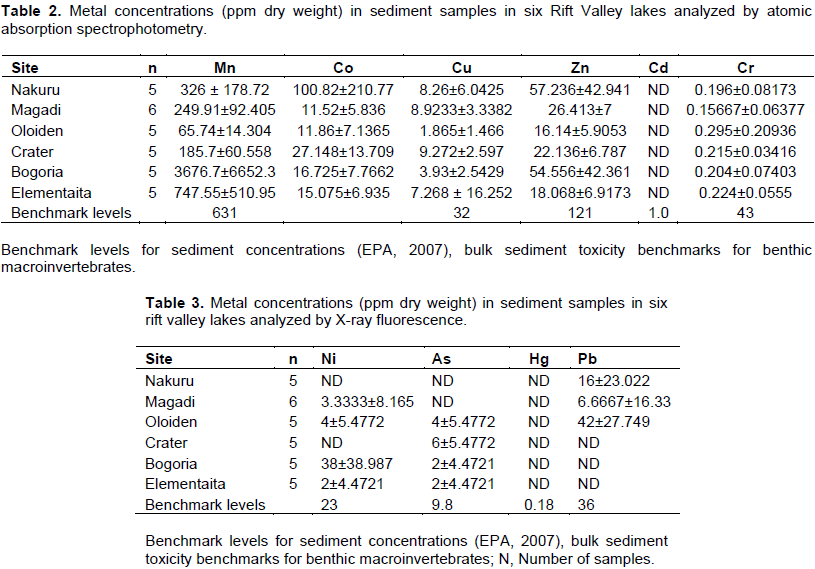
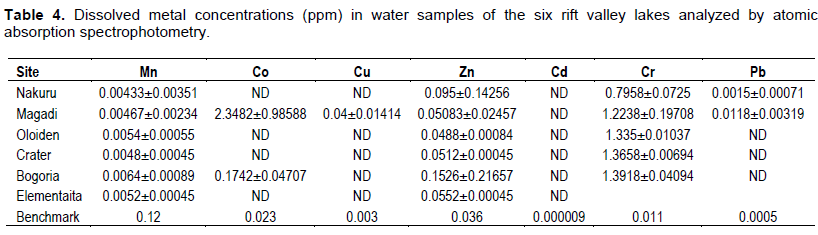
In water samples, high mean concentrations of Cr and Zn is above the threshold limits (0.011 and 0.036 ppm), respectively, according to EPA (2007) limit, were recorded in all the six lakes with highest Cr levels in (1.335 ppm) Table 3. These levels are much higher compared to those obtained by Ochieng et al. (2007) who obtained a maximum of 0.188 ppm in Lake Nakuru but lower than ranges (10 to 280 ppm) recorded by Nelson et al. (1998) in Lake Nakuru sediments. Highest Zn levels were recorded in Lake Bogoria (0.1526 ppm) (Table 3). Lake Magadi water samples had high levels of both Co (2.3482 ppm) and Cu (0.04 ppm) which were above the benchmark levels given by EPA (2007) limits (Table 3). Lake Bogoria also had high Co levels in water samples (0.1742 ppm). Mn levels were low in water samples from all the six lakes. Apart from Zn, all other metal elements (Pb, Co, Mn, Cr, Cd, Fe and Cu) varied significantly (P<0.05) in water samples among the six lakes. The noted high levels of metal elements in water columns from the lakes is a major concern since these lakes support many water birds, wild animals, fish among other biological organisms.
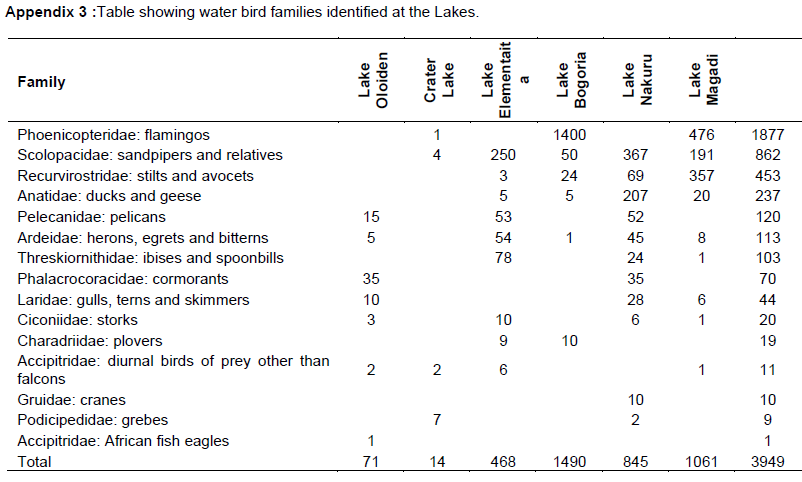
A total of 15 water bird families were identified across the six lakes (Table 5, Appendix 3). Phoenicopteridae family, which comprises flamingos, was the most abundant with an estimate of 1877 lesser flamingos (Phoeniconias minor), followed by Scolopacidae (862) and Recurvirstridae (453) families. The distribution of water bird families for lakes Nakuru, Magadi, Elementaita, Oloiden, Bogoria and Crater were11, 9, 9, 7, 6 and 4 respectively. African fish eagle of the family Accipitridae was sighted at Lake Oloiden.
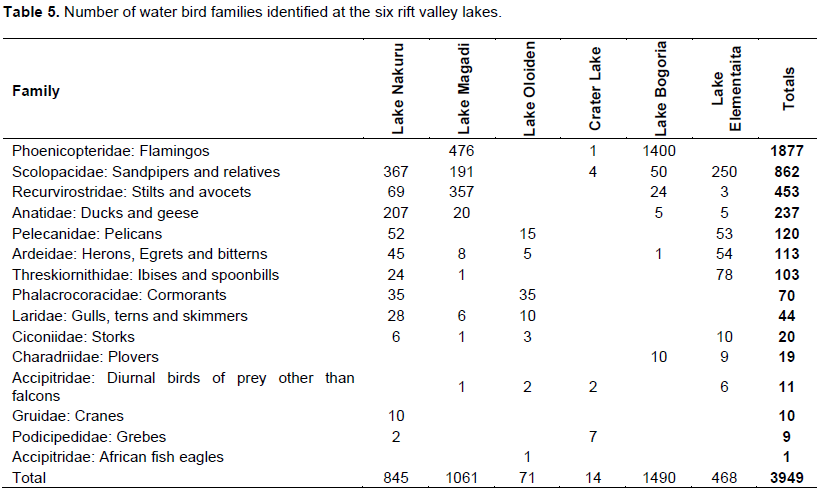
The test of association between mean heavy metal concentrations and water bird families distribution in the six lakes was performed using Chi-square test in Statistical Package for the Social Sciences (SPSS) version 24. There was no significant influence (P>0.05) (Appendix 4) of heavy metals on water bird distribution in all the lakes. This implies that other factors different from heavy metal concentrations affect the ever changing distribution of water birds in Kenya Rift Valley lakes.
The authors have not declared any conflict of interests.
REFERENCES
|
ATSDR-Agency for Toxic Substances and Disease Registry (2000). Toxicological profile for Manganese. Atlanta GA: Agency for Toxic Substances and Disease Registry: Profile updates.
|
|
|
|
Begon M, Townsend CR, Harper JL, (2006). Ecology: From individuals to ecosystems. (4th ed) Blackwell. ISBN 1405111178
|
|
|
|
EPA-Environmental Protection Agency (2007). Bulk sediment toxicity benchmarks for benthic macroinvertebrates.
|
|
|
|
Gavin FB, Marco AO (2008). Sediment – bound heavy metals as indicators of human influence and biological risk in coastal water bodies. ICES J. Mar. Sci. 68:1407-1413.
|
|
|
|
Guynup S (2002). Mysterious Kenya Flamingo Die-Offs tied to Toxins, Stud. National Geographic News on October, 28, 2010.
|
|
|
|
Hahladakis J, Smaragdaki E, Vasilak G, Gidarakos E (2013). Use of Sediment Quality Guidelines and pollution indicators for the assessment of heavy metals and PH contamination in Greek surficial sea and lake sediments. Environ. Monit. Assess. 185:2843-2853.
Crossref
|
|
|
|
Jakimska A, Konieczka P, Krzysztof S, Namiesnik J, (2011). Bioaccumulation of Metals in Tissues of Marine Animals, Part 1: the Role and Impact of Heavy Metals on Organisms. Pol. J. Environ. Stud. 20:1117-1125
|
|
|
|
Motelin GK (2000). An ecotoxicological study of the potential roles of metals, pesticides and algal toxins on the 1993/5 Lesser Flamingo mass die-offs in Lake Bogoria and Nakuru, Kenya. - East African Environmental Forum. Nairobi: East African Environmental Network. pp. 11-12.
|
|
|
|
Motelin GK (1995).The mysterious Lesser Flamingo deaths in Lake Nakuru: A cross-sectional ecotoxicological study of the potential roles of algal toxins, heavy metals and pesticides. Nakuru: World Wildlife Fund.
|
|
|
|
Nelson YM, Thampy RJ, Motellin GK, Raini JA, Disante CJ, Lion LW (1998). Model for Trace metal exposure in filter-feeding flamingos at an alkaline Rift Valley lake in Kenya. Environ. Toxicol. Chem. 17:2302-2309.
Crossref
|
|
|
|
Ochieng EZ, Lalah JO, Wandiga SO (2007). Analysis of Heavy Metals in Water and Surface Sediments in Five Rift Valley Lakes in Kenya for Assessment of Recent Increase in Anthropogenic Activities. Bull. Environ. Contam. Toxicol. 79:570-576.
Crossref
|
|
|
|
O'Doherty G, Caro TM (1999). On the use of surrogate species in conservation biology. Conserv. Biol. 13:805-881.
Crossref
|
|
|
|
Ogden JC, Baldwin JD, Bass OL, Browder JA, Cook MI, Frederick PC, Frezza PE, Galvez RA, Hodgson AB, Meyer KD, Oberhofer LD (2014). Waterbirds as indicators of ecosystem health in the coastal marine habitats of Southern Florida: 2. Conceptual ecological models. Ecol. Indic. 44:128-147.
Crossref
|
|
|
|
Ouko MC, Odhiambo AM, Mark B (2016). Assessment of Hydrological Impacts of Mau Forest, Kenya. Hydrol. Curr. Res. 7:223
|
|
|
|
Paul BT, Clement GY, Anita KP, Dwayne JS (2012). Heavy metal toxicity and the environment. In. Molecular, clinical and environmental toxicology. Springer Basel. 101:133-164
|
|
|
|
Reena S, Neetu G, Anurag M, Rajiv G (2011). Heavy metals and living systems: An Overview. Indian J. Pharmacol. 43:246-253
Crossref
|
|
|
|
Samir MS, Ibrahim MS (2008). Assessment of Heavy Metals Pollution in Water and Seiments and Their effect on Oreochromis niloticus in the Northern delta lakes, Egypt. 8th International Symposium on Tilapia in Aquaculture 2008, Central lab. For aquaculture research, Agricultural research Center. Limnology Dept.
|
|
|
|
Stokes PM, Campbell PGC, Schroeder WH, Trick C, France RL, Puckett KJ, LaZerte B, Speyer M, Hanna JE, Donaldson J (1988).
|
|
|
|
Tenai BC (2015). Ecotoxicological Assessment of Rift Valley lakes in Kenya and The Potential Health Impact on the Lesser Flamingo Population. Nairobi. University of Nairobi Repository.
|
|
|
|
Zimmerman DA, Donald AT, David JP (1999). Birds of Kenya and Northern Tanzania: Field Guide Edition.
|
APPENDIX