ABSTRACT
Worldwide understorey nesting bird species such as bulbuls can also be highly vulnerable to nest predation in disturbed landscapes because they breed mainly on the lower stage of the forest. We test the following hypotheses: the transformation of forests into alternative land use systems and the vegetation’s variables at the nesting sites will affect the understorey nest predation rates. The nests of 12 understorey bird species were surveyed and vegetation variables were measured within five types of habitats along a gradient of increasing forest destruction in the north-eastern peripheral zone of the Korup National Park in Cameroon. Only the open-cup nest type suffers from predation, mostly egg predation. The general linear mixed model analysis suggests that the types of habitat do not affect nest daily predation rate which decreases with increasing trees and understorey plant density. The most deleterious impact of deforestation in this study area is the reduction of nesting sites whose characteristics remain unchanged across the landscape. These results underscore the need to give understorey nesting species, as well as other particularly sensitive groups, special consideration within conservation strategies such as the reduced-impact logging techniques.
Key words: Cameroon, deforestation, land-use system, nest predation, understorey birds.
Tropical forests have been destroying in at an alarming rate (Sodhi et al., 2004). Yet, the mechanisms of how forest modification affects the biodiversity destruction of African tropical rainforest regions are less well known (Norris et al., 2010; Newmark and Stanley, 2011; Newbold et al., 2015). These regions with high wildlife species richness and abundance have severe extinction rates because of habitat loss and overexploitation (Bradshaw et al., 2009; Cordeiro et al., 2015). There is evidence that habitat loss and alterations of species interaction are the major impacts of land use changes on bird populations (Cordeiro et al., 2015). Accordingly, extinctions of some forest birds resulting from direct or indirect consequences of deforestation have been recorded from various tropical regions (Castelletta et al., 2000; Sodhi et al., 2004). Understorey birds are within the most vulnerable of the forest bird communities because their nests are the most exposed to diverse predators species (Bellamy et al., 2018)as compared to canopy nesters in some study areas (Martin, 1993a). Furthermore, some evidence in tropical forests showed that off-ground nests are generally less predated than ground nests (Pangau-Adam et al., 2006; Bobo, 2007).
Nest survival within a tropical understorey bird community in a fragmented landscape is affected by many environmental factors (Newmark and Stanley, 2011; Aldinger et al., 2015). Nest predation seems to be the primary cause of nest failure among birds. Many studies have registered increased rates of nest predation in fragmented habitats (Githiru et al., 2005; Newmark and Stanley, 2011)due to higher densities of predators and reduced food availability in that landscapes (Chalfoun et al., 2002). Predation is also an important and ubiquitous selective force that can determine habitat preferences of prey species (Chalfoun and Martin, 2009)and it has been considered as an influential force in the evolution of an avian life-history trait (Bradley and Marzluff, 2003).
Evaluating the theory of the negative roles of tropical forests fragmentation on avian nesting success has always been a complex task because nests are concealed in tropical forests and are very difficult to localize (Tewksbury et al., 2006; Newmark and Stanley, 2011). From this, artificial nest experiments have often been used (Posa et al. 2007; Vergara and Simonetti 2004). These studies compare disturbed and non-disturbed habitats. They do not look at the effect of a gradient of increasing habitat destruction on predation. Also, they do not evaluate the negative or positive influences of vegetation variables on predation. Although, many studies on the bird nest predation have been carried out in Americas (Bradley and Marzluff, 2003; Vergara and Simonetti, 2004; Debus, 2006; Tewksbury et al., 2006), only a few of them are devoted in Africa (Djomo et al., 2014; Githiru et al., 2005; Newmark and Stanley, 2011). To the best of our knowledge, only two studies have been carried out in the “Guineo Congolese” area (Djomo et al., 2014) which considered predation on artificial nests. So, the real effects of forest destruction on natural nests remains less clear. The purpose of our research is to examine the effects of types of habitats on understorey avian nest predation within an Afrotropical understorey bird community. In this analysis, we compare the number of active nests found and the predation rates of open-cup nests between natural forests (near primary forest and old secondary forest) and modified habitats (disturbed forest, cocoa/coffee plantations and annual crop food fields) in one hand and between breeding phases (which are egg laying, incubation and nestling phases) in another hand. Additionally, the study investigates the influences of the vegetation parameters on daily predation rate. Based on other studies in similar ecosystems in Africa (Githiru et al., 2005) and South America (Vergara and Simonetti, 2003; Brawn et al., 2011), our hypothesis is that nest predation rates will increase with the gradient of increasing forest destruction and nest loss will be greatest in the nestling stage. In accordance with other studies (Dion et al., 2000; Estrada et al., 2002; Debus, 2006), we also hypothesize that in our study area, some nest sites features such as the density of trees, the canopy cover, etc. will significantly affect the depredation levels with more concealed nest sites being less predated (Vergara and Simonetti, 2004).
Study area
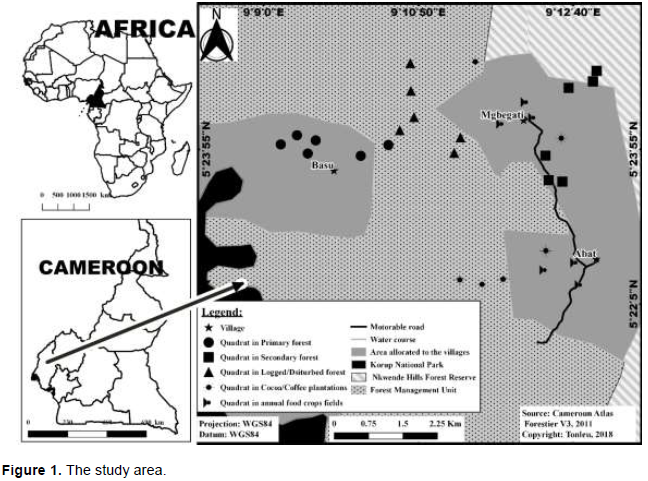
The study sites (Mgbegati, Abat and Basu), found between 5°21’18”-5°25’38” N and 9°06’29”-9°15’07” E, are in the Northeastern peripheral zone of Korup National Park (KNP) Southwest Region of Cameroon and are a legal entity in the management of KNP (Figure 1). It is the only extensive forest of western central Africa that originally spread from the Niger delta eastwards to Cameroon and South through Equatorial Guinea and Gabon. Located in the centre of the Guinea Congolese forest refugium, Korup is made up of four different forests (Atlantic Biafran Forest, Swamp Forest, Piedmont Forest and Sub-montane Forest) (Thomas, 1995). Our study sites are in the Piedmont Forest. Shifting cultivation is practised on farming areas which are associated with different forest types. Both food crops and cash crops are produced.
The study area encompasses the following broadly defined types of habitat: (1) primary forest (PFO), which is the natural forest with about 570 trees/ha (Waltert et al., 2005) and very little or no anthropogenic activities; the dominant tree species are Oubanguia alata, Gilbertiodendron demonstrans and Dichostema glaucescens; (2) old secondary forest (OSF), with about 530 trees/ha (Waltert et al. 2005)and where anthropogenic impacts are present but more than in PFO; Elaeis guineensis, Rauvolfia vomitoria, Pycnanthus angolensis and Barteria fistulosa constitute the main tree species; (3) disturbed forests (DFO), where logging was executed within 5 years prior to the study period; (4) cocoa/coffee plantations (CCP), with about 377,8 trees/ha (cocoa/coffee trees excluded) (Waltert et al., 2005) and where the land has been used for cocoa/coffee production, with few natural trees remaining; the dominant tree species are Coffea/Theobroma, Elaeis guineensis, Dacryodes edulis, Rauvolfia vomitoria and Funtumia elastic; (5) annual crops fields (ACF), where the land has been used for subsistence crops production (cassava, yams, maize, groundnut, etc.), with about 107,8 trees/ha (Waltert et al., 2005); E. guineensis, Ricinodendron heudelotii and Rauvolfia vomitoria are the main tree species. Each of the above habitats constitutes a stratum (Figure 1). The avifauna is a typical lowland rainforest, with more than 184 species restricted to this biome (Fishpool and Evans, 2001)and 420 species recorded (Rodewald et al., 1994). Particularly diverse groups are flycatchers (Muscicapidae), Old World Warblers (Sylviidae), Bulbuls (Pycnotidae), Sunbirds (Nectariniidae), and Weavers (Ploceidae).
Study design and data collection
The study was based on a total of 30 200 m x 200 m plots, six plots in each of five pre-classified habitats, primary forest, secondary forest, disturbed forest, cocoa/coffee plantations and annual culture fields (Figure 1). The plots were demarcated randomly within each habitat with at least 0.5 km between plots (Bobo, 2004). The period from April to August 2013 (within the wet season) corresponds to the nesting season of understorey nesting bird of this region (Serle, 1981). Nests between 0 and 2 m height were localized using direct searching in the shrub and lianas in accordance with the observed behaviour of adult birds. Each nest was monitored until the nesting attempt failed or the young fledged successfully. Nests searching had been carried out for five days per week. Each located nest was marked with flags at least 5 m from the nest (Dion et al., 2000)and visited every three days (Buler and Hamilton, 2000). At each visit, nests were examined for signs of predation, including missing, dead, or partially consumed young, broken eggs and disturbed nest bowls. Predation was presumed to be the cause of nest failure when the nest had disappeared, was torn apart or when the entire contents of the nest were absent (Newmark and Stanley, 2011). It is assumed that the young would have fledged if signs of predation were absent and if nestlings were close to fledging during the previous visit (Dion et al., 2000). We specially named as “Egg + chick predation” a case of predation in which we could not determine which stage it happened. Bird species were identified with a reference field guide to African birds (Borrow and Demey, 2008).
To test the hypothesis that the vegetation parameters would influence the daily predation rate of the understorey bird nests, nest habitat features over two scales were measured. One scale consisted of the plants that supported the nests, the nest's position inside the plants and the immediate surroundings of the nesting site (microhabitat). The second scale covered the vegetation patch surrounding the nest (mesohabitat) (Mezquida, 2004). At the level of the microhabitat, information was collected based on the modification of the method of (Dion et al., 2000)and the method of Wray and Whitmore (1979)so as to note three indicators of nest-site vegetation since only shrub nests were found. At each nest depredated or fledged young, four 1 m bamboo poles serving as sample sticks were positioned horizontally on the ground around the nest. These bamboo poles formed a square plot with a nest at its centre. The first indicator was the percentage of vegetation cover around the nests which was visually estimated at the nearest 5%. The second was the maximum height of the vegetation above the nest which was estimated by observing the last contact of the plant with a one-metre pole placed on the nest and the third was the horizontal density of the trees based on the number of stems in the above-defined square. The following set of variables were also measured for all nests. These include the type of the supporting plant (liana or shrub), the height (estimated with the metre) of the nest on the supporting plant and the total visibility of the nest or concealment category (subjective score ranked from 0 = low to 3 = high). The latter was obtained as a sum of horizontal and vertical concealment (each scored as 0 = nest well visible from most directions; 1 = intermediate; 2 = nest not visible in any direction from a distance of c. 1 m) (Weidinger, 2002; Remeš, 2005). Finally, nest concealment scores (that is after the sum) were up to 10 (the highest score) for some nests
At the mesohabitat scale, the methods of Bobo et al. (2006)were adapted to sample the plant's parameters such as density and the basal area. Firstly, each of the above plots was divided into 400 (10 m x 10 m) subplots for overstorey plants data recorded. Then, 20 study plots were chosen systematically from the previous subplots so that, the distance between two subplots of the same line is 30 m and the distance between two lines containing the study subplots is
50 m (Additional File Figure S1). This resulted in a total of 600 subplots covering a sampling area of 60 000 m². Overstorey plants are defined as all trees of more than 10 cm in diameter at 1.3 m height (DBH) and understorey plants being all vascular plants of less than 1.3 m height as well as grasses etc. One m² (1 m × 1 m) quadrat was demarcated at the centre of each study subplot to collect understorey plants data. In agroforestry sites, cocoa/coffee trees were not measured, but their numbers (based on 3 m × 3 m as space for a cocoa/coffee tree) and size classes were estimated for each plot. All plants species were counted and identified at the morpho-species level. Only the most common trees and understorey plants were identified at the species level.
The classes of canopy cover were described as Clark and Clark (1992). Therefore, crown illumination indexes (or classes) were recorded for all trees placed at the corners (four) and the centre of each study subplot and the most encountered index were then adopted for the entire subplot. This index scores the source and relative amount of crown lighting. They are broadly equivalent to the estimation of canopy closure and are measured on an ordinal scale (Jennings et al., 1999)that is: Class 1, Class 1.5, Class 2, Class 2.5, Class 3, Class 4 and Class 5 where Class 1 is highest canopy cover and Class 5 is no canopy cover (Jennings et al., 1999)(Additional file Explanation Notes).
Statistical analyses
During the analyses, a nest was considered as an individual and only the predation of open nests types was considered because the sample size of enclosed nests was very small (only 4). Although these are built by different species, these nests were generally cup-shaped and were constructed on either shrubs or lianas so they would likely experience similar predation rates (Auer et al., 2007). Also, there were not significant differences between the clutch sizes of these understorey-nesting birds’ species (Kruskal-Wallis χ2= 17.261, df = 10, p = 0.068).
The daily predation rates (DPR) in different types of habitat were estimated using the adaptation of the logistic exposure model (Shaffer, 2004). The latter was implemented in R using a complementary log-log link with the package MASS (Venables and Ripley, 2002). The predation rates were also performed using the adaptation of the nesting success formula (Mayfield, 1975)as the predation of nests being the result of exposure events.
The Kruskal-Wallis rank sum test was performed to compare the mean number of nests between habitat types (Hollander et al., 2014). This test was also used to compare the mean DPR between types of habitat as well as to verify the dependence between types of predation (eggs and nestlings) and the types of habitat (Hollander et al., 2014). The Chi-Squared Test of equality of proportions with Yates’ correction (Yates, 1934)was conducted to compare the proportions of predated nests between different types of habitats and breeding stages (Wilson, 1927).
The effects of habitats and features of habitats on birds’ nests predation were assessed using the generalized linear mixed models (GLMM) in which species had random effects and vegetation variables and habitat types (selected in the best model using the AIC) viewed as fixed effects (Table 1) (McCullagh and Nelder, 1989). Only the vegetation variables that appeared in the best model was included in the GLMM. Vegetation variables included in the models were first transformed using the square root transformation techniques after Kolmogorov test (Conover, 1972). Mixed-effects models were created using package lme4 (Bates et al., 2014), with the explanatory variables incorporated as nested (hierarchical) Fixed effects and types of habitats as random effects (Table 1).
Akaike’s information criterion (AIC) Burnham and Anderson, (2003)was used to derive best fit top models in package AICcmodavg (Mazerolle, 2016)that is, models having variables that best explain the variation of the DPR. This calculated an AICc (bias-adjusted AIC for small sample sizes), ΔAICc (AICc of the alternative model – AICc of best models) and Akaike weight (wi) for each candidate model. We, first of all, choose several models by AIC in a Stepwise Algorithm (Venables and Ripley, 2002). Top fit models were chosen where there was enough strength of evidence to reject the alternative models. This was defined as the best model having an Akaike weight greater than that of the alternative models (Symonds and Moussalli, 2011).
Furthermore, to indirectly evaluate the factors stimulating the nesting sites choice of these birds along the gradient of increasing forest destruction, the mean trends of vegetation parameters having significant influences on the DPR were accessed using the Jonckheere-Terpstra test, an ordered non parametric test (Terpstra, 1952; Jonckheere, 1954)with the package “clinfun” version 1.0.14 (Seshan, 2017). All these analyses were performed in the R-Core software version 3.4.1 (R Foundation for Statistical Computing, 2017).
Active nests monitored in different types of habitat
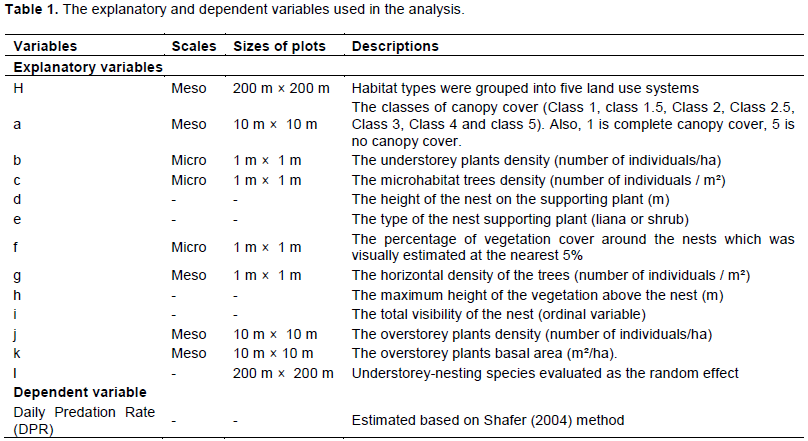
During the 2013 breeding season, we located and monitored 60 active nests of 12 understorey nesting bird species distributed inconsistently across the five habitats under studied (Table 2). All these nests were off the ground and were open-cup (56 nests) or enclosed (4 nests). No ground nests were found. The 56 open-cup nests surveyed belong to 11 different species and did not differ significantly along the gradient of increasing forest destruction (Kruskal-Wallis χ2 = 8.5195, df = 4, p = 0.074) although they seemed to be highest in the secondary forest.
Nest predation rates according to types of habitat and breeding phases
About 241 observations were made on the nests and only the open-cup nests were predated. About 47% of the open-cup nests monitored were predated. The proportions of predated nests did not vary significantly between types of habitats (χ2 = 4.192; df = 4; p = 0.381). However, the highest number of predated nests (43%) was found in the old secondary forest and the lowest in Annual culture farms (4%) (Figure 2A). Egg predation (77.78%), chick predation (18.52%), chick and egg predation (3.7%) were recorded in the study area (Figure 2B). There was a significant difference between the main types of predation in the study area (χ2= 137.051; df = 2; p < 0.0001). Also, variation on predation rates of eggs (χ2 = 5.556; df = 4; p = 0.234) and chicks (χ2 = 1.356; df = 4; p = 0.851) did not exist between types of habitats. Moreover, the frequency of predation varied significantly along the nesting phases (χ2 = 97.915; df = 2; p < 0.0001), with the nests being mostly predated during the incubation phase (71.43%) (Figure 2C).
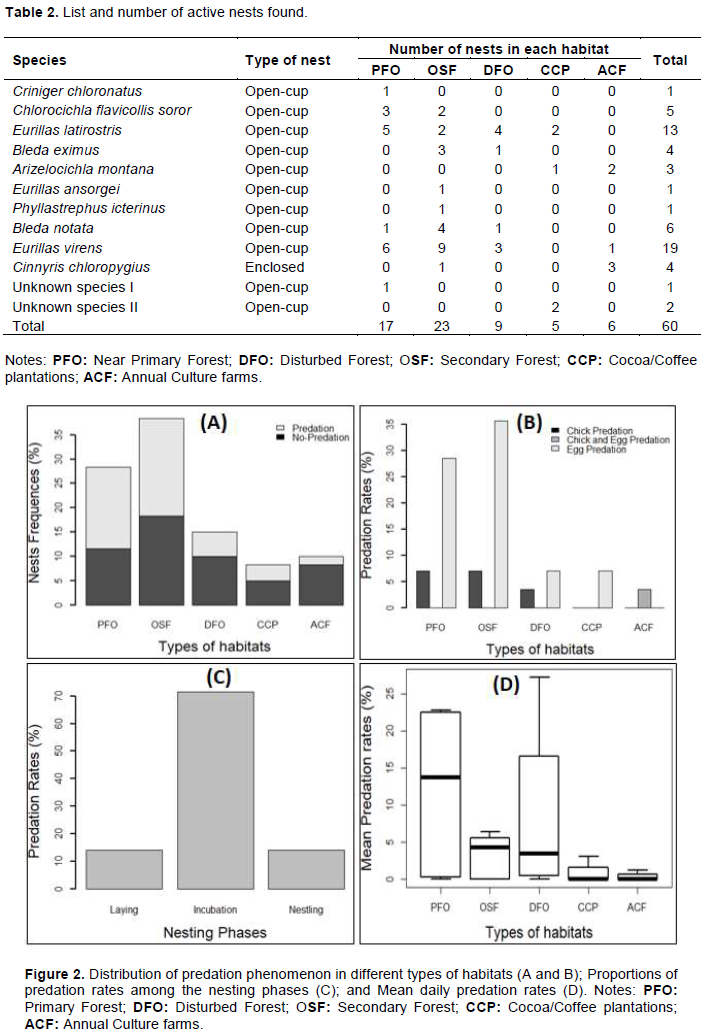
The average predation rate across the ecosystem varied between Ë‚ 0.001 and 27.18% but was not influenced significantly by the types of habitats (Kruskal- Wallis χ2 = 5.955, df = 4, p = 0.203). Nevertheless, this average predation rate seemd to be highest in primary forest (11.867%) and smallest in the cocoa/coffee plantations (1.539%), compared to other types of habitats (Figure 2 (D)). Also, when pooling the data into two habitats (that is natural forests vs disturbed habitats), the average predation rate did not vary significantly (Kruskal-Wallis χ2 = 2.188, df = 1, p = 0.139).
Influences of habitat features on the daily predation rate
The daily predation rate (DPR) of these understorey nesting birds varied between 0.019 and 0.964 but did not vary significantly amongst types of habitats (Kruskal-Wallis χ2 = 5.955, df = 4, p = 0.203). This daily predation rate did not vary significantly between natural forests and modified habitats (Kruskal-Wallis χ2 = 2.188, df = 1, p = 0.139). Table 2 shows top models according to changes in delta AIC as described by (Burnham and Anderson 2003). The best fit model, which is the one having the smallest value of the Aikake Information Criterion (AIC = 207.23) was chosen (Additional file Table S1). This model included quantitative variables (understorey plant density, microhabitat trees density) and a qualitative variable (classes of canopy cover) were then introduced as fixed effects in the generalized mixed effects model with the understorey-nesting species evaluated as the random effect (Table 3).
The density of trees in the microhabitat and the density of understorey plants had significant negative effects at the 95% significant level. To this effect, when the density of trees (n/m²) in the microhabitat and the density of the understorey plants (n/ha) increased, probabilities of the open-cup nest to be predated decreased for about 3.912e-01 and 8.749e-05 respectively (Figure 3A – B). Furthermore, the influences of the canopy cover classes and the types of nests support (shrub or liana) on the daily predation rate at 95% confidence interval were not significant, although the canopy cover classes 1.5 and 5 had negative effects. However, the canopy cover class 1 and the support had positive influences on the daily predation rate (Table 3). Moreover, very low correlations existed between most of the Fixed effects parameters on the daily predation rate (Additional file Table S2).
Furthermore, the trend in the average density of the trees in the microhabitat did not differ significantly along the gradient of increasing forest destruction (JT = 492.5, p = 0.301) whereas the average density of the understorey plants increased significantly (JT = 249.5, p = 0.011) along this gradient (Table 4). This indicates that the choice of the nesting sites depended on the presence of these vegetation parameters.
Number of active bird nests found across the types of habitat
The average number of open-cup nests varies significantly according to the types of habitats with the most degraded having the smallest number of nests. Similar results are found by numerous studies elsewhere in the world (Canaday, 1996; Castelletta et al., 2000). Even if derived forest habitats like cocoa plantations are not a habitat substitute for the forest, they provide habitat for many species, which depend to some degree on forests (Reitsma et al., 2001). Similarly, Van Bael et al. (2007)find that shaded cocoa farms can provide habitat for a wide variety of resident and migratory bird species. However, in the present study, it is not the case for nesting birds, because the less the habitat is disturbed, the more nests are present. This implies that the human activities reduce the probability of understorey nesting bird species of having available nesting sites. Finally, the absence (or rarity) of ground nests in our study area may be due to the presence of highly specialized ground nest predators or to the absence of ground-nesting bird species during sampling time. It could also be due to ground nests being difficult to find.
Predation rates across types of habitat and nesting phases
Predation is the main cause of nest failure in this study.
Similar results have been found in temperate (Debus, 2006; Tewksbury et al., 2006)and tropical regions (Githiru et al., 2005; Newmark and Stanley, 2011). In this study area, less than half of the nests are predated. This proportion is less than the one found outside the tropics (Mitchell et al., 1996; Tewksbury et al., 1998; Braden, 1999; Mezquida, 2004). These results, however, corroborate those of (Tewksbury et al., 2006)in temperate zones. Several reasons may explain this fact as the diversity of predators, because predation models depend on the response of different predator species, on the composition of the landscape and on the relative effects of these predators on bird species (Tewksbury et al., 2006).
The proportions of predated nests and the daily predation rate do not also vary significantly among types of habitat in our study area. Although the proportion of nests predated in the secondary forest seem to be highest while the proportion found in the annual crop fields was the smallest. This result can be associated with the higher number of active nests found in secondary forest. Moreover, the primary forest seems to have the highest daily predation rate while the secondary forest had the third-highest predation rate. This supports the results of Tewksbury et al. (1998)in temperate forests and contradicts those of Morse and Robinson (1999) in the neotropical forests in which rates of nest predation were significantly lower in the older forest than within even-aged clear-cuts. Moreover, when using artificial nests placed in the forest interior, at the edge and at the clear-cuts in the temperate ecosystem, (Rudnicky and Hunter Jr, 1993)have reported a similar trend. However, this predation model seems to contradict the general assumption that nest predation rate increases with ecosystem disruption due to the influx of predators from neighbouring habitats owing to the best conditions created by ecosystem degradation, as the birds will be more in danger in the heavily modified habitats than in the less degraded ones (Githiru et al., 2005; Pangau-Adam et al., 2006; Tewksbury et al., 2006). Furthermore, predation can be a problem in human-modified habitats if food supplies or nesting sites are reduced but can cope with high predation rates in natural systems (Martin and Clobert, 1996; Wesolowski and Tomialojc, 2005). However, the landscape of Korup National Park (the present study area) is different from other study sites (Githiru et al., 2005; Pangau-Adam et al., 2006; Tewksbury et al., 2006)because the dominant habitats are forests (primary and secondary forests) in terms of area and distribution and are less (or not) disturbed. So, the results obtained here could be completely different due to this landscape structure. This implies that predators arrive rather from modified areas, thus creating a surplus in abundance and diversity of these predators in the natural areas.
Over the course of the nesting cycle, we have found the greatest rate of nest loss in the incubation stage and least in the egg laying and nestling stages. Similar results were obtained in the forest understorey in South America (Ryder et al., 2008). But some studies recorded increasing rate of predation as nesting proceeded (Ryder et al., 2008; Brawn et al., 2011; Fu et al., 2016; Jiang et al., 2017). Increased proportion of predation (DPR) in the incubation phase suggests that nest losses can be ascribed to visually oriented predators as the eggs and chick predation type was recorded over the course of the study.
It can be also explained by lower nest attentiveness and by nesting birds in the early incubation phase. Moreover, decreased proportions of the predation in the laying and nestling stages suggest that, although the number of female trips increases during the nestling phase, nestlings are completely silent even during feeding bouts (Ryder et al., 2008).
Influences of habitat features on predation rates
Generally, habitat quality appears to affect breeding success (Debus, 2006)and birds are mostly scrupulous in their choice of nesting sites (Martin, 1993b). It seems that anthropogenic land management disturbs habitat quality by removing key elements for nesting individuals (Debus, 2006). Many studies have shown that several characteristics (grass cover, height of the nest, percentage of sky visible, detectability index, forb cover, vegetation height around the nest, etc.) of nesting sites have no effect on nest predation rates (Braden 1999; Dion et al., 2000; Githiru et al., 2005; Posa et al., 2007). However, Fu et al. (2016)report that the nest-site-selection variables (tree cover, bamboo cover, liana abundance, etc.) are positively associated with predation. In our study area, only the density of the microhabitat trees and the density of the understorey plants have a significant negative correlation with the daily predation rate.
Moreover, the density of the microhabitat trees does not vary significantly according to the gradient of the increase of forest destruction whereas the density of the understorey plants increases sharply according to this gradient. This explains why annual crop fields, despite their very high level of degradation, might have some secured nesting sites as well. This implies that birds always choose the best nesting site regardless of the degree of disturbance (Martin, 1993b). These results also suggest that bird nest predators in the understorey are more active in relatively stable habitats such as primary forest and secondary forest. These can also be due to the fact that nests might be easier to be found in the natural forests as compared to modified habitats.
In conclusion, our findings support the prediction that well-hidden bird nests are less subjected to predation (Vergara and Simonetti, 2004)and nest-site selection is non-random. As opposed to other findings (Dion et al., 2000; Estrada et al., 2002; Debus, 2006), the predation of understorey bird nests in our study area seems not to be affected by the gradient of increasing habitat destruction. Only the density of trees in the microhabitat and the density of the understorey trees have significant negative effects on the daily predation rates of the open-cup nests.
These observations also suggest that this support zone adjacent to the Korup National Park is an important additional breeding habitat for the understorey bird species. Furthermore, in order to consider understorey nesting birds conservation in Korup, sets of timber harvesting guidelines designed to mitigate the deleterious environmental impacts of tree felling, yarding, and hauling known as “reduced-impact logging” techniques (Sist et al., 2003; Putz et al., 2008)must be applied for future trees harvesting practices. Although the effectiveness of reduced-impact logging in reducing tree destruction is limited under high felling intensity (>8 trees/ha) these techniques are better than the conventional techniques (Sist et al., 2003).
The authors have not declared any conflict of interests.
The authors appreciate the support of the Volkswagen Project, “Managing Forest Wildlife for Human Livelihoods in the Korup-Oban Hills region, West- Central Africa” as well as the support from IDEA WILD. They are also grateful to the inhabitants of the three villages (Mgbegati, Abat and Basu) around our study area for their contributions. Final thanks to Mr Haman Missa and some anonymous reviewers for comments and critics.
REFERENCES
|
Aldinger K, Terhune II, Wood P, Buehler D, Bakermans M, Confer J, Flaspohler D, Larkin J, Loegering J, Percy K (2015). Variables associated with nest survival of Golden-winged Warblers (Vermivora chrysoptera) among vegetation communities commonly used for nesting. Avian Conservation and Ecology 10:6.
Crossref
|
|
|
|
Auer SK, Bassar RD, Fontaine JJ, Martin TE (2007). Breeding biology of passerines in a subtropical montane forest in northwestern Argentina. The Condor 109:321-333.
Crossref
|
|
|
|
|
Bates D, Mächler M, Bolker B, Walker S (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823.
|
|
|
|
|
Bellamy PE, Burgess MD, Mallord JW, Cristinacce A, Orsman CJ, Davis T, Grice PV, Charman EC (2018). Nest predation and the influence of habitat structure on nest predation of Wood Warbler Phylloscopus sibilatrix, a ground-nesting forest passerine. Journal of Ornithology 159:493-506.
Crossref
|
|
|
|
|
Bobo KS (2004). Birds as indicators of biodiversity change in tropical landscapes. A case study from the Korup Region, Western Cameroon. Master thesis, The University of Gottingen.
|
|
|
|
|
Bobo SK (2007). From forest to farmland: Effects of land use on understorey birds of Afrotropical rainforests. PhD thesis, The University of Gottingen.
|
|
|
|
|
Bobo KS, Waltert M, Sainge NM, Njokagbor J, Fermon H, Mühlenberg M (2006). From forest to farmland: species richness patterns of trees and understorey plants along a gradient of forest conversion in Southwestern Cameroon. Biodiversity and Conservation 15:4097-4117.
Crossref
|
|
|
|
|
Borrow N, Demey R (2008). Guide des oiseaux de l'Afrique de l'Ouest. Delachaux et Niestlé.
|
|
|
|
|
Braden GT (1999). Does nest placement affect the fate or productivity of California Gnatcatcher nests? The auk 116:984-993.
Crossref
|
|
|
|
|
Bradley JE, Marzluff JM (2003). Rodents as nest predators: influences on predatory behavior and consequences to nesting birds. The Auk 120:1180-1187.
Crossref
|
|
|
|
|
Bradshaw CJ, Sodhi NS, Brook BW (2009). Tropical turmoil: a biodiversity tragedy in progress. Frontiers in Ecology and the Environment 7:79-87.
Crossref
|
|
|
|
|
Brawn JD, Angehr G, Davros N, Robinson WD, Styrsky JN, Tarwater CE (2011). Sources of variation in the nesting success of understory tropical birds. Journal of Avian Biology 42:61-68.
Crossref
|
|
|
|
|
Buler JJ, Hamilton RB (2000). Predation of natural and artificial nests in a southern pine forest. The Auk 117:739-747.
Crossref
|
|
|
|
|
Burnham KP, Anderson DR (2003). Model selection and multimodel inference: a practical information-theoretic approach. Springer Science and Business Media.
|
|
|
|
|
Canaday C (1996). Loss of insectivorous birds along a gradient of human impact in Amazonia. Biological Conservation 77:63-77.
Crossref
|
|
|
|
|
Castelletta M, Sodhi NS, Subaraj R (2000). Heavy extinctions of forest avifauna in Singapore: lessons for biodiversity conservation in Southeast Asia. Conservation Biology 14:1870-1880.
Crossref
|
|
|
|
|
Chalfoun AD, Martin TE (2009). Habitat structure mediates predation risk for sedentary prey: experimental tests of alternative hypotheses. Journal of Animal Ecology 78:497-503.
Crossref
|
|
|
|
|
Chalfoun AD, Thompson FR, Ratnaswamy MJ (2002). Nest predators and fragmentation: a review and meta-analysis. Conservation biology 16:306-318.
Crossref
|
|
|
|
|
Clark DA, Clark DB (1992). Life history diversity of canopy and emergent trees in a neotropical rain forest. Ecological monographs 62:315-344.
Crossref
|
|
|
|
|
Conover WJ (1972). A Kolmogorov goodness-of-fit test for discontinuous distributions. Journal of the American Statistical Association 67:591-596.
Crossref
|
|
|
|
|
Cordeiro NJ, Borghesio L, Joho MP, Monoski TJ, Mkongewa VJ, Dampf CJ (2015). Forest fragmentation in an African biodiversity hotspot impacts mixed-species bird flocks. Biological Conservation 188:61-71.
Crossref
|
|
|
|
|
Debus SJS (2006). Breeding-habitat and nest-site characteristics of Scarlet Robins and Eastern Yellow Robins near Armidale, New South Wales. Pacific Conservation Biology 12:261-271.
Crossref
|
|
|
|
|
Dion N, Hobson KA, Larivière S (2000). Interactive effects of vegetation and predators on the success of natural and simulated nests of grassland songbirds. The Condor 102:629-634.
Crossref
|
|
|
|
|
Djomo Nana E, SedláÄek O, Vokurková J, HoÅ™ák D (2014). Nest position and type affect predation rates of artificial avian nests in the tropical lowland forest on Mount Cameroon. Ostrich 85:93-96.
Crossref
|
|
|
|
|
Estrada A, Rivera A, Coates-Estrada R (2002). Predation of artificial nests in a fragmented landscape in the tropical region of Los Tuxtlas, Mexico. Biological Conservation 106:199-209.
Crossref
|
|
|
|
|
Fishpool LD, Evans MI (2001). Important Bird Areas in Africa and associated islands: Priority sites for conservation. BirdLife International Cambridge, UK, Cambridge, UK.
|
|
|
|
|
Fu Y, Chen B, Dowell SD, Zhang Z (2016). Nest predators, nest-site selection and nest success of the Emei Shan Liocichla (Liocichla omeiensis), a vulnerable babbler endemic to southwestern China. Avian Research 7:18.
Crossref
|
|
|
|
|
Githiru M, Lens L, Cresswell W (2005). Nest predation in a fragmented Afrotropical forest: evidence from natural and artificial nests. Biological Conservation 123:189-196.
Crossref
|
|
|
|
|
Hollander M, Wolfe DA, Chicken E (2014). Nonparametric Statistical Methods, 3rd Edn. John Wiley and Sons.
|
|
|
|
|
Jennings SB, Brown ND, Sheil D (1999). Assessing forest canopies and understorey illumination: canopy closure, canopy cover and other measures. Forestry: An International Journal of Forest Research 72:59-74.
|
|
|
|
|
Jiang A, Jiang D, Zhou F, Goodale E (2017). Nest-site selection and breeding ecology of Streaked Wren-Babbler (Napothera brevicaudata) in a tropical limestone forest of southern China. Avian Research 8 p.
Crossref
|
|
|
|
|
Jonckheere AR (1954). A distribution-free k-sample test against ordered alternatives. Biometrika 41:133-145.
Crossref
|
|
|
|
|
Martin TE (1993a). Nest predation among vegetation layers and habitat types: revising the dogmas. The American Naturalist 141:897-913.
Crossref
|
|
|
|
|
Martin TE (1993b). Nest predation and nest sites. BioScience 43:523-532.
Crossref
|
|
|
|
|
Martin TE, Clobert J (1996). Nest predation and avian life-history evolution in Europe versus North America: a possible role of humans? The American Naturalist 147:1028-1046.
Crossref
|
|
|
|
|
Mayfield HF (1975). Suggestions for calculating nest success. The Wilson Bulletin 87:456-466.
|
|
|
|
|
Mazerolle MJ (2016). AICcmodavg: Model selection and multimodel inference based on (Q) AIC (c)[Software].
|
|
|
|
|
McCullagh P, Nelder AJ (1989). Generalized Linear Models, Second Edition (Chapman and Hall/CRC Monographs on Statistics and Applied Probability). Chapman. and Hall, London., London.
Crossref
|
|
|
|
|
Mezquida ET (2004). Nest site selection and nesting success of five species of passerines in a South American open Prosopis woodland. Journal of Ornithology 145:16-22.
Crossref
|
|
|
|
|
Mitchell MC, Best LB, Gionfriddo JP (1996). Avian nest-site selection and nesting success in two Florida citrus groves. The Wilson Bulletin pp. 573-583.
|
|
|
|
|
Morse SF, Robinson SK (1999). Nesting success of a neotropical migrant in a multiple-use, forested landscape. Conservation Biology 13:327-337.
Crossref
|
|
|
|
|
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, Choimes A, Collen B, Day J, De Palma A, Díaz S, Echeverria-Londo-o S, Edgar MJ, Feldman A, Garon M, Harrison MLK, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, Kleyer M, Correia DLP, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HRP, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, Mace GM, Scharlemann JPW, Purvis A (2015). Global effects of land use on local terrestrial biodiversity. Nature 520:45.
Crossref
|
|
|
|
|
Newmark WD, Stanley TR (2011). Habitat fragmentation reduces nest survival in an Afrotropical bird community in a biodiversity hotspot. Proceedings of the National Academy of Sciences 108:11488-11493.
Crossref
|
|
|
|
|
Norris K, Asase A, Collen B, Gockowksi J, Mason J, Phalan B, Wade A (2010). Biodiversity in a forest-agriculture mosaic–The changing face of West African rainforests. Biological conservation 143:2341-2350.
Crossref
|
|
|
|
|
Pangau-Adam MZ, Waltert M, Mühlenberg M (2006). Nest predation risk on ground and shrub nests in forest margin areas of Sulawesi, Indonesia. Biodiversity and Conservation 15:4143-4158.
Crossref
|
|
|
|
|
Posa MRC, Sodhi NS, Koh LP (2007). Predation on artificial nests and caterpillar models across a disturbance gradient in Subic Bay, Philippines. Journal of Tropical Ecology 23:27-33.
Crossref
|
|
|
|
|
Putz FE, Sist P, Fredericksen T, Dykstra D (2008). Reduced-impact logging: challenges and opportunities. Forest ecology and management 256:1427-1433.
Crossref
|
|
|
|
|
R Foundation for Statistical Computing (2017). R: The R Project for Statistical Computing. https://www.r-project.org/
|
|
|
|
|
Reitsma R, Parrish JD, McLarney W (2001). The role of cacao plantations in maintaining forest avian diversity in southeastern Costa Rica. Agroforestry Systems 53:185-193.
Crossref
|
|
|
|
|
Remeš V (2005). Nest concealment and parental behaviour interact in affecting nest survival in the blackcap (Sylvia atricapilla): an experimental evaluation of the parental compensation hypothesis. Behavioral Ecology and Sociobiology 58:326-332.
Crossref
|
|
|
|
|
Rodewald PG, Dejaifve P-A, Green AA (1994). The birds of Korup National Park and Korup Project Area, Southwest Province, Cameroon. Bird Conservation International 4:1-68.
Crossref
|
|
|
|
|
Rudnicky TC, Hunter Jr ML (1993). Avian nest predation in clearcuts, forests, and edges in a forest-dominated landscape. The Journal of wildlife management 57:358-364.
Crossref
|
|
|
|
|
Ryder TB, Durães R, Tori WP, Hidalgo JR, Loiselle BA, Blake JG (2008). Nest survival for two species of manakins (Pipridae) in lowland Ecuador. Journal of Avian Biology 39:355-358.
Crossref
|
|
|
|
|
Serle W (1981). The breeding season of birds in the lowland rainforest and in the montane forest of West Cameroon. Ibis 123:62-74.
Crossref
|
|
|
|
|
Seshan VE (2017). clinfun: Clinical Trial Design and Data Analysis Functions version 1.0.14 from CRAN.
|
|
|
|
|
Shaffer TL (2004). A unified approach to analyzing nest success. The Auk 121:526-540.
Crossref
|
|
|
|
|
Sist P, Sheil D, Kartawinata K, Priyadi H (2003). Reduced-impact logging in Indonesian Borneo: some results confirming the need for new silvicultural prescriptions. Forest Ecology and Management 179:415-427.
Crossref
|
|
|
|
|
Sodhi NS, Liow LH, Bazzaz FA (2004). Avian extinctions from tropical and subtropical forests. Annual Review of Ecology, Evolution, and Systematics 35:323-345.
Crossref
|
|
|
|
|
Symonds MR, Moussalli A (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behavioral Ecology and Sociobiology 65:13-21.
Crossref
|
|
|
|
|
Terpstra TJ (1952). The asymptotic normality and consistency of Kendall's test against trend, when ties are present in one ranking. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen 55:327-333.
Crossref
|
|
|
|
|
Tewksbury JJ, Hejl SJ, Martin TE (1998). Breeding productivity does not decline with increasing fragmentation in a western landscape. Ecology 79:2890-2903.
Crossref
|
|
|
|
|
Tewksbury JJ, Garner L, Garner S, Lloyd JD, Saab V, Martin TE (2006). Tests of landscape influence: nest predation and brood parasitism in fragmented ecosystems. Ecology 87:759-768.
Crossref
|
|
|
|
|
Thomas D (1995). Botanical Survey of the Rumpi Hills and Nta Ali. Report to Korup Project.
|
|
|
|
|
Van Bael SA, Bichier P, Ochoa I, Greenberg R (2007). Bird diversity in cacao farms and forest fragments of western Panama. Biodiversity and Conservation 16:2245-2256.
Crossref
|
|
|
|
|
Venables WN, Ripley BD (2002). Modern Applied Statistics with S, W.N. Venables, Springer. Springer, Oxford OX1 3TG Australia England bill, Oxford OX1 3TG Australia England bill.
|
|
|
|
|
Vergara PM, Simonetti JA (2003). Forest fragmentation and rhinocryptid nest predation in central Chile. Acta Oecologica 24:285-288.
Crossref
|
|
|
|
|
Vergara PM, Simonetti JA (2004). Does nest-site cover reduce nest predation for rhinocryptids? Journal of Field Ornithology 75:188-191.
Crossref
|
|
|
|
|
Weidinger K (2002). Interactive effects of concealment, parental behaviour and predators on the survival of open passerine nests. Journal of Animal Ecology 71:424-437.
Crossref
|
|
|
|
|
Wesolowski T, Tomialojc L (2005). Nest sites, nest depredation, and productivity of avian broods in a primeval temperate forest: do the generalisations hold? Journal of Avian Biology 36:361-367.
Crossref
|
|
|
|
|
Wilson EB (1927). Probable inference, the law of succession, and statistical inference. Journal of the American Statistical Association 22:209-212.
Crossref
|
|
|
|
|
Wray TII, Whitmore RC (1979). Effects of vegetation on nesting success of Vesper Sparrows. The Auk pp. 802-805.
|
|
|
|
|
Yates F (1934). Contingency tables involving small numbers and the χ 2 test. Supplement to the Journal of the Royal Statistical Society 1:217-235.
Crossref
|
|