ABSTRACT
Among baboon species, Papio anubis is the most broadly distributed species, ranging through most of central sub-Saharan of Africa. Papio anubis live in troops but the size varies in different area. There are three troops of P. anubis in Trigni forest with 149 to 180 members in each troop. The objective was to assess the abundance of Anubis baboon (AB) and their conflict with humans with respect to the livelihood of local farmers in Trigni forest of Gida Ayana district, Western Ethiopia. Counting was conducted early morning at 6:00 to 7:00 am and late afternoon at 5:00 to 6:00 pm when the baboons were at the site of their sleeping. Six trained data collators were arranged during census of AB. These six data collators were divided into three sites (Harawa, Gendo, and Kotam cliffs). To increase accuracy and minimize error, counting was also done when the baboons left their sleeping site and went to foraging area, and in the afternoon when AB return to their sleeping site. Population count was carried out three times during each dry and wet season. For the human-anubis baboon conflict (HABC) assessment, participatory techniques were used. Data were analyzed using descriptive statistical methods, using SPSS version 20. The dry and wet populations of AB were 484 and 511, respectively. The distance of farmland from forest edge and problems of HABC were directly correlated. Results showed that AB is physically strong enough to attack livestock. About 62.9% of the respondents indicated that they lost their livestock by AB during 2015/2016. Most of the respondents reported that they rely on dogs and children to protect AB from raiding crops and this require time and energy. The majority (78.5%) of the respondents strongly agree that habitat disturbance is the main cause of HABC in the study area. More than 93.1% of the livelihood of local farmers of Gute Gudina kebele, adjacent to the forest reserve was affected by conflict between humans and AB. AB spent most of their time along the road and entered the agricultural fields. They can also attack and injure humans especially females and babies if provoked. Therefore, leaving sufficient buffer zones between crop productions and forest that allow free movements of AB and avoid high guarding investments (easy for guarding) is recommended.
Key words: Cliffs, human-wildlife conflict, Papio anubis, primate habitat.
The Anubis baboons (Papio anubis) is a member of the order Primates (Cawthon Lang, 2006; Codron et al., 2006). Anubis baboons (AB) are one of 4 species of savannah baboons found in different parts of Africa (Johnson et al., 2012.). They are generally light to dark brown with a brindle pattern, weighing up to 50 kg (Bergman and Beehner, 2004; Strum, 1991). AB are ecologically flexible based on the factors influencing in that they use wide variety of foods and can live in a variety of habitats (Codron et al., 2006). The main reason why they are able to adapt to these numerous habitats could be their flexibility in foraging strategies and ability to extract food and nutrients from almost all strata of the environment (Strum, 1991; Johnson et al., 2015). AB are highly social animals; with a complex multi-male, multi-female social structure. They capture different animals by using group power (Zinner et al., 2001). Members of a troop travel, forage, and sleep together. When availability of food decreases, troops are divided in small groups and search for food in different directions (Kuns and Linemair, 2007). Females mostly stay in troops throughout their life while males sometimes move from the groups and search for food individually. Within the troop, there is social structure with according to their age; the dominant adult males lead the troop (Johnson et al., 2015). Reproduction in AB is linked with the social structure of this species. Since AB lives in multi-male, multi-female troops their mating is promiscuous, with both males and females mating with multiple partners. Predators like hyena, lion, leopard, and human kill AB. Because of these predators many AB are killed every year. Due to intensive agricultural expansion, interaction between AB and human being continue to increase from time to time due to natural habitat loss and degradation by anthropogenic power (Amaja et al., 2016). This interaction leads to HABC from rapid agricultural activities and declining of forest, the conflict between human-anubis baboon is one of the most interesting topical problems in countries that depend on agriculture (Datiko and Bekele, 2013). In Ethiopia, AB is found throughout the country in all habitats, forest woodland, desert and human habitat area. Since most of the population of Ethiopians depend on agriculture for their livelihood, conflict between HABC is common. This conflict create negative attitude toward the conservation of AB, and different farmers kill a lot of AB from time to time (Datiko and Bekele, 2013). As in other parts of Ethiopia, in Gida Ayana, AB have been causing damage to agricultural crops and plantations. There are wide varieties of herbivores, primates, and small mammals causing damage to crops and small ruminant and chicken livestock but, AB cause serious damage to agricultural crops in different parts of this district. In spite of a generally good understanding of HABC, there is no consistent, reliable inventory, well-studied and documented information with regarding to this area. Therefore, this research study aim was to assess the abundance of AB (P. anubis) and conflict with humans around Trigni forest of Western Ethiopia. Consequently, the second objective of this research is to investigate the major effect of HABC on socio-economic characteristics (crops and domestic animals that are mainly damaged) of farmers around the study area and conservation of Trigni forest (TF).
Description of study area
The study was carried out in TF which is located in Gida Ayana district, Western Ethiopia, located within 9°49.5’-9°59.6’N and 36°40-36°43’E. It is located about 433 km west of Addis Ababa and 112 km west of Nekemte (Figure 1). The altitude ranges from 2183 to 2268 m above sea level. The forest covers an area of 57 ha. The average yearly rainfall is 1739.15 mm, with a rainy season during May to September and a relatively dry period from December to March. There is no high variation of temperature throughout the year but the maximum temperature is in February and March. TF is bordered by Gendo village on the East and South, Lelise village on the West, Golole Harawa on the North. All of these villages are found in Gute Gudina kebele. The main crops produced in this Kebele include maize, teff, wheat, barley, peas, beans, potatoes, sorghum, onion and various types of oil seeds.
Sampling methods of data collection
Based on preliminary survey, Gute Gudina Kebele was purposively selected because of the presence of serious HABC in the area. This Kebele contains 12 villages, out of these 3 villages, namely Lelise, Golole Harawa and Gendo were purposively selected based on the distance of their farmlands from TF. The total household living in the selected village as documented by the Kebele administration was 720. The sample size for the study was specified by using the following formula (Cochran equation, 1977 cited in Amaja et al., 2016):
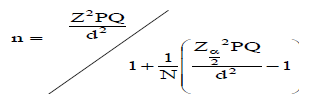
Where: n = desired sample size when population less than 10000, Z = standard normal deviation (1.96 for 95% confidence level), P = 0.1 (proportion of population to be included in sample, that is, 10%), q = is 1-P, that is, (0.9), N = is total number of population and d = is degree of accuracy desired (0.05). Total of 116 sample population were selected using simple random sampling techniques from the total population of 720 (217 from Lelise, 253 from Gendo and 250 from Golole Harawa). Within each village, households were randomly selected using probability proportional to size. Accordingly, the numbers of HH studied were 35 from Lelise, 41 from Gendo and 40 from Golole Harawa. Gender composition composition of sample population was 84 (72.4%) males while 32 (27.6%) were females. Regarding to the age groups of sample population, data were mainly got from matured age groups (41-50 years with 39 (33.6%), 31-40 years with 27 (23.3%), 51-60 years with 23(19.8%), below 30 years with 19 (16.4) and above 61 years with 8 (6.9). For the HABC assessment, participatory techniques (focal group discussions and key informant interview), structured questionnaire survey of households. In addition, relevant written information was gathered from kebele and districts agricultural offices.
Population estimation of Anubis baboons (AB)
Data was collected from December 2015 to June 2016. Direct count method for surveying was used to estimate the abundance of AB in TF (Bergman and Beehner, 2004; Strum, 1991). Direct count was carried out at 4 different sites, namely Harawa, Gendo, and Kotam cliffs. This direct counting was conducted early morning at 6:00 to 7:00 am and late afternoon at 5:00 to 6:00 pm when the baboons were at the site of their sleeping by human counter using distances ranging from 15 to 50 m looking from side to side (Admassu et al., 2014). Population count was carried out 3 times during each dry and wet season. Six trained data collators were arranged during census of AB. These six data collators were divided into three sites (Harawa, Gendo, and Kotam cliffs). To increase accuracy and minimize error, direct counting was also done when the AB left to their sleeping site and went to foraging area and in the afternoon when AB return to their sleeping site. Population count was carried out three times during each dry and wet season. The population density of AB was calculated by the following formula. . Where: D = density Anubis baboon in the Trigni forest, N = Numbers of Anubis baboons counted in the forest and A = Areas of Trigni forest in hectare (Admassu et al., 2014).
Data analysis
Data were analyzed using SPSS version 20 software and MS Excel. Accordingly, descriptive statistics in a form of percentage and frequency were used for the analysis of the types of crops cultivated by farmers, types of crops mostly damaged by AB, the main causes that increase HABC, effect of HABC on livelihood of farmers nearby TF and responses were compared using chi-square test. To compute the variation among population of AB student’s paired t-test was used.
Population size of Anubis baboons (AB) in Trigni forest (TF)
There were 3 cliffs namely Gendo Cliff, Harawa Cliff and Kotam Cliff. Almost similar results were obtained from each counting site and statistically no significant difference between these three counting sites (Tables 1 and 2). The result indicated that each of the cliffs contain single baboon troop and they cannot mix even when they search for food. In both seasons, the numbers of adult females are more than adult males. During the dry season, the individual count at TF showed 15.9% adult male, 24.2% adult female. There was a significant difference between the number of male and female AB population in the study area in both dry and wet seasons (t = 68.69, df = 1, p < 0.05 and t = 70.95, df =1 p < 0.05) for dry and wet seasons respectively. The ratio of male to female is 1:1.6 in both seasons.
Human Anubis- baboon conflict (HABC)
Crops, small ruminant and chickens livestock mostly attacked by AB
This result indicated that AB damage almost all types of crops, but beans, maize, barleys, peas and potatoes were ranked as the most frequently damaged crops (93.1, 90.5, 81, 75.9 and 67.2%, respectively) by AB (Tables 3 to 4). This AB raid stands of beans and maize significantly more frequently than all other crops and they cause series damage on these two crops than the others. Maize is one of the most frequently cultivated crops around this area and one of the most preferred crops by AB. Most of the respondents reported that beans were sensitive crop as highly damaged by AB and their response was significantly different (t = 40, df = 1, p <0.05). Table 3 also shows some of the crops not mostly raided by AB, such as onions and anchote.
In the 3 villages, most farmers have good experience on crop damage and beans maize and barleys were the most frequently raided crops by AB (Table 4). There was no significant difference in the three villages in case types of crops mostly AB.
This study indicated that there is conflict between the local people and AB in TF. About 87.7% of the respondents strongly agree that AB raid crops and kill goat and sheep around this area (Table 5). The result indicate that there was significant difference among different groups of respondents (c2 = 9.546, df = 8 and P < 0.05). The result of interview and focus group discussion also confirm this result.
The distance of farmland from forest edge and problems of HABC are directly related. Table 6 shows that farmers that have farmland nearest to TF strongly agree that AB raided their crops highly than those that have a farmland far from TF.
Small ruminant livestock that are mostly depredation by AB in the study area were sheep (28.4%) and goat (23.3%). Table 7 indicates that this primate also kills domestic foul (14.65%). There was significant difference between the respondents (c2 = 8.81, df = 2, P < 0.05).
Table 8 shows time of raiding by AB is relatively high in the morning and in the afternoon, with 60.3% of the respondents strongly agreeing that AB raid crops mostly at the morning time. The result also indicated that the level at which AB raids crops is high during the afternoon (Table 8) and their response was significantly different (c2 = 17.73, df = 8, p < 0.05). The level of crop raiding by AB was low at night.
HABC was not seasonal since the results of this study show that the AB raids crops and attack small ruminant and chicken livestock throughout the year (Table 9). The majority of the respondents (76.7%) said that AB raid crops and attack small ruminant and chicken livestock in both wet and dry seasons. The result indicated that there is statistically significance difference between the groups of respondents on the season at which AB mostly raids crops and attack small ruminant and chicken livestock.
AB raid crops at different stage but, the level of damage varies based on the types of crops. Figure 2 indicates that AB raid matured crops more frequently than early maturation and seedling. AB damages some crops like maize, beans and peas throughout their life cycle. This show that the stages of crops damaged by AB depend on the types of crops.
Causes of human-anubis baboon conflicts (HABC) nearby Trigni forest (TF)
The study showed that the major causes of HABC in the study area were habitat disturbance (due to expansion of subsistence agriculture around forest edge), proximity of farmlands to natural forest and the associated behavioral changes of AB (Table 10 and Figure 3). The majority (78.5%) of the respondents strongly agree that habitat disturbance is the main causes of HABC around the study area. This result showed that there is no statistically significant different among different groups of respondents regarding to the cause of HABC.
Effect of HABC on socio economic activity of farmers and conservation of Trigni forest
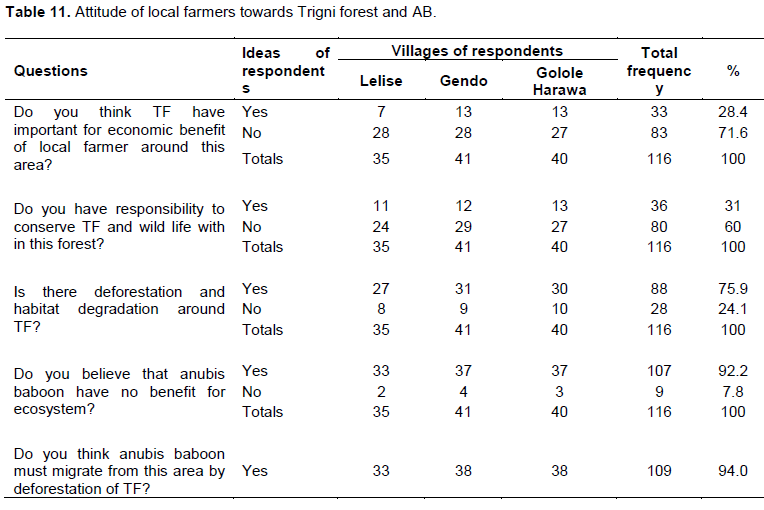
This study show that HABC cause deforestation of TF and dangerous to the conservation of wildlife. About 75.9% of respondent reported that deforestation and habitat degradation is common in Trigni forest. About 93.96% of respondents said that AB must migrate from this area by deforestation of TF (Table 11). This deforestation threatens other wildlife in addition to AB. The respondents also showed that AB did not have any ecological benefit. However, all organisms have their own ecological benefit.
The most significant determinant of livelihood was availability of food for household consumption. More than 79.3% of the respondents strongly agree and 10.3% agree (Table 12) that they face decrease in their agricultural income since crops and livestock were destroyed by HABC. Most of respondents reported that HABC highly affects income (c2 = 12.06, df = 8 and P < 0.05). Regarding sending of their children to school, 54.3% of the respondents strongly agree and 21.6% of the respondent agrees (Table 12) that their children were not going to school because of HABC. Majority of the respondents strongly agree that their children did not go to school since they protect AB from their properties guarding and there was statistically significant difference among different groups of respondents (c2 = 18.6, df = 8 and P<0.05).
Traditional methods used by farmers to defend Anubis baboon from their properties
Traditional methods of protecting AB from crop dammage were also given in Figures 4 and 5. Farmers nearby TF protect their properties from AB by using different traditional methods. Guarding takes the highest frequency (56.9%) followed by shooting (23.3%) and scarecrow (13.8%). Fencing is uncommon to protect AB from crop raiding (Figure 4). Most of the respondents reported that they use dogs and children for guarding and this consumes time and energy.
Table 13 shows that there is statistically significant difference in terms of methods used to protect crops and small ruminant and chickens livestock from attack by AB (c2 = 8.59, df = 8 and p = 0.037> 0.05). In the three studied villages guarding is common and contains more frequency than other methods.
AB was choosing sleeping sites based on the factors influencing. This study showed that AB in TF has been using three sleeping cliffs. In addition to cliffs, some AB use moist large trees such as Croton macrostachys (Birbirsa) and Ficus vasta (Kiltu). According to Cawthon Lang (2006) reported that AB prefers cliffs and when cliff is not available they were use trees as sleeping site. This study indicated that there were 3 troops of AB in TF with 149 to 180 individuals. AB lives in groups of 15 to 150. In this study, the individual numbers of AB was high when we compare with the results of (Cawthon Lang, 2006). The numbers of individuals that were found in the 3 cliffs was almost the same in both seasons and the number of female AB was higher than the males. This showed that adult males move from their group to search for food and raid crops. Higher number of female AB is good to increase the population of AB in the future. In their social organizational single male can mate with many females and produce juvenile. According to Strum (1991) the hierarchies of AB were based on age groups and mostly adult males dominate the groups. This study also agrees with this notion. All of the baboon troops leave TF during the morning time and returns to their cliffs during afternoon. The large groups of troops are divided into smaller sub-groups when they forage and enter into human habitat. This finding agrees with Bergman and Beehner (2004). The study showed that serious interaction of AB with human resource and habitat. Observation during data collection indicated that AB become aggressive when they enter human habitat area and this behavior was very dangerous for both populations. Aggressive behavior in primates usually occurs during intra and intergroup competition for limited resources, dominance status and social partners.
Most of the respondents (65.5%) depend on mixed farming and some (23.3%) get their income from crop production only. The dominant crops in the study area were teff, maize, potatoes, barley, wheat and beans. About 80.2% of respondent responded that they cultivated teff and 63.8% of respondents cultivated maize on their farmland during 2015/2016. The study showed that AB does not equally damage all crops. Based on sensitivity levels, some crops are highly damaged and others are less damaged. From these results, AB in the study area mostly damaged beans and maize. This agrees with the studies carried in Nigeria and Uganda. According to Eniang (2011), of all the crops grown by the farmers, maize sustains the most frequent damage. Maize was the most vulnerable crop to crop raiders followed by beans and sweet potato. Maize is the first ranked crop that is damaged by baboons. Anchiote and Onion are types of crops that are least damaged by AB in the study area. Both agricultural damage and livestock depredation were reported in the study area, but crop loss due to AB was the most serious problem in the study sites (Yigrem et al., 2016).
The local farmers are killing AB to revenge from their raided crop, sheep goats and chickens. The level of conflict differs from village to village depend on distance from the sleeping sites and farmland. The study indicated that farmers who have farm nearest to sleeping sites were highly exposed damage. This study agrees with Wallace and Hill (2012). They reported that the distance of farmland from forest edge is directly related to human wild life conflict. This also agree with (Datiko and Bekele, 2013) which reported as people who live close/near the park area generally faced many problems than those living far from the park. This study showed that the proximity of farms to the edge of wildlife habitat has a direct effect on the proportion of crop damage and livestock depredation in the study area. AB is diurnal animals and they are active during the daytime. This primate raid crops and kill domestic animals only during the daytime. This study showed that the conflict between AB and human occurred through the day. These results agree with the result of Warren (2009) which reported crop raiding by baboon was high during the mid-day (11.00-12.00 pm). In the study area AB forages specially during the morning and afternoon but, during the mid-day they spent their time on resting and this is why crop raiding by AB was relatively low during the mid-day. According to this result crops which were nearest to forest edge raided seriously at the morning time and during afternoon when AB return to their cliffs.
The levels of crop raiding during the dry and wet seasons by the AB are affected by abundance or distribution food resource. According to Adeola et al. (2014) nutritional content, abundance, distribution, and seasonal availability of food eaten by AB have a major impact on their feeding. In the study area, the crops were mainly cultivated starting from May and become matured from September to December. Most of the farmers responded that even though crop raiding was high during the dry season, the conflict between human and AB occurred throughout the years. This confirmed that AB raid crops and kill domestic animals throughout the year. AB damage crops at different stage but, it depend on the types of crops. Some crops like maize and sorghum were damaged throughout their life cycle (starting from seedling up to maturity) and some others like teff, bean and peas were mainly damaged when they become matured. In this study area, AB prefers crops which contain high contents of carbohydrates and proteins. This indicated that there was a diet preference of AB based on nutritional value. As energy and nutrition are the main factors in diet preference and time spent for the foraging of AB, local farmers protect AB from their properties by using different methods. In this study, the most commonly used methods to protect AB from crop raiding and small ruminant and chickens livestock was guarding. Since this primates are diurnal, guarding is one the effective methods which is used to protect AB from damaging crops and small ruminant and chickens livestock. Guarding can be done by watching or even by using dogs. Because of the aggressive behavior of AB some respondents reported that children and females fear to protect AB by guarding. Next to guarding shooting and scarecrow were the second and third method used by farmers respectively. Shooting is very dangerous for AB since farmers kill specially young males during crops raiding and even it increase aggressiveness of AB. Scarecrow is one of the traditional indigenous methods in which farmers used different models that may looks humans but, since AB are the intelligent primate this method is not effective. Fencing and smoking were not common to protect crop raiding and small ruminant and chickens livestock depredation by AB (Akosim et al., 2010). The main causes that increase HABC around TF were habitat disturbance, proximate to forest edge and increment of AB population respectively. The result of observation indicated that around Trigni forest there was disturbance and fragmentation of forest by agricultural activity. This increase the interaction between human and AB which increase the conflict and change the feeding behavior of Anubis baboons. This result was agreed with (Akosim et al., 2010) who reported increased habitat disturbance as caused of human wild life conflict in Uganda. The result showed that natural habitats of AB decline from time to time for agricultural purpose. This situation make primate became aggressive and even difficult to protect from crop damage and domestic animals depredation. Most the farmers around the study area did not have positive attitude toward TF and wild animals. This is one of the main wild life conservation problems in the study area. The current study confirmed that HABC seriously affect the livelihood of the farmers nearby TF. More than 93.1% of respondents strongly agree that their livelihood were affected by HABC. This associate with food shortage, prevent children from school, make poor social life and others problems.
CONCLUSION AND RECOMMENDATION
This study indicated that AB found in TF is threatened due to HABC. AB in the surrounding areas has aggravated HABC because of crop raiding and depredation of livestock. Beans, maize, barley, peas and potatoes are the main crops mostly damaged by AB in the study area. The types of livestock that are mostly attacked by AB are sheep goats and chicken. Since, agricultural activities have been carried out near the forest and the original habitats of the AB were disturbed and fragmented. This is confirmed by direct observation and interview during data collection. This habitat disturbance and reduction of forest increase HABC in this area. The main causes, which increase HABC, were disturbance AB habitats and proximity of forest and farmland. The most common method used by farmers to protect AB from their properties was guarding. Even though, AB raids crops and kill sheep and goats throughout the daytime, at the morning and afternoon there was a series crop raiding in the study area. HABC have negative effects on TF, wild animals and livelihood of farmers around this area. According to this study most of the respondents responded that AB must migrate from this area by deforestation of TF. Therefore, this study indicated that local peoples have negative attitude to toward TF and AB. In other case, farmers nearby this area faced many problems because of HABC. They lose agricultural incomes by crop raiding, chicken and small ruminant livestock depredation, preventing their children from school and poor relationship with their neighbors since guarding is throughout the day times. Therefore, from this finding, the following suggestions were recommended:
1. Leaving sufficient buffer zones between crops and forest that allow freely moments of AB and easily for guarding.
2. To avoid high guarding investments, encouraging farmers to grow unpalatable crops to AB, cooperatively keep their crop and changing their means of farming.
3. Further research is needed on aggressive behavior of AB and methods of protecting crops raiding and livestock depredation with the aim of introducing the successful and cost effective ones to farmers.
The authors have not declared any conflict of interests.
The authors would like to thank everybody who contributed to the sampling campaigns and the useful comments provided for the revision of the technical report.
REFERENCES
|
Adeola AJ, Apapa AN, Adeyemo AI, Alaye SA, Ogunjobi JA (2014). Seasonal variation in plants consumption pattern by foraging Olive Baboons (Papio anubis. Lesson, 1827) inside Kainji Lake National Park. J. Appl. Sci. Environ. Manage. 18(3):481-484.
|
|
|
|
Admassu M, Mamo Y, Bekele A (2014). Abundance of hamadryas baboon (Papio hamadryas hamadryas) and conflict with humans in Awash National Park, Ethiopia. Int. J. Biodivers. Conserv. 6(3):200-209.
Crossref
|
|
|
|
|
Akosim C, Joseph J, Egwumah PO (2010). Assessment of feeding behaviour of baboons (Papio anubis) in Hong hills Adamawa State, Nigeria. J. Res. For. Wildl. Environ. 2(1):60-72.
|
|
|
|
|
Amaja LG, Feyssa DH, Gutema TM (2016). Assessment of types of damage and causes of human-wildlife conflict in Gera district, south western Ethiopia. J. Ecol. Nat. Environ. 8(5):49-54.
Crossref
|
|
|
|
|
Bergman TJ, Beehner JC (2004). Social system of a hybrid baboon group (Papio anubis× P. hamadryas). Int. J. Primatol. 25(6):1313-1330.
Crossref
|
|
|
|
|
Cawthon Lang KA (2006). April 18. Primate Factsheets: Olive baboon (Papio anubis) Taxonomy, Morphology, & Ecology.
View.
|
|
|
|
|
Codron D, Leeâ€Thorp JA, Sponheimer M, de Ruiter D, Codron J (2006). Interâ€and intrahabitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C, δ15N, and %N. Am. J. Phys. Anthropol. 129(2):204-214.
Crossref
|
|
|
|
|
Datiko D, Bekele A (2013). Conservation Challenge: Human-herbivore Conflict in Chebera Churchura National Park, Ethiopia. Pak. J. Biol. Sci. 16(23):1758-1764.
Crossref
|
|
|
|
|
Eniang EA, Ijeomah HM, Okeyoyin G, Uwatt AE (2011). Assessment of human–wildlife conflicts in Filinga range of Gashaka Gumti National Park, Nigeria. Prod. Agric. Technol. J. 1:15-35.
|
|
|
|
|
Johnson C, Piel AK, Forman D, Stewart FA, King AJ (2015). The ecological determinants of baboon troop movements at local and continental scales. Mov. Ecol. 3(1):14.
Crossref
|
|
|
|
|
Johnson C, Swedell L, Rothman J (2012). Feeding Ecology of Olive Baboons (Papio anubis) in the Kibale Forest, Uganda: Preliminary Results on Diet and Food Selection. Afr. J. Ecol. 50(3):367-370.
|
|
|
|
|
Kunz BK, Linsenmair KE (2007). Changes in baboon feeding behavior: maturity-dependent fruit and seed size selection within a food plant species. Int. J. Primatol. 28(4):819-835.
Crossref
|
|
|
|
|
Strum SC (1991). Weight and age in wild olive baboons. Am. J. Primatol. 25(4):219-237.
Crossref
|
|
|
|
|
Wallace GE, Hill CM (2012). Crop damage by primates: quantifying the key parameters of crop-raiding events. PloS one 7(10):e46636.
Crossref
|
|
|
|
|
Warren Y (2009). Crop-raiding baboons (Papio anubis) and defensive farmers: a West African perspective. West Afr. J. Appl. Ecol. 14(1):1-11.
Crossref
|
|
|
|
|
Yigrem K, Wondimagegnehu T, Hailu M (2016). Conservation Challenge: Human-Herbivore Conflict in Sodo Community Managed Conservation Forest, Wolaita Sodo Zuriya District, Southern Ethiopia. J. Cult. Soc. Dev. 18:7-16.
|
|
|
|
|
Zinner D, Pelaez F, Torkler F (2001). Distribution and habitat associations of baboons (Papio hamadryas) in Central Eritrea. Int. J. Primatol. 22(3):397-413.
Crossref
|
|