ABSTRACT
Broodstock development, spawning, fertilization, embryonic development, fecundity, “hatching rate and deformation rates” of five species (Amphiprion ocellaris, A. percula, A. melanopus, A. clarkii and A. nigripes) of anemone fish were studied in captive condition. The egg diameter, parental care, fecundity rate, fertilization rate, hatching rate, deformation rates and size of eggs were quantified. In addition, the courtship behaviors and parental care of each broodstock were noted. The day wise embryonic developments from 1st day to 8th day were observed under microscope and major morphological and functional features of embryos were documented. The egg size ranged from 2.2 to 3.1 mm in length and width of 1.1 to 1.3 mm. The numbers of spawned eggs were varied between 405 to 810 numbers. Spawning was achieved in every 15 to 20 days of interval in summer and in monsoon it was increased ranging from 20 to 40 days. The clutch diameter ranged from 5.2 to 14. 3 cm in length and width of 4.8 to 11.5 cm.
Key words: Anemone fish, embryonic development, fecundity, fertilization, hatching, deformation.
Tropical and subtropical countries are exports marine ornamental species for the aquarium trade (Olivotto et al., 2003). Today, owing to the increasing petition, many studies was carried on ornamental fishes, particularly, larval rearing and nutrition have been performed (Avella et al., 2007) and due to their notable colour and some remarkable behavioral features provides the important place in aquarium trade such as symbiotic with sea anemones (Fautin and Allen, 1997), formation of a group consisting monogamous pairs and protandrous hermaphrodites (Ross, 1978). Because of their main characteristic features, they won hearts of millions of people such as adaptability, easiness to be fed with artificial diets and their fascinating display behavior (Ignatius et al., 2001). However, among the coral reef fishes, anemone fishes belonging to the family, Pomacentridae and subfamily Amphiprioninae are abundant and about 30 species have been recognized under two genera, Amphiprion and Premnas (Allen et al., 2010).
The development of hatchery production techniques, it is necessary to understand the entire ontogeny of a particular fish in the aquarium. Ontogeny of fish can be divided into several periods of time such as embryonic (laying to Hatching), larval, juvenile, sub-adults and adult. For researchers, embryonic development of a particular fish is very important in terms of reproduction biology (Balon, 1981: Urho, 2002). Dhaneesh et al. (2009) documented that breeding patterns and behavioural aspects of many tropical and subtropical Pomacentrid fishes have been recognized well and the description of eggs, embryological development, larval rearing and juvenile production are available for relatively few Pomacentrid fishes, and Ajith Kumar et al. (2012) were resulted his breeding experiments in Lakshadweep waters. Falk-Petersen (2005) resulted that, development of early life stage, from fertilization to embryo formation among Teleost ï¬sh, generally follows the same pattern.
Spawning and behavioural aspects of many Pomacentrid species have been well documented by previous researchers (Alva and Gomes, 1989; Wilkerson, 2001; Overton et al., 2008; Dhaneesh et al., 2009; Ajith Kumar and Balasubramanian, 2009; Swagat et al., 2012; Uthaya Siva et al., 2014). However, description of ova, embryonic development, larval rearing and production of juveniles are available only for a few Pomacentrids and are scarce in the case of species dwelling in Indian waters. Hatching and chronological flow of embryonic developmental stages were differing depending on the species and speciï¬c environmental conditions in which they take place (Dhaneesh et al., 2009). Chen stated that the free embryo phase begins when the embryo is free of egg membranes and the beginning of the larval period is indicated when the larvae are able to capture feed objects (Chen, 2005).
Culture practice is the only eco-friendly way to conserve ornamentals. It will hopeful to understand about egg development, importance of parental and their high trade value. Since the study notably on anemone fish embryonic development are not much popular. In the present study provide basic information on day wise embryonic development to enhance captive production of anemone fishes.
Collection and pair formation of anemone fish
Anemone fish species (10 individuals for each species) of Amphiprion ocellaris, A. percula, A. melanopus, A. clarkii and A. nigripes were (5.2 to 7.2 cm TL) procured from the ornamental fish traders. Immediately after fish arrival in the hatchery, they were acclimatized in 5000 L rectangular cement tank filled with 1000 L water and sea anemones, Stichodactyla haddoni and Heteratics magnifica were also introduced in the same tank. Pair of A. ocellaris, A. percula, A. clarkii and A. nigripes with Stichodactyla haddoni and Amphiprion melanopus with Heteratics magnifica was obtained, after three to eight months of rearing and they were transferred into the Fiberglass Reinforced Plastics (FRP) tanks with 750 L (Swagat et al., 2012).
Water quality and feeding habits
The estuarine water was filtered through sand filter and followed with UV treatment and quality water was purified by flowing. The water quality parameters were maintained as salinity 24-30 ± 1 ppt, pH 8.1 ± 0.2, temperature 26.5 ± 0.5°C, dissolved oxygen <5.0 ± 0.4 ml/L, and ammonia 0.01 ppm. The fishes were fed three times in a day at 07:00, 11:00, 16:00 at 5% of their body weight and they were fed with mixed diet (boiled oyster, shrimps meat). After feeding, excess feeds and waste materials were confiscated from the tank through siphoned. The water exchange was done once in a week (30-40%) for elimination of fouling organisms like barnacles, algal communities and other dust materials settled in the tank. Sea anemones S. haddoni and Heteratics magnifica were fed with post larval form of shrimps in a day interval.
“Embryonic development”
The eggs were sampled randomly (5 eggs/day) to document the embryonic development. They were placed on sterile glass slide with UV filtered brackish water for record morphological development based of method of Swagat et al. (Ignatius et al., 2001; Swagat et al., 2012). The photographs were taken with a digital camera (Sony cyber-shot G, China) under the light microscope (Novex, Holland) from day 1 to 8 for documenting major morphological and functional features of embryos. Milky white eggs found in the sample were considered as dead or unfertilized and were removed.
“Estimation of fecundity, fertilization, hatching and deformation rate”
Developed broodstock were tested for their efficiency of fecundity, fertilization, hatching and deformation rate using the formula (Gunasekara et al., 1996):
Fecundity rate =π (R1+R2) ×Number of eggs/1cm
Where R1= clutch width and R2= clutch length.
Fertilization rate = No of eggs fertilized / No of total eggs laid × 100
Hatching rate = No of eggs hatched / No of total eggs fertilized ×100
Deformation rate= No of deformed (dead or unhealthy) larvae / No of larvae hatched ×100
“Broodstock development”
After obtaining successful pair formation of five species (Figure 1) in the period of 6 to 8 months and brooders were spawned every 15 days intervals. It was observed that bigger fish (female) in the tank always dominant and occupied the earthen pots, which they defended aggressively and also fed first during the feeding. Females of A. nigripes and A. percula matured shortly and it stimulated the male by bruising (cleaning) many locations near by hiding place such as sea anemone to showed the willingness. Each spawning tank was provided with substratum like tiles, dead corals and pipes for egg deposition which copies the natural environment. Before spawning, the pairs show their courtship behaviours which are precise in the male showed morphological and behavioral changes. Courtship began
2 days before spawning especially A. Sebae and A. clarkii, with the female initiating by displaying a range of behaviours that included vertical dives, ferocious swimming signals and clinging to the bottom of the rearing tank and no colour changes were observed during spawning.
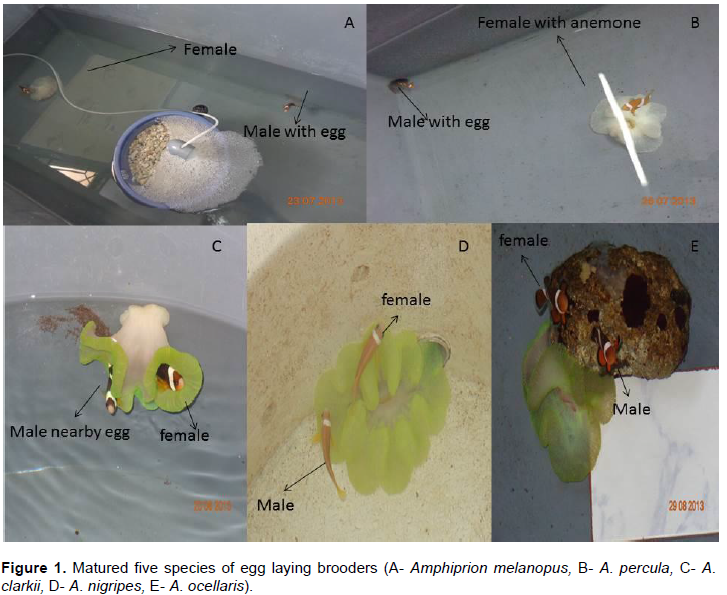
“Spawning and parental care”
The brooders were brushed the algae and debris, when thought to lay their eggs, which is more closely to the sea anemone. There was a result all the anemone fishes preferred nearby sea anemone and at time of before spawning, the abdomen of female became larger and its took large quantity of feed. Most of the spawning occurred in the morning (8:00-9:00 h) for A. melanopus and A. nigripes and in some fishes were in the afternoon (15:00-16:00 h) in case of A. clarkii, A. percula and A. ocellaris. While female deposit their eggs and followed by the male releasing spermatozoa for fertilization. The spawning process was extended 50 min to 1 h for completing the entire clutch fertilization. The colour of newly laid eggs was varied from bright orange to yellowish in most of the anemone fishes but in case of A. melanopus dark orange to reddish in colour was observed on first day.
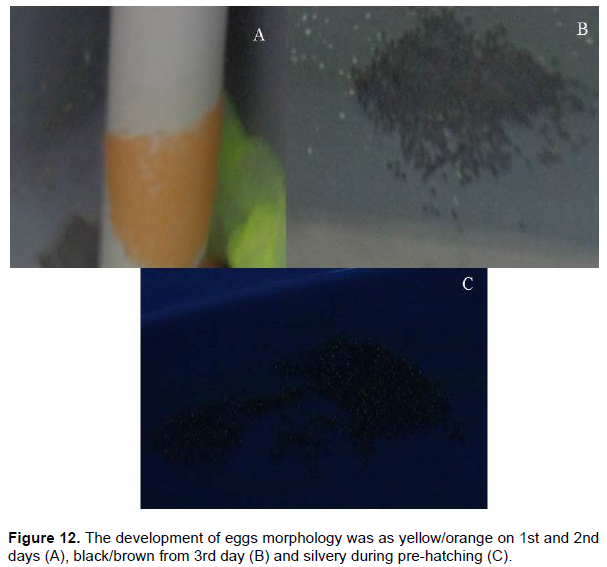
The development of eggs were noted morphologically on color changes, were yellow/orange on 1st and 2nd days, black/brown from 3rd day and silvery during pre-hatching. The eggs were adhesive, covering with transparent chorion with narrow perivitalline space. Egg diameters were measured and surface of the egg capsule was smooth. Thereafter major role in parental care (fanning or mouthing) was mainly demonstrated by males in all the species and partially done by female, which created a cooling effect as well as suitable environment to the clutch that reduced the damage of egg mass. Continuous fanning was observed by male but in case of female only 2 attempts / hour (during first 3 days) was observed. The longest and shortest duration of single time fanning by the males (includes mouthing) were 1.25
and 0.21 min. In the case of females, maximum and minimum fanning duration were 0.57 and 0.40 min respectively. During the fanning and mouthing the unfertilized, dead eggs and dust particles were also removed by the process of mouthing.
“Egg size, shape and clutch diameter”
All the five species laid their eggs in spherical shape of clutches. The egg size ranged from 2.2 to 3.1 mm in length with a width of 1.1 to 1.3 mm (Table 1). The number of eggs produced varied between 405 and 810 numbers. Per spawning for per pair and spawning was archived at every 15 to 20 days in summer and in monsoon it was varied between 20 and 40 days. The clutch diameter ranged from 5.2 to 14. 3 cm in length and width of 4.8 to 11.5 cm.
“Fecundity and fertilization rate”
The estimated fecundity and fertilization rate was presented in the Figures 7 and 9. Maximum fecundity rate was observed in A. clarkii and minimum was observed in A. melanopus after spawning. Fertilization rate was more or less similar in all the five species of anemone fishes and it was maximum in A. clarkii and minimum in A. percula.
“Embryonic development” of anemone fishes
The general pattern of day wise embryonic development of five anemone fishes (Figure 2 to 6), from fertilization to hatching was classified into 7 to 8 days. During the development of the embryo is entirely depends on the nutrition provided by the mother, mainly by the form of yolk.
First day
The cytoplasm of the fertilized egg was clear in day 1 embryo, yolk and different sizes of maximum number of oil globules were dispersed in it. One end of the egg was identified as animal pole attached to the substrata (earthen pot and tiles) with a tuft of filamentous stalk or cilia with glutinous substance and cell cleavage observed at animal pole (Dhaneesh et al., 2009).
Second day
After 24 h, the cleavage was completely stopped and oil globules were moved at opposite end from the animal pole. On the bottom of yolk, head bud, below head bud and perivitalline space was clearly observed in A. ocellaris, A. nigripes, and A. clarkii but in case of A. melanopus and A. percula, head bud was not observed. It is the gastrula stage in which the blastomeres were extended towards the vegetal pole and the numbers of fat globules decreased.
Third day
The embryo showed the clear visible head, mytosomes and tail. The body was transparent having no muscular structure and heart beet was clearly observed in all the four species except A. percula in which head bud and perivitalline space only developed.
Fourth day
The embryo exhibited eye, melanopores development and the tail became separated from the yolk and moved freely but the body was still attached to the yolk. On the microscope, utilization of yolk was clearly observed while, clear blood circulation.
Fifth day
The mouth and eye were evident for blood circulation through the vessels could be observed. It indicated the functioning of circulating system of the embryo.
Sixth day
In embryo,head and tail were clearly separated from the yolk. Thereafter, embryo was further enlarged and occupied most of the space within the capsule; pectoral fin development was also observed.
Seventh day
On the day, yolk sac became quite small and covered by the abdomen of the embryo. The melanophores are distributed throughout the body. Fins and eyes are well developed in A. clarkii and egg clutch showed silvery in colour. On the day night 19:00 to 20:00 h and the larva are free from the capsule.
Eighth day
Fins and eyes are well developed; in this stage the embryo began to hatch by moving itself vigorously to break the capsule.
Ninth day
Embryo was free from the capsule and became a larva on day 9. The dorsal fin, caudal fin and anal fin were continuous in a longitudinal line.
“Hatching”
Hatching was took place on 8 to 9th days of post- fecundation during the first 2 h of darkness. The dorsal, caudal and anal fins were continuous in a longitudinal line. The distal end of the chorion becomes soft and pliable and the movements of the embryo became more frequent and violent until the weakness chorion gives way. The newly hatched larva had a transparent body, large eyes, open mouth and a small yolk sac. Immediately after hatching, the larvae were found floating on the surface vertically with up head position and larval size ranging from 3.7 to 4.4 mm in length. After 3 to 5 h, the larvae were transferred to 50 L FRP larval rearing tanks with stocking density of 5 individuals l-1 and it could consume rotifer (B. plicatilis-130-340 µm) and zooplankton as its primary food.
“Incubation time, hatching rate and deformation rate”
The estimated incubation time, hatching rate and deformation rate were presented in the Figure 8, 10 and 11. The incubation time was varied between the species, A. clarkii showed minimum incubation time than others and maximum incubation time was noted in A. melanopus. Hatching rate was maximum in A. percula and minimum in A. nigripes. Deformation rate was maximum in A. nigripes and minimum in A. melanopus.
Breeding of anemone fishes in hatchery holds minimum challenges as compared to the other marine ornamental fishes and many breeding experiments conducted successfully in hatcheries on different species of anemone fishes (Alva and Gomes, 1989; Wilkerson, 2001; Overton et al., 2008; Dhaneesh et al., 2009; Ajith Kumar and Balasubramanian, 2009; Swagat et al., 2012; Uthaya Siva et al., 2014). However, there would be certain limitations in culture practices of anemone fish in private aquaria. Therefore, the present study was analyzed about embryonic development and spawning efficiency of five different anemone fish as fecundity rate in captivity. Boby Ignatius et al. (2001) observed that the brooders fed with clam meat and marine polycheate attained the sexual maturity within 3 months of maintenance and spawned repeatedly when compared with those pairs fed with minced fish meat alone (Dehasque et al., 1995). In the present study, boiled clam, shrimp meet and commercial pellet feeds were provided for maturation of brooders to balance the broodstock nutrition.
Dehasque et al. (1995) stated from his result that feed consumption showed the capacity of egg laying power of brooders. On the other hand, co-feeding of live feeds supplemented with lipid and vitamin formulation helped to increase the nutritional reserves of broodstock and hence to improve the overall egg and larval quality (Allen, 1974) because in aquaculture spawning and proper embryo development was related to broodstock nutrition and high quality breeders were essential for successful larval rearing (Fautin and Allen, 1997; Jobling, 2002). The present study resulted that fertilization, hatching and deformation rate of larvae were depends on several factors (feed quality, brooder health and environmental parameters) not only because of brooders. Although, the brooders were maintained in the backwater (with salinity variation) showed similar fecundity and spawning frequency to those kept in running seawater (Alva and Gomes, 1989; Wilkerson, 2001; Overton et al., 2008; Dhaneesh et al., 2009; Ajith Kumar and Balasubramanian, 2009; Swagat et al., 2012; Uthaya Siva et al., 2014).
In addition, several factors such as temperature, salinity, ammonia level, dissolved oxygen, pH, light intensity and photoperiod were known to affect the growth and development of anemone fish (Alva and Gomes, 1989). In the extant study also said that the water quality parameters were influenced on growth as well as breeding of anemone fish thus, present study maintained in optimum level as temperature 27±2°C, salinity 29±3 ppt, ammonia level 0.001 ppm, dissolved oxygen 4±2 ppm, pH 7.7±0.2, light intensity 18 µ mol-1sec-1and photoperiod 13 L: 11 D. Unless other parameters, temperature was mainly greatly influenced on embryo development. During summer, incubation was observed at 7 days but in monsoon it was extended to 8 days because development of embryo taken additional time than summer, it indicated temperature (summer-27°C, monsoon-24°C) was the important environmental parameter involved in embryonic development. Spawning ratio was maximum in summer because normal temperature was observed and minimum in monsoon.
According to Jobling (2002) and Moyle and Cech (2004) the embryonic developmental period was the phase between fertilization and commencement of organogenesis. The contour of eggs in different species of Pomacentridae ï¬sh varies from oval to capsule shape (Moyer and Nakazono, 1978; Pathiyasevee, 1994; Balamurugan et al., 2013; Hoff, 1996; Uthayasiva et al., 2014). Similarly, the clutches of anemonefishes were in oval shape. The clutch diameter demonstrated the fecundity rate of a particular species was found as per formula. Balamurugan et al. (2013) documented the development of eggs were noted morphologically on color changes, were yellow/orange on 1st and 2nd days, black/brown from 3rd day and silvery during pre-hatching. No color change in the eggs on 3rd and 4th days was considered immature/decayed (Hoff, 1996; Melville and Griffiths, 1997). Similarly, the egg clutches of anemone fish have been found as light/dark orange in color on1st and 2nd days. Light/ dark brown was observed from 3rd to 4th/6th days and silvery in pre-hatching (Figure 12).
Hoff (1996) reported that the length of anemone ï¬sh eggs were ranged from 2.0 to 2.4 mm but in the case of present study, it varied from the earlier reports (Table 1). While, Swagat et al. (2012) documented that other species of cultured anemone fish in aquaria make different size of clutches, such as A. sebae (8.5-9 cm), A. percula (6.5-7.0 cm), A. clarkii (8.5-9.5 cm), A. ocellaris (4.5- 5.5 cm) and P. biaculeatus (7.5 cm) but in this study have been observed clutch diameter was frequently high then previous reports (Table 1). The above two statement was occurred because of the size of females and age. Earlier Dhaneesh et al. (2009) researches demonstrated that the fecundity rate was increased with size of the female. Same observation has recorded in current study also in A. percula and A. clarkia female was found bigger size than A. melanopus, A. ocellaris and A. nigripes. However; fecundity rate of A. melanopus, A. ocellaris and A. nigripes were lesser than A. percula and A. clarkii. Hence, the fecundity rate of the anemone fishes only depends on the size of female and also its age.
After fertilization, especially the male taken care of eggs by fanning with their pectoral fins and cleaned the clutch gently through fanning and mouthing until hatching (Swagat et al., 2012). Similar observations were reported in other anemone species also (Overton et al., 2008; Melville and Griffiths, 1997; Madhu et al., 2006a) however, there were some variations in fanning and embryonic development periods related to anemone fish species. Mariscal (1970) reported the embryonic development rate of fertilized eggs were varied depending on ambient temperature and dissolved oxygen content of water in the tank. Similarly, the present result demonstrated that hatching occurred in different types of incubation hours due to the temporal variations. From this observation it could be suggested that, embryonic development of anemone fishes were depending on temperature and parental care.
Wilkerson (2001) reported that the Amphiprion embryo formed a heart, brain and spinal cord on the 3 day after deposition, and blood circulation was clearly visible on day 4 in A. chrysopterus (Jobling, 2002). Whereas in the study, it was stated that blood circulation was observed in 3 days after the fertilization in all the species. In the study of Ignatius et al. (2001), Pomacentrids fish male parent also exhibited culling by unfertilized or dead embryos and was made to produce other embryos but in case of present study, in anemone fishes male alone cared for the eggs during incubation. In some species, female (A. clarkii and A. melanopus) cared in early stages of egg development and other does not. Silvery coloration with distinct visible eyes is usually a good indication for the hatch out within 12 h, because the matured eyes showed this color. These observations are also similar to those reported for other anemone species (Pathiyasevee, 1994; Madhu et al., 2006a). In this study, on 7th day A. clarkii eggs have got silvery and 8th day completely silvery of all other four species (A. percula, A. clarkii, A. melanopus, A. nigripes and A. ocellaris) because A. clarkii eggs got matured within 7 days than other species.
Appearance was noted and it is due to variations in the fertilization and incubation time from the beginning of spawning and also it indicates the parental care of the anemone fishes during incubation. Incubation time varied because of seasonal variations. So, temperature is important factor for development of embryo (Uthayasiva et al., 2014). The embryonic development of five different species provides a better idea on their development stages and this information helped the mass production of this tropical coral reef ornamental fish in captivity. Briefly, the increasing popularity and demand of marine ornamental fish, especially anemone fish in market sustainably increased worldwide.
The present study conclude that there is less influence of size variations of breeders on the fecundity, parental care, incubation periods, hatching, and deformation rate of anemone fishes would help the stockholders to culture the particular species to earn high price. Estuarine water is adequate for sustainable production, this will help the coastal fisher folk to enhance their livelihood through setting up of backyard hatcheries and significantly, it will help conserve the precious marine ornamental fish biodiversity.
The authors have not declared any conflict of interests.
REFERENCES
|
Ajith Kumar TT, Balasubramanian T (2009). Broodstock development, spawning and larval rearing of the false clown fish, Amphiprion ocellaris in captivity using estuarine water. Curr. Sci. 97(10):1483-1486.
|
|
|
|
Ajith kumar TT, Gopi M, Dhaneesh KV, Vinoth R, Ghosh S, Balasubramanian T, Sanmugaraj T (2012). Hatchery production of the clownfish Amphiprion nigripes at Agatti Island, Lakshadweep, India. J. Environ. Biol. 33:623-628.
|
|
|
|
|
Allen GR, Drew J, Fenner D (2010). Amphiprion pacificus, a new species of anemonefish (Pomacentridae) from Fiji, Tonga, Samoa, and Wallis Island. Aquaculture 16:3-15.
|
|
|
|
|
Allen GR (1974). The Anemonefishes: The classification and biology, 2nd edn. TFH Publication, Inc. USA. 352.
|
|
|
|
|
Alva VR, Gomes LAO. (1989). Breeding marine aquarium animals: the anemone fish. The Nega, ICLARMQ. pp. 12-13.
|
|
|
|
|
Avella MA, Olivotto I, Gioacchini G, Maradonna F, Carnevali O (2007). The role of fatty acids enrichments in the larviculture of false percula clownfish. Amphiprion ocellaris Aquaculture 273:87-95.
Crossref
|
|
|
|
|
Balamurugan RJ, Ajith Kumar TT, Balasubramanian T (2013). Influence of host anemone (Stichodactyla haddoni, Saville-Kent, 1893) locomotion on its resident anemone ï¬sh reproduction. Anim. Reprod. Sci. 140:103-107.
Crossref
|
|
|
|
|
Balon EK (1981). Additions and amendments to the classification of reproductive style in fishes. Environ. Biol. Fishes 6:377-389.
Crossref
|
|
|
|
|
Boby ignatius G, Jagadis I, Kandasami D, Victor ACC. (2001). Spawning and larval rearing technique for tropical clown fish Amphiprion sebae under captive condition. J. Aquac. Trop. 16(3):241-249.
|
|
|
|
|
Chen YF (2005). Induced ovulation and embryonic development of ocellated puffer, Takigugu ocellatus. J. Appl. Ichthyol. 1(2):136-140.
Crossref
|
|
|
|
|
Dehasque M, Candreva P, Carrascosa M, Lavens P (1995). Comparison of an artificial diet and live food as broodstock maturation diets for sea bream (Sparusaurata): effects on spawning and egg quality In: Lavens, P., Jaspers, E. and Roelants, I. (Eds.) Larvi' '95 Gent, Belgium. Eur. Aquac. Soc. 24:16-21.
|
|
|
|
|
Dhaneesh KV, Ajith Kumar TT, Shunmugaraj T (2009). Embryonic
|
|
|
|
|
Development of Percula Clownï¬sh Amphiprion percula (Lacepede, 1802). Middle-East J. Sci. Res. 4(2):84-89.
|
|
|
|
|
Falk-Petersen IB (2005). Comparative organ differentiation during early life stages of marine ï¬sh. Fish Shellï¬sh Immunol. 19:397-412.
Crossref
|
|
|
|
|
Fautin DG, Allen GR (1997). Anemone fishes and their host sea anemones. Western Australian Museum-160.
|
|
|
|
|
Gunasekara RM, shim KF, lam TJ (1996). Effect Protein Level on Spawning Performance and Acid Amino Composition of Eggs of Nile tilapia, Oreochromis niloticus. Aquaculture 146:121-134.
Crossref
|
|
|
|
|
Hoff FH (1996). Conditioning, spawning and rearing of fish with emphasis on marine clown fish. Dade city: Aquaculture Consultants 212.
|
|
|
|
|
Ignatius B, Rathore G, Jagdis I, Kandasami D, Victor ACC (2001). Spawning and larval rearing technique for tropical clownï¬sh Amphiprion sebae under captive conditions. J. Aquac. Trop. 16(3):241-249.
|
|
|
|
|
Jobling M (2002). Environmental factors and rates of development and growth. In: Hart PJB and Reynolds JD. (Eds.). In: Hand book of Fish Biology and Fisheries, vol.1. Fish Biology Blackwell Science Ltd. Blackwell Publishing Company.
Crossref
|
|
|
|
|
Madhu K, Madhu R, Krishnan L, Sasidharan CS, Venugopalan KM (2006a). Spawning and larval rearing of Amphiprion ocellaris under captive conditions. Mar. Fish. Infor. Serv. T&E Ser. 188.
|
|
|
|
|
Madhu K, Rema M, Retheesh T (2012). Broodstock development, breeding, embryonic development and larviculture of spine-cheek anemonefish, Premnas biaculeatus (Bloch, 1790). Indian J. Fish. 59(1):65-75.
|
|
|
|
|
Mariscal RN (1970). The nature of symbiosis between Indo-Pacific anemonefishes and sea anemones. Mar. Biol. 6:58-65.
Crossref
|
|
|
|
|
Melville K, Griffiths S (1997). Recent developments in disease management. Bull. Aquac. Assoc. Canada 2:1-19.
|
|
|
|
|
Moyer JT, Nakazono A (1978). Protandrous hermaphroditism in six species of the anemonefish genus Amphiprion in Japan. Jap. J. Ichthyol. 25:101-106.
|
|
|
|
|
Moyle PB, Cech JJJR (2004). Fishes: An introduction to ichthyology, 5th ed. Pearson, Prentice-Hall, Inch. Upper Saddle River, N J. 726.
|
|
|
|
|
Olivotto I, Cardinali M, Barbaresi L, Maradonna F, Carnevali O (2003). Coral reef fish reading: The secrets of each species. Aquaculture 224:69-78.
Crossref
|
|
|
|
|
Overton JL, Bayley M, Paulsen H, Wang T (2008). Salinity tolerance of cultured Eurasian perch, Percaflu viatilis L: Effects on growth and on survival as a function of temperature. Aquaculture 277:282-286.
Crossref
|
|
|
|
|
Pathiyasevee U (1994). Egg-laying behaviour and growth of false clown anemoneï¬sh Amphiprion ocellaris (Cuvier, 1830). Annual seminar on 1994. National Institute of Coastal Aquaculture Department of Fisheries, Bangkok, Thailand pp. 393-412.
|
|
|
|
|
Ross RM (1978). Territorial behaviour and ecology of the anemone fish Amphiprion melanopus on Guam. Z. Tierpsychology 46:71-83.
|
|
|
|
|
Swagat G, Ajith Kumar TT, Nanthinidevi K, Balasubramanian T (2012). Reef fish breeding and hatchery production using brackishwater, a sustainable technology with special reference to clarkii clownfish, Amphiprion clarkii (Bennett, 1830). Int. J. Environ. Sci. Dev. 3:1.
|
|
|
|
|
Urho L (2002). Characters of larvae-what are they? Folia Zool. 51:161-186.
|
|
|
|
|
Uthaya Siva M, Balamurugan J, Ajith Kumar TT, Badhul Haq MA (2014). Domestication of Anemone Fishes with High Fecundity and Spawning Efficiency under Captive Condition for Indian Scenario. Theriogenology Insight. Int. J. Reprod. Anim. 4(1):9-16.
|
|
|
|
|
Uthayasiva M, Balamurugan J, Kumar TTA, Haq MAB (2014). Seasonal Disparity in Fecundity and Embryonic Development of Cinnamon Anemone Fish, Amphiprion melanopus (Bleeker, 1852) In Captivity. J. Mar. Biol. Oceanogr. 3:3.
Crossref
|
|
|
|
|
Wilkerson DJ (2001). Clownï¬shes: A guide to their captive care breeding and natural history, 1stedition., Microsom Ltd. USA. pp. 240-260.
|
|