ABSTRACT
Natural forest regeneration is the renewal of a forest crop by self-sown seed or by sprouting of stumps. However, there have been few studies on sprouting in the Cameroon tropical rainforest. The main objectives of this study were to examine the influence of stump sprouting of timber species in natural forest regeneration in the Akak forest area in Cameroon. In this study, stumps were located in forest compartments that had been selectively logged in 2013, 2015 and 2017. Stumps attributes: (1) species, (2) the diameter at the top of the mother stump, (3) the height of the mother stump, (4) the number of sprouts, both living and dead, (5) the height of the tallest sprout, (6) the basal diameter of the tallest sprout, and (7) the extent of the decay of the stump were recorded at every sprouted stump using a Global Positioning System (GPS) device. Thirteen of the 20 tree species had some stumps which had sprouted. Stumps of Nauclea diderrichii, Pterocarpus soyauxii species, Terminalia ivorensis, and Piptadeniastrum africanum sprouted most frequently, with N. diderrichii having the greatest number of stumps with sprouts. Principal component factor analysis for all the species together showed that the first factor contributed 34.1% and the second factor contributed 19.1% of the observed variation, with a communality of 66.6% while for N. diderrichii alone showed that the first two axes of the PCA explained 62.8% of the variance suggesting that sprouting dynamics could only be partially explained by the attributes that were recorded. Multiple linear regression shows that the diameter of the tallest sprout can be used to predict the height of the tallest sprout for all the species combined (p=0.000). These predicting models could help in predicting the future growth rate and stand of a tropical rainforest.
Key words: Natural forest recovery, timber species, tropical rainforest.
The importance of tropical rainforest ecosystems in terms of global biodiversity, environmental protection, human welfare, and more recently for carbon sequestration and climate stability, cannot be overestimated (Lamprecht, 1989; FAO, 2003). Tropical forests have shrunk in area by 35 to 50% since pre-industrial times (Wright and Muller-Landau, 2006). If losses continue at current rates, the last remnants of primary tropical forest will probably disappear sometime between 2100 and 2150, although global climate change (if unchecked) will undoubtedly accelerate the process (Wright and Muller-Landau, 2006). Despite greater general awareness and efforts made towards the sustainable management of these valuable natural resources, global tropical forest degradation persists (FAO, 2007).
After the first timber harvest in tropical rainforests, there is generally a qualitative and/or quantitative decline in subsequent harvests, and inadequate natural regeneration of some commercially valuable timber species in the residual stand. Securing sufficient natural regeneration of commercial tree species after logging is critical for sustainable forest management (Nzogang, 2009).
Most studies of tropical forest regeneration focus on tree recruitment from seeds. Consequently, regeneration is often viewed as depending on seed production, seed dispersal, seed viability, and the environmental requirements for seed germination and seedling establishment (Holl, 1999; Dalling and Hubbell, 2002; De Steven and Wright, 2002). However, sprouts from cut stumps (coppicing) may also contribute to the regeneration of tropical forests. Sprouting is a common response to tissue damage by woody plants and is a source of regeneration that contributes to the composition and development of many forest ecosystems (Bond and Midgley, 2001; Del Tredici, 2001). Sprouts can have a competitive advantage over other sources of forest regeneration (White, 1991; Dietze and Clark, 2008; Vickers et al., 2011) because rapid early growth of sprouts is supported by an established root system with stored carbohydrates (Del Tredici, 2001). Additionally, regeneration from sprouts can contribute to sustaining pre-disturbance species composition (Dietze and Clark, 2008). Several studies have shown that sprouts can constitute the majority of the dominant trees within a regenerating cohort after harvesting (Boring et al., 1981; Beck and Hooper, 1986; Arthur et al., 1997), although the capacity to sprout varies by species and site conditions. Furthermore, in forests in general, and in dry forests in particular, tree seedlings that do become established often grow more slowly than sprouts (Miller and Kauffman, 1998; Khurana and Singh, 2001).
Cameroon is well-endowed with tropical forest areas estimated at 21.5 million ha (Nasi et al., 2006; FAO, 2007). Efforts are underway to better understand forest regeneration after harvesting. However, there have been relatively few studies on stump sprouting in Cameroon’s tropical rainforest. This study is intended to help fill that gap.
The objectives of this study were to examine the role of stump sprouting of timber species in natural forest regeneration in the Akak forest area in Cameroon, specifically: (i) to identify tree species that sprout after logging and mapped the distribution of sprouted stumps; and (ii) determined relationships between sprouting characteristics (number of sprouts per stump, height of the dominant sprout, diameter of the dominant sprout, height, and diameter of mother stump) for all the species and the species with the highest number of stump sprouts (Nauclea diderrichii). This study was to provide
baseline information on the sprouting ability of harvested tree species in the Akak forest area of Cameroon, as well as to provide recommendations for management in the Akak forest area to promote sprout development.
Location of the study area
The Akak forest area of Cameroon is located between 5°20′ - 5°25’ N latitude and 9° 12’ - 9°30’ E longitude (Figure 1). Akak is comprised a semi-deciduous lowland rainforest of the Guineo-Congolian type (Kenfack et al., 2006). Precipitation is unimodal, with an annual average of around 4100 mm (Nchanji and Plumptre, 2003), with a three-month dry season from December to February (Groenendijk, 2015). The topography is relatively flat. Human interventions, primarily establishing large plantations of cash crops (palm oil, coffee), as well as natural factors, such as elephant disturbance and windfalls, have created large gaps in these forests. Logged forest sites are located in the “heart” of the Mukete Plantations Limited (MPL) concession and the forest in this area has undergone logging, both formal and informal, from 1995 to the present.
Sample design
The three areas sampled were logged in 2013, 2015, and 2017, respectively. Opportunistic sampling technique was used to select the three areas, since we had pre-information from community people living in Akak village (Former-workers of the logging concession, hunters and Non Timber Forest Products collectors) that they have observed some logged stumps in these areas sprouting. The 2013 site was logged by Transformation Reef Cameroon and local chainsaw millers. The 2015 and 2017 sites were logged by SEFECAM and local chainsaw millers. Trees selected for harvesting were widely scattered (less than 1 ha-1 in most places) and it proved difficult to locate stumps using any formal sampling design. Consequently, stumps were located by active searching of the logged areas. All stumps located, whether they had sprouts or not, were recorded.
Both sprouted and non-sprouted stumps were observed, but only sprouted stumps were marked and measured. The diameter and basal diameter for both stumps and sprouts were measured using a diameter tape at 1.3 m breast height while a 30 m measuring tape was used to measure the height for both stumps and sprouts. Once a stump with sprouts was located, its location was recorded using a Global Positioning System (GPS) device so that these stumps could be relocated for future follow-up. The attributes recorded for each sprouted stump were: (1) species; (2) the diameter at the top of the mother stump; (3) the height of the mother stump; (4) the number of sprouts, both living and dead; (5) the height of the tallest sprout; (6) the basal diameter of the tallest sprout; and (7) the extent of the decay of the stump.
Data analysis
The field data were imported into QGIS 2.8 so that the coordinates of the sprouted stumps could be digitized. Baseline information from the Cameroon official forest data and the Cameroon atlas were collected before any data manipulation commenced. After all digitizing was completed, a quality check was performed to ensure that no points or lines were missing. To ensure all of the data would display correctly, all of the shapefiles were projected to UTM 84, zone 32 N.
Multivariate factor analysis without rotation was done to explore relationships among the variables. Two factors were extracted and their contribution to explaining the resulting data patterns was determined by the percent variation and communality. This was followed by applying the Spearman rank correlation.
Step-wise regression was used to examine the relationships between (1) the heights of the tallest sprouts and number of sprouts per stump and stump diameter; (2) the heights of the tallest sprouts and the largest basal sprout diameter and the number of sprouts per stump; and (3) the heights of the tallest sprouts and the number of sprouts per stump and stump height. The data (all variables) were log-transformed to achieve a normal distribution for the residuals. These analyses were done independently for data collected in 2013, 2015 and 2017, and then for the entire study to determine the overall effects of the stump characteristics on the sprout characteristics.
All statistical analyses were done using Minitab Version 17 Statistical Package (Minitab Inc., PA, USA). The significance level (α) was set at 0.05.
Timber species that sprout in the Akak forest after logging and distribution of sprouted stumps
A total of 643 stumps from 20 tree species were located. Of these stumps, 56 (9%) had sprouted. Stumps from 13 different tree species (65% of the species that had been harvested) had sprouts (Figure 2 and Table 1). N. diderrichii, Piptadeniastrum africanum, Terminalia ivorensis, and Pterocarpus soyauxii were the most frequent sprouts. Collectively, these four species contributed almost 79% of the total number of sprouts observed.
The number of sprouts varied by species and period (Figure 3). N. diderrichii had the most sprouts (30) in 2017, 11 sprouts in 2015, and 12 sprouts in 2013, while P. soyauxii had 9 sprouts in 2013, 7 sprouts in 2015, and 2 sprouts in 2017. T. ivorensis had 4 sprouts in 2013 and 17 sprouts in 2015 and no sprouts in 2017.
The mean diameter of the sprouted stumps varied by species and ranged from 20.0 to 102.9 cm (Figure 4).
Generally, most sprouts originated from stumps
with mean diameters between 70 and 100 cm. N. didderrichii and T. ivorensis sprouted from stumps with the highest mean stump diameters (102 and 103 cm, respectively). Diospyros crassiflora sprouted from stumps with a mean diameter of 20 cm and Milicia excels sprouted from stumps with a mean diameter of 30 cm. Both stump diameters are below the minimum diameter for logging as stated in the 1994 forestry law of Cameroon.
Most of the sprouts were found on stumps with diameters between 81 and 100 cm, but stumps between 41 and 60 cm had the highest number of sprouts (23) in a single year (2015) (Figure 5). The numbers of sprouts within the smaller stump diameter classes were small, as were those in the larger classes.
Generally, the mean height of sprouts increased with years since logging and varied among species (Figure 6).
One exception was Pycnanthus angolensis, which showed a mean sprout height of 55 cm 5 years after logging, 70 cm 3 years after logging, and 60 cm 1 year after logging. Similarly, T. ivorensis had a mean sprout height of 301 cm 3 years after logging, but 152 cm 5 years after logging. These differences could be due to competition amongst sprouts, stump characteristics or several other factors. Stump diameter classes of 101-120 and 81-100 had the highest mean sprout height while the two smallest diameter classes had the lowest mean sprout height (Figure 7).
Most sprouted stumps were recorded in the compartments logged in 2015 and 2017 (21 stumps each) while in 2013 only 14 sprouted stumps were found (Figure 8). Also, the compartment from 2017 had more different species sprouting than the other two compartments.
Relationships between sprouting characteristics (number of sprouts per stump, height of the dominant sprout, diameter of the dominant sprout, height, and diameter of mother stump) for all the species and species with the highest number of stump sprouts (N. diderrichii)
The year of harvesting correlated negatively with the other variables (Figure 9). The correlation between the year of logging and sprout decay was weakly negative, but it had a significant effect (P <0.005). Also, the correlation between the height of the tallest sprouts and stump diameter was weak but significant (P <0.032). There was a highly significant relationship (P <0.0005) between the height of the tallest sprouts and their basal diameter with a moderate correlation. There was also a significant relationship between the diameter of the tallest sprouts and the decaying state of the sprouts (P <0.001) signifying that as the diameter of the tallest sprout increased so did the decay of other sprouts (Table 2).
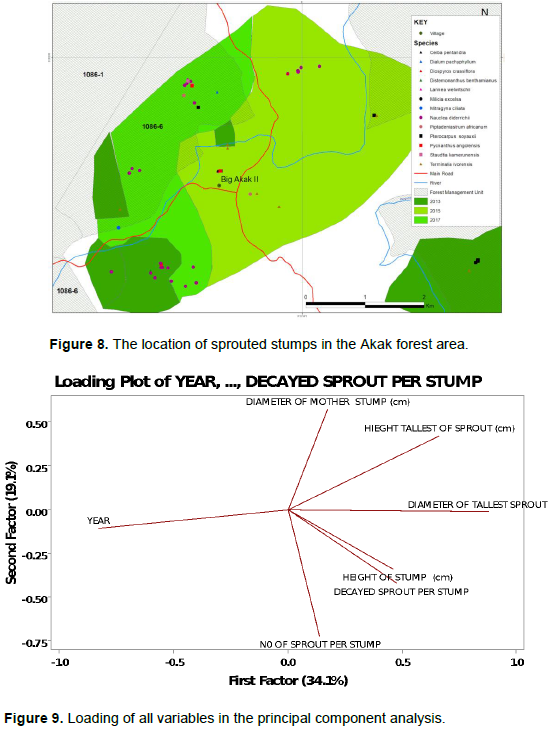
Principal component analysis (PCA) showed that the first factor contributed 34.1% and the second factor contributes 19.1% of the observed variation, with a communality of 66.6% (Figure 9 and Table 3) suggesting that the sprouting dynamics of the observed stumps could only be partially explained by the parameters measured.
The first two axes of the PCA for N. diderrichii explained 62.8% of the variance (Table 4 and Figure 10). The height of the stump correlated moderately negatively with the height of the tallest sprout indicating that as stump height increased sprout height decreased and the diameter of mother stump correlated moderately with decayed sprouts. These interactions were significant (P<0.002) and (P<0.031) respectively (Table 5). The correlations amongst stump sprouting parameters were not statistically significant (Table 8).
Neither the equation for predicting the number of sprouts for all species together or for N. diderrichii (Plate 1a and b) by itself were significant, although the latter relationship was stronger (Table 6). Thus, it is likely that stronger relationships might have been found if there were enough observations to separate the overall data by species or species cohort.
The diameter of the tallest sprout can be used to predict the height of the tallest sprout for all the species together (Table 7). Similarly, the diameter of the mother stump, the diameter of the tallest sprout, the height of the stump, and the decay level of the sprouts can predict the height of the tallest sprout for N. diderrichii. Again, it is likely that stronger relationships could be established if there were sufficient observations to allow species-specific equations to be fit for all species that sprouted.
The decaying state of the sprouts and the height of the tallest sprouts can be used to predict the diameter of the tallest sprouts for all species together (Table 8). Similarly, for N. diderrichii, the diameter of the stump, the height of the tallest sprouts, the height of stump and the decaying state of the sprouts can be used to predict the diameter of tallest sprout.
Timber species that sprout in the Akak forest after logging and distribution of sprouted stumps
Thirteen of the twenty harvested tree species sprouted after logging. Sprouting differed among stump diameter classes, with the highest number of sprouts found in stumps with 81 to 100 cm top diameters (Figures 2, 3 and 5). The highest number of sprouts was recorded 3 years after logging from stumps with 41 to 60 cm top diameters (Figure 5). This in contrasts with Gould et al. (2007) who indicated that most hardwood species sprout from small diameter stumps. The least number of sprouts were observed in the 121 to 140 cm diameter class. This is in line with Johnson (1977) and McGee (1978) who postulated that as the stump diameter increases, the number of sprouts reduces.
N. diderrichii, P. soyauxii, T. ivorensis, and P. africanum displayed considerable variability in sprouting response after logging (Figure 2). This result agrees with Benjamin et al. (2017) who found high sprouting probabilities across at least some parent tree sizes in their study. The small number of sprouts for Distemonanthus benthamia, Lannea welwitschi, Staudtia Kamerunesis, Mitragyna ciliate, and M. excels agrees with Wolfe and Pittillo (1977), Barnes (1985) and Stanturf et al. (2001) who found relatively low sprouting probabilities or low sprout persistence in their studies.
Johnson (1977) showed that four-year shoot elongation of the tallest stem among white and black oak increased as stump diameter increased up to a threshold of 6 inches (15.24 cm), and that, among black and white oak stump sprouts examined in southern Indiana, white oak had a marginal height advantage (Johnson, 1977). The present results showed a relatively high mean height with stump diameter that ranges from 121 to 140 for the year 2013, followed by the year 2015 as opposed to the year 2017 with relatively low sprout height.
Relationships between sprouting characteristics (number of sprouts per stump, height of the dominant sprout, diameter of the dominant sprout, height, and diameter of mother stump) for all the species and species with the highest number of stump sprouts (N. diderrichii)
It was found that the stump diameter can be used to predict the height of the tallest sprouts and the diameter of tallest sprouts N. diderrichii (Tables 3 and 4). This result agrees with many other studies. Sprouting ability is influenced by the amount of accumulated reserves in the stump and/or the activity of underground buds. Bigger stumps have more reserves and/or more active underground buds; therefore, bigger stump tends to produce more sprouts (Cirne and Scarano, 2001; Miura and Yamamoto, 2003; Ickes et al., 2003).
It was found that stump height and stump diameter influenced the height of the sprouts (Table 6). Sander (1971) noted that the early height growth of sprouts present after clear-cuts depended on the size of the stumps from which they originate, with sprouts from the largest stumps growing the fastest, with the most sprouts. For N. diderrichii, the height of the sprouts decreased with an increase in stump height (Table 8), in line with the findings of Johnson (1977), McGee (1978) and Gould et al. (2007).
None of the regression equations for predicting the number of sprouts were significant for all species together and for N. diderrichii alone (Table 2). This is in contrast to DeBell (1971) and El Houri (1977) who found an increase in sprouting with an increase in stump height working with other species. Harrington (1984) also reported that sprouting in red alder (Alnus rubra) was poor when the stumps were cut low. This may be due to the number of dormant or trace buds being small on such stumps (Hook and DeBell, 1970).
The first two axes of the PCA explained 62.8% of the variance for N. diderrichii (Figure 8). This indicates that there are other unmeasured parameters, such as soils, predation, and climate that could influence sprouting (Poskin, 1949). Stump height correlated moderately negative with the height of the tallest sprout (P<0.002). Shackleton (2001) also noted a negative correlation between the stump size and the shoot coppice lengths in the regrowth of an indigenous savannah tree species (Terminalia sericea). Khan and Tripathi (1986), working in northeast India, found decreasing coppicing with increasing stump size for four sub-tropical forest species.
They thought this to be caused by the increased bark thickness of larger stems hindering the emergence of buds.
In general, sprouting contributes to a life history strategy of persistence that has been associated with root carbohydrate storage, relatively high root to shoot ratios, slow initial rates of shoot growth for true seedlings, large seeds, and relatively low seed production (Kruger et al., 1997; Bellingham and Sparrow, 2000; Bond and Midgley, 2001). Sprouting is important for promoting regeneration, especially of logged timber species, and for the overall dynamics of forest stands (Putz and Brokaw, 1989; Kammesheidt, 1998; Miura and Yamamoto, 2003). Thirteen of the 20 species harvested were found sometimes to produce sprouts, although sprouting was not consistent among the three logging areas and years examined. This could be due to anthropogenic, ecological, and environmental factors that were not measured (Poskin, 1949; Salk et al., 2011).
While stump sprouting appears to be an important mechanism of regeneration of some tropical timber species, seedling regeneration, either natural or artificial, must occur for the site to completely recover. Forest managers could have reduced the cost of artificial seedling regeneration by applying silvicultural treatments on sprouting species such as N. diderrichii, P. soyauxii, P. africanum and T. ivorensis which has a high ability of sprouting. Long-term research is necessary to monitor the growth and examine several unknowns concerning the role of stump sprouts in successful site recovery following harvesting. These include long-term sprout survival (including the impact of stump damage on survival), the quality of sprout-produced trees, and environmental and ecological factors influencing tropical tree species ability to sprout.
Clear cutting often prevents stumps from reaching an age and diameter in which sprouting declines rapidly (Johnson et al., 2002). Stump diameter is known to be an important predictor of sprouting probability (Johnson et al., 2002; Dey and Jensen, 2002).
Stump sprouts have the potential to grow quickly within the first ten years due to the large root mass and stored carbohydrates. Multiple flushes in growth can occur even under drought conditions, whereas most other growth forms are subject to single flushing (Johnson et al., 2002). Thinning of sprouts relatively early after harvest may improve growth rates of high-quality sprouts, resulting in mature stems with better growth form for future harvest and rapidly creating oaks capable of mast production (Johnson et al., 2002). Similar to other regeneration methods, site preparation methods aimed at reducing competitive vegetation before harvest, may be vital even for stump sprout regeneration. Harvesting timber at a relatively high diameter between 81 and 141 cm may have a greater probability of becoming the primary constituent in the future forest composition.
In this study, it was found out that 13 timber species sprouts in the Akak rain forest, N. diderrichii was the most frequent sprouted. Principal component analysis tells us that the relationship between sprouting parameters both for all the species combined and N. diderrichii could only partially be explained by the variables measured. Sprout diameter, stump diameter, stump height and sprout decay could be used to predict the height of the tallest sprout while sprout height, stump diameter and sprout decay could be used to predict the diameter of the tallest sprout. These predicting models could help in predicting the future growth rate and stand of the tropical rainforest.
The authors have not declared any conflict of interest.
REFERENCES
|
Arthur MA, Muller RN, Costello S (1997). Species composition in a Central Hardwood forest in Kentucky 11 years after clear- cutting. American Midland Naturalist 137(2):274-281.
Crossref
|
|
|
|
Barnes WJ (1985). Population dynamics of woody plants on a river island. Canadian Journal of Botany 63(3):647-655.
Crossref
|
|
|
|
|
Beck DE, Hooper RM (1986). Development of a Southern Appalachian hardwood stand after clear-cutting. Southern Journal of Applied Forestry 10(3):168-172.
Crossref
|
|
|
|
|
Bellingham PJ, Sparrow AD (2000). Resprouting as a life history strategy in woody plant communities. Oikos 89(2):409-416.
Crossref
|
|
|
|
|
Benjamin O, Matthew G, Daniel C (2017). Early Stump Sprout Development after Two Levels of Harvest in a Midwestern Bottomland Hardwood Forest. Forest Science 63(4):377-387.
Crossref
|
|
|
|
|
Bond WJ, Midgley JJ (2001). Ecology of sprouting in woody plants: the persistence niche. Trends in Ecology and Evolution 16(1):45-51.
Crossref
|
|
|
|
|
Boring LR, Monk CD, SWANK WT (1981). Early regeneration of a clear-cut Southern Appalachian forest. Ecology 62(5):1244-1253.
Crossref
|
|
|
|
|
Cirne P, Scarano FR (2001). Resprouting and growth dynamics after fire of the clonal shrub Andira legalis (Leguminosae) in a sandy coastal plain in south-eastern Brazil. Journal of Ecology 89(3):351-357.
Crossref
|
|
|
|
|
Dalling JW, Hubbell SP (2002). Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. Journal of Ecology 90(3):557-568.
Crossref
|
|
|
|
|
De Steven D, Wright SJ (2002). Consequences of variable reproduction for seedling recruitment in three neo tropical tree species. Ecology 83(8):2315-2327.
Crossref
|
|
|
|
|
Del Tredici P (2001). Sprouting in temperate trees: A morphological and ecological review. The Botanical Review 67(2):121-140.
Crossref
|
|
|
|
|
Dey DC, Jensen RG (2002). Stump sprouting potential of oaks in Missouri ozark forests managed by even-aged and uneven-aged silviculture proceedings of the second Missouri Ozark Forest Ecosystem Symposium: Post-treatment results of the landscape experiment. General Technical Reports 227:102-113.
|
|
|
|
|
Dietze M, Clark J (2008). Rethinking gap dynamics: the impact of damaged trees and sprouts. Ecological Monographs 78(3):331-347
Crossref
|
|
|
|
|
El Houri A (1977). The effects of stump heights on the coppicing power of Eucalyptus microtheca. Sudan Silvia 3(22):90-105.
|
|
|
|
|
Food and Agriculture Organization (FAO) (2003). Sustainable management of tropical forest in Central Africa. In search for excellence. FAO forestry paper n° 143. Roma. p 128.
|
|
|
|
|
Food and Agriculture Organization (FAO) (2007). Global Forest Resources Assessment 2005: progress towards sustainable forest management. FAO Forestry Paper 147. FAO, Rome. p 350.
|
|
|
|
|
Gould PJ, Fei S, Steiner KC (2007). Modeling sprout-origin oak regeneration in the central Appalachians. Canadian Journal of Forest Research 37(1):170-177.
Crossref
|
|
|
|
|
Groenendijk P (2015). Long-term trends in tropical tree growth: a pantropical study.
|
|
|
|
|
Harrington A (1984). Factors influencing initial sprouting of red alder. Canadian Journal of Forestry Research 14(3):357-361.
Crossref
|
|
|
|
|
Holl KD (1999). Factors limiting tropical rain forest regeneration in abandoned pasture: Seed rain, seed germination, microclimate, and soil. Biotropica 31(2):229-242.
Crossref
|
|
|
|
|
Hook D, DeBell DS (1970). Factors influencing stump sprouting of swamp and water tupelo seedlings. Res. Pap. SE-57. Asheville, NC: U.S. Depart- ment of Agriculture, Forest Service, Southeastern Forest Experiment Station 57:9.
|
|
|
|
|
Ickes K, Dewalt SJ, Thomas SC (2003). Resprouting of woody saplings following stem snap by wild pigs in a Malaysian rain forest. Journal of Ecology 91(12):222-233.
Crossref
|
|
|
|
|
Johnson P (1977). Predicting stump sprouting and sprout development in theMissouri Ozarks. USDA For Forest Service Research Paper, NC-149. P 11.
|
|
|
|
|
Johnson P, Shifley S, Rogers R (2002). The Ecology and Silviculture of Oaks. CABI New York, NY 503:100-106.
Crossref
|
|
|
|
|
Kammesheidt L (1998). The role of tree sprouts in the restoration of stand structure and species diversity in tropical moist forest after slash-and-burn agriculture in Eastern Paraguay. Plant Ecology 139(2):155-165.
|
|
|
|
|
Kenfack D, Thomas DW, Chuyong G, Condit R (2006). Rarity and abundance in a diverse African forest. Biodiversity and Conservation 16:2045-2074.
Crossref
|
|
|
|
|
Khan ML, Tripathi RS (1986). Tree regeneration in a disturbed sub-tropical wet hill forest of northeast India: effect of stump diameter and height on sprouting of four tree species. Forest Ecology and Management 17(2-3):199-209.
Crossref
|
|
|
|
|
Khurana E, Singh J (2001). Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A review. Environmental Conservation 28(1):39-52.
Crossref
|
|
|
|
|
Kruger LM, Midgley JJ, Cowling RM (1997). Resprouters vs reseeders in South African forest trees; a model based on forest canopy height. Functional Ecology 11(1):101-105.
Crossref
|
|
|
|
|
Lamprecht H (1989). Silviculture in the tropics: tropical forest ecosystems and their tree species; possibilities and methods for their long-term utilisation. Gesellschaft für Technische Zusammenarbeit (GTZ) GmbH, Eschborn, Deutschland P 296.
|
|
|
|
|
Mcgee CE (1978). Size and age of tree affect white oak sprouting. Deptartment of Agriculture, Forest Service, Southern Forest Experiment Station 239:2.
|
|
|
|
|
Miller PM, Kauffman JB (1998). Seedling and sprout response to slash-and-burn agriculture in a tropical deciduous forest. Biotropica 30(4):538-546.
Crossref
|
|
|
|
|
Miura M, Yamamoto S (2003). Structure and dynamics of a Castanopsis cuspidata var. Sieboldii population in an old-growth, evergreen, broad-leaved forest: The importance of sprout regeneration. Ecological Research 18(2):115-129.
Crossref
|
|
|
|
|
Nasi R, Cassagne B, Billand A (2006). Forest management in Central Africa: where are we? International Forestry Review 8(1):14-20.
Crossref
|
|
|
|
|
Nchanji AC, Plumptre AJ (2003). Seed germination and early seedling establishment of some elephant dispersed species in Banyang-Mbo Wildlife Sanctuary, south-western Cameroon. Journal of Tropical Ecology 19(3):229-237.
Crossref
|
|
|
|
|
Nzogang A (2009).Tropical forest dynamics after logging - natural regeneration and growth of commercial tree species - in southeast Cameroon. Freiburg in Breisgau, Germany P 170.
|
|
|
|
|
Poskin A (1949). Traite de sylviculture. Gembloux, Librairie agricole pp. 392-420.
|
|
|
|
|
Putz FE, Brokaw NV (1989). Sprouting of broken trees on Barro Colorado Island, Panama. Ecology 70(2):508-512.
Crossref
|
|
|
|
|
Salk CF, McMahon SM, Oecologia (2011). Restoration of tropical dry forest: A review. Environmental Conservation 28:39-52.
|
|
|
|
|
Sander I (1971). Height growth of new oak sprouts depends on size of advance reproduction. Journal of Forestry 69(11):809-811.
|
|
|
|
|
Shackleton CM (2001). Managing regrowth of an indigenous savanna tree species (Terminalia sericea) for fuel wood: the influence of stump dimensions and post-harvest coppice pruning. Biomass and Bioenergy 20(4):261-270.
Crossref
|
|
|
|
|
Stanturf JA, Schoenholtz SH, Schweitzer CJ, Shepard JP (2001). Achieving restoration success: Myths in bottomland hardwood forests. Restoration Ecology 9(2):189-200.
Crossref
|
|
|
|
|
Vickers LA, Fox TR, Loftis DL, Boucugnani DA (2011).Predicting forest regeneration in the Central Appalachians using the REGEN expert system. Journal of sustainable forestry 30(8):790-822.
Crossref
|
|
|
|
|
White AS (1991). The importance of different forms of regeneration to secondary succession in a Maine hardwood forest. Bulletin of the Torrey Botanical Club 118(3):303-311.
Crossref
|
|
|
|
|
Wolfe CB, Pittillo JD (1977). Some ecological factors influencing the distribution of Betula nigra L. in western North Carolina. Castanea 42(1):18-30.
|
|
|
|
|
Wright SJ, Muller-Landau HC (2006). The future of tropical forest species. Biotropica: The Journal of Biology and Conservation 38(3):287-301.
Crossref
|
|