Full Length Research Paper
ABSTRACT
INTRODUCTION
MATERIALS AND METHODS
RESULTS
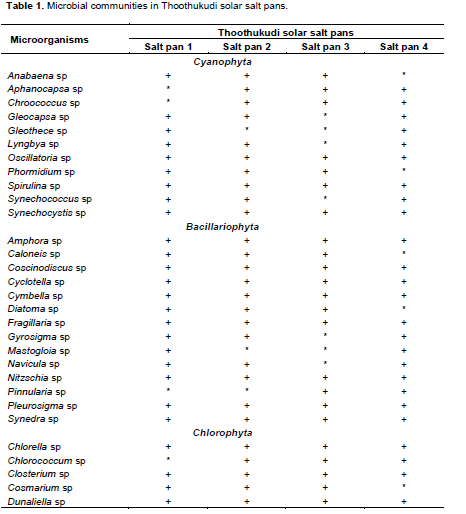
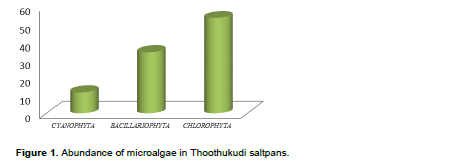
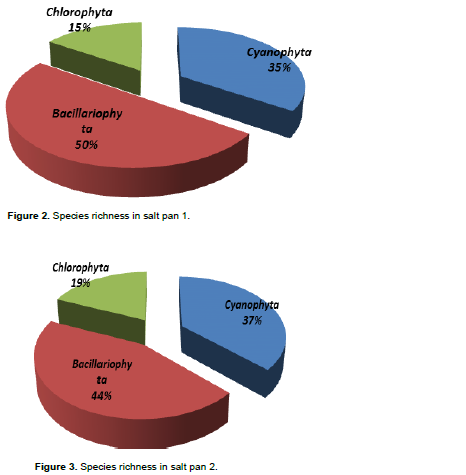
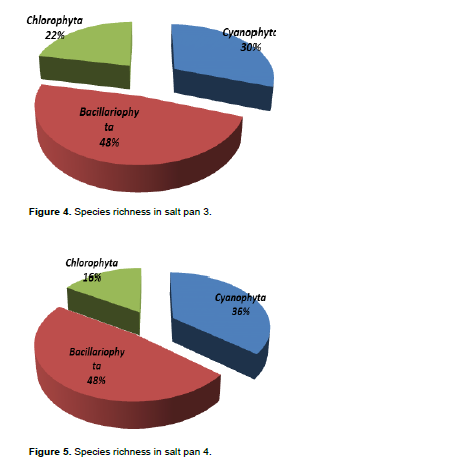
DISCUSSION
CONFLICT OF INTEREST
REFERENCES
| Ashok Kumar N, Viji D, Baluswami M (2011). A short term study of algal flora of a salt pan near Chennai. Int. J. Recent Sci. Res. 2(12):292–296. | ||||
|
Borowitzka LJ (1981). The microflora: Adaptation to life in extremely saline lakes. Hydrobiologia 81:33–46. Crossref |
||||
| Davis JS (2000). Function and management of the biologicalsystem for seasonal solar salt work. Global Nest. 2:217-226. | ||||
|
Giordano M, Davis JS, Bowes G (1994). Organic carbon release by Dunaliella salina under different growth conditions of CO2, nitrogen and salinity. J. Phycol. 30:249- 257. Crossref |
||||
|
Golubic S (1980). Halophilic and halotolerance in cyanophytes. Original Life 10:169–183. Crossref |
||||
| Hendey NI (1964). An introductory account of the smaller algae of British coastal waters, Part V: Bacillariophyceae (diatoms), Her Majesty's Stat. Office, London. P. 317. | ||||
|
Javor BA (1989). Hypersaline environments. -Microbiology and Biogeochemistry. Springer – Verlag, Berlin, Germany. Crossref |
||||
|
Kabilan M, Bhakti S, Deepthi D, Judith MB (2012). Community solar salt production in Goa. India Aquatic Biosystems. 8:30. Crossref |
||||
| Komarek J, Anagnostidis K (2005). Cyanoprokaryota 2.Teil/2nd part: Oscillatoriales, [in:] Budel B, Krienitz L, Gartner G. Schagerl M. (eds.), Susswasser flora von Mitteleuropa, 19(2), Elsevier/Spektrum, Heidelberg. P. 759. | ||||
| Korovessis NA, Lekkas TD (2000). Solar saltworks production process evolution – wetland function, [in:] Saltworks: Preserving saline coastal ecosystems, N.A. Korovessis and T.D. Lekkas (eds.), 6th Conf. Environ. Sci. Technol. Pythagorion, Samos, 1 September 1999, Global NEST, Athens. | ||||
| Lakshmanan C (2008). Biogeochemical studies for increased salt production in the Tuticorin Coast, Tamil Nadu. Ph.D. Thesis submitted to ManonmaniamSundaranar University, Tirunelvely. | ||||
| Michael J, Sullivan C, Currin A (2000). Community Structure and Functional Dynamics of Benthic Microalgae in Salt Marshes. Concepts and Controversies in Tidal Marsh Ecology. pp. 81-106. | ||||
|
Olson JM (2006). Photosynthesis in the archean era. Photosynth Res. 88:109–117. Crossref |
||||
| Oren A, Seckbach J (2001).Oxygenic photosynthetic microorganisms in extreme environments. Nova Hedwigia, Beiheft 123, Garcia-Pichel, F., Castenholz, R. W. Charac-terization and biological implications of scytonemin, a cyanobacterial sheath pigment. J. Phycol. 1991(27):395-409. | ||||
| Oren A (2000). Salt and brines. In: Whitton, B.A. & Potts, M. (eds): The ecology of cyanobacteria: Their diversity in time and spaceKluwer Academic Publishers. Dordrecht. pp. 281–306. | ||||
|
Oren A, Stambler N, Dubinsky Z (1992). On the red coloration of saltern crystallizer ponds. Int. J. Salt Lake Res.1:77-89. Crossref |
||||
| Prescott GW (1951). Algae of the western Great Lakes area, Cranbrook Inst. Sci. Bloomfield Hills P. 946. | ||||
| Reginald M, LailaBanu NR (2009). Role of microalgae for quality salt production in south Indian solar salt production Proceedings of the 2nd International Conference on the Ecological Importance of Solar Saltworks (CEISSA2009) Merida, Yucatan, Mexico. pp. 26–29. | ||||
| Reginald M, Kitto MR, Raj ADS (2004). It Pays to Grow Dunaliella, a sea vegetable Fishing Chimes 24(10):116-117. | ||||
| Reginald M, Helen Diana Y (2008). Role of the micro alga Dunaliellasalina in salt biotechnology. Seaweed Res. Utilin. 30(Special issue):273-277. | ||||
| Sarma YSRK, Khan M (1980). Algal taxonomy in India. Today and tomarrow. Book agency. New Delhi. pp. 153-169. | ||||
| Sorgeloos P (1983). Brine Shrimp Artemia in coastal saltworks. Inexpensive source of food for vertically integrated aquaculture. Aquaculture 9:25-27. | ||||
| Thajuddin N, Subramanian G (2005). Cyanobacterial biodiversity and potential applications in biotechnology. Current Sci. 89:1. | ||||
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0