ABSTRACT
Biodiesel is produced from edible/non-edible plants oil. However, alkaline transesterification of these oils makes the process challenging due to the presence of large amount of free fatty acids (FFA), which forms soap on reaction with alkali. Hence, it is necessary to reduce FFA present so that alkaline catalyzed transesterification can be carried out. In this work, biodiesel fuel was produced using diphenylamine functionalized magnetic mesoporous silica SBA-15 as catalyst for the esterification of free fatty acid (FFA) present in neem oil (NO) and its effect on esterification reaction was studied. Optimum catalyzed esterification was achieved using 1 g diphenylamine functionalized magnetic mesoporous silica SBA-15 as a solid base catalyst with a methanol to oil ratio of 9:1, at 60°C and reaction time of 1.25 h. During this process, FFA was converted into fatty acid methyl esters. The acid value of NO oil was reduced to 7.34 mg KOH/g from 52.45 mg KOH/g, accounting for 86% conversion efficiency. Consequently, this pretreatment reduces the overall complexity of the process and reduces the cost of producing biodiesel fuel. Pretreated NO was converted to biodiesel by a process of alkaline catalyzed transesterification using 1% KOH in methanol.
Key words: Biodiesel, esterification, transesterification, free fatty acid, neem oil.
Increasing demand for energy due to boosting population across the globe has created huge burden on the economy of many nations particularly considering environmental challenges posed by emission of green-house gases from the burning of fossil fuels. Hence, the need for an alternative fuel that is renewable and ecofriendly becomes imperative.
Bio-diesel is a fuel comprised of mono-alkyl esters of long chain fatty acids derived from vegetable oils or animal fats. It is a domestic, clean-burning and renewable liquid fuel. As a result of molecular similarities, bio-diesel can be used instead of petroleum-based diesel with little or no engine modifications (Panigrahi et al., 2012). Price hike and uncertainties concerning petroleum availability contributed immensely to the interest of vegetable oil fuels for diesel engines (Mishra et al., 1995).
Neem tree with botanical name Azadirachta indica, which belongs to the mahogany Meliaceae, is a widely grown crop in India and its even termed as divine tree due to its wide relevance in many areas of research. Its oil is derived from seeds after being pressed. The seeds produce up to 45% oil, which because of its high density, viscosity, flash point and low calorific value cannot be directly used in engines. It needs to be converted into biodiesel to make it consistent with fuel properties of a diesel (Heroor and Rahul, 2013). An important aspect in conversion process is getting rid of the high amount of free fatty acid which forms soap, thereby making the product separation very difficult (Anya et al., 2012; Sathya and Manivannan, 2013).
Mesoporous ordered silica materials are versatile and present a wide variety of applications. SBA-15 is a very important solid catalyst, and because of its large pore size, it is especially suitable for catalysis. In this work, diphenlyammine was supported onto mesoporous silica SBA15 via siloxane linkages and its catalytic esterification activity was evaluated (Helen et al., 2010).
The aim of the present work is to produce biodiesel using diphenylamine functionalized magnetic mesopo-rous silica SBA-15 as catalyst for the esterification of free fatty acid (FFA) present in neem oil (NO) and to study its effect on the esterification reaction.
Pluronic acid P123 (EO20-PO70-EO20, Aldrich) was obtained from Sud-Chemie India Ltd., Mumbai, India. Diethyl ether, ethanol, methanol, ferric chloride, ferrous sulphate and phenolphthalein were obtained from Qualigens Fine Chemicals Ltd., Mumbai, India. Tetraethyl orthosilicate (TEOS) was supplied by Aldrich india, while non-edible grade neem oil was obtained from local market, Chennai Tamil Nadu, India.
Preparation of SBA-15
SBA-15 was synthesized by traditional hydrothermal method using the triblock copolymer P123 (EO20-PO70-EO20) as the surfactant (Woodford,2013). The structure directing agent, pluronic P123 (10 g) was dissolved in water (75.5 cm3) and 2 M hydrochloric acid (291.5 cm3) with stirring at 35°C. 15.5 cm3 of tetraethylorthosilicate (TEOS) was added and left for 20 h with agitation. The resulting gel was hydrothermally treated under sealed conditions for 24 h at 100°C without agitation. The solid was filtered, washed with 1000 cm3 of distilled water and dried at room temperature before calcination at 500°C for 6 h.
Preparation of magnetic nanoparticle coated with SBA-15
The chemical precipitation technique was used to prepare particles with homogeneous composition and narrow size distribution (Panneerselvam et al., 2011). A complete precipitation of Fe3O4 nanoparticles was achieved under alkaline condition while maintaining a molar ratio of Fe2+ to Fe3+, 1:2, under inert condition. To obtain 2 g of magnetic particles, 2.1 g of FeSO4.7H2O and 3.1 g of FeCl3.6H2O were dissolved under inert atmosphere in 80 ml of double distilled water with vigorous stirring. While the solution was heated to 80°C, 10 ml of ammonium hydroxide solution (25%) was added. To ensure complete growth of nanoparticles crystal, the solution was then added to 4 g of SBA-15 and the reaction was carried out for 30 min at 80°C under constant stirring. The resulting suspension was cooled down to room temperature and then washed with distilled water to remove the unreacted chemical. The reactions that occurred in the production are shown in chemical Equations 1 and 2:
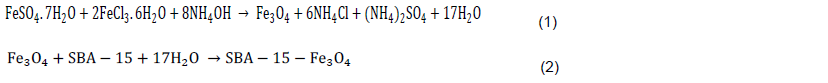
Synthesis of diphenylamine functionalized Fe3O4-SBA-15
2 g of prepared Fe3O4-SBA-15 particles were first evenly dispersed in 50 ml toluene and then 2 g of diphenylamine was added and the mixture was refluxed for 24 h. The solid phase was filtered and washed 3 times with acetone (20 ml each) to remove the impurities and dried at room temperature. The product obtained was referred to as Fe3O4-SBA-15 diphenylamine sample.
Catalyzed esterification
The neem oil was poured into three-necked flask and heated to 60°C. To this, 1 g of SBA-15 solid catalyst was added, followed by methanol (methanol to oil ratio is 4:1) and the reaction was carried out for 2 h at a constant stirring rate of 200 rpm using a mechanical stirrer. This procedure was repeated for 1 g Fe3O4-SBA-15 and Fe3O4-SBA-15-diphenylamine respectively while still maintaining the same methanol to oil molar ratio. Fe3O4-SBA-15-diphenylamine appears to be the most promising and hence it was optimized.
The effect of molar ratios of methanol-to-oil was investigated over a range of 4:1-11:1. The progress of the reaction was routinely monitored by measuring the acid value at an interval of 15 min in each case. The conversion of FFA to methyl ester was calculated using the acid value. The final reaction mixture was centrifuge after removal of excess methanol to separate the solid catalyst and used for further processing by base-catalyzed reaction.
Base-catalyzed transesterification
The pretreated oil was poured into the reaction flask and heated. The solution of KOH in methanol (1%) was thermostated at 60°C, and the pretreated oil was added. The molar ratio of methanol-to-oil was 6:1 and the reaction mixture was stirred by a mechanical stirrer at 200 rpm. The reaction was stopped after 2 h and the mixture was poured into a separating funnel and left for 12 h to separate. The lower layer containing glycerol and impurities was drawn off. The upper layer is the biodiesel and was washed with hot distilled water three times. The lower layers were discarded, and after the third washing, the upper became biodiesel product. The biodiesel product was kept in a hot air oven at 110°C for 1 h to remove water. The transesterification reaction for bio-diesel synthesis is shown in Figure 1.
Analytical methods
The acid value (AC) of the reaction mixture was determined by the acid-base titration technique (Panneerselvam et al., 2011).
Catalyst characterization
Fourier transform infrared spectroscopy
FTIR studies were conducted by using FTIR spectrophotometer (model: FTIR Bruker IFS 66V). Spectra obtained in the range of 400 to 4000cm-1 were analyzed. A spectrum of synthesized and calcined SBA-15 is shown in Figure 2a. In the spectrum, intense broad peak at 3434 cm-1 was due to OH stretching vibration of water and defective OH groups. Its bending vibration occurred at 1719 cm-1. The asymmetric stretching vibration of the CH2 group locked in template occurred at 2360 cm-1. The asymmetric vibration of Si-O-Si group gave an intense broad band at 1631 cm-1, while corresponding symmetric vibration was seen at 1083 cm-1. The bending vibration of Si-O-Si was noted at 464 cm-1. Peak at 959 cm-1 was due to defective Si-O-H vibration. These OH groups were the anchoring sites for functionalization of magnetic nanoparticle in Figure 2b. When comparing the peaks in Figure 2a and b, Figure 2b shows that there were various functional groups detected on the surface of SBA-15-Fe3O4 and a small peak was observed in the Fe-O group. There are some peaks that were shifted, disappeared and new peaks were produced. Significant band decrease of functional group on the SBA-15- Fe3O4 are shown in Figure 2c, which corresponded to the bonded C=O stretching, stretching in quinones, stretching alkanes and C-O stretching in ethers, respectively. The newer peak at 465 cm-1 observed in Figure 2b is related to the Fe-O group, and the peak around 3430 cm-1 in curve a was assigned to the –OH group on the surface of the magnetite. These two significant bands in the spectrum indicate the possible involvement of that functional group on the surface of SBA 15- Fe3O4.
SEM studies
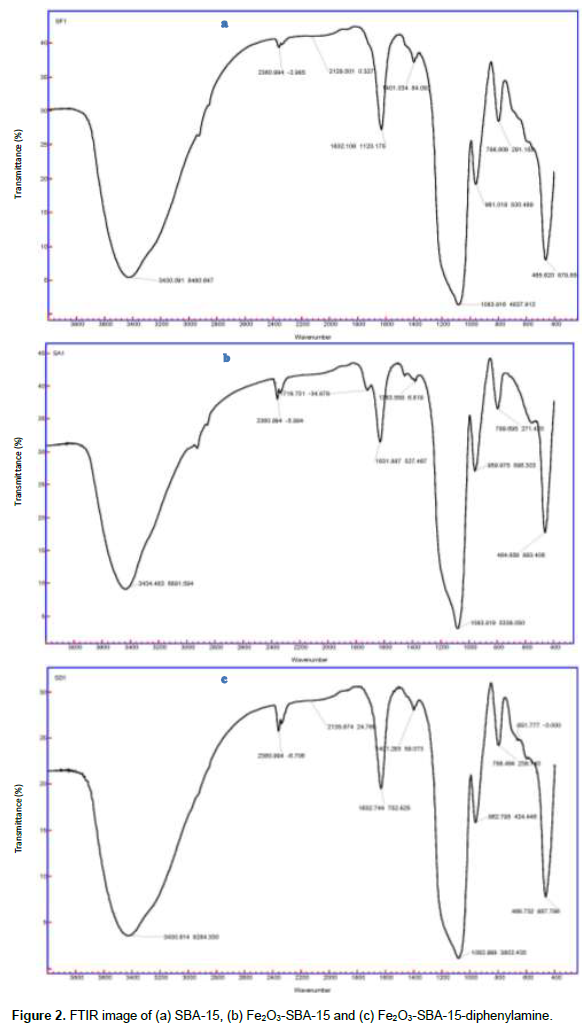
SEM is widely used to study surface morphology. The SEM image of SBA-15 is shown in Figure 3a. Some of the particles showed spherical sponge like morphology, but others were of thick rock like structure. These particles were of different sizes. In addition, small irregular crystallites were also seen. Such type of morphologies is important to lower dielectric constant, as they can hold lot of air voids between the particles. The SEM image of SBA 15- Fe3O4 is shown in Figure 3b. The large spherical sponges of parent SBA-15 were reduced to small crystallites. The decrease in size was attributed to coating with magnetic nanoparticle applied during functionalization. Such size reduction could admit enhanced magnetic nanoparticle functionalization as compared to the parent bulk particles. The SEM image of Fe2O3-SBA-15-diphenylamine is shown in Figure 3c. The crystals were of large irregular shape. The terrace of the crystals was not so smooth and the sides revealed piling of plates.
Catalyzed esterification
Comparison of different modified forms of a catalyst
The conversion efficiency of the three different functionalized forms of the catalyst were examined using 1 g each of the catalyst at 60°C while maintaining 4:1 methanol to oil ratio. They both show higher activities with Fe3O4-SBA-15-diphenylamine showing highest conversion efficiency. This is because of its amphoteric nature since the silicate backbone is acidic while the Fe3O4 and diphenylamine are basic in nature and esterification reaction is generally catalyzed by both acids and bases. Hence, the conversion efficiency increases due to acid-base catalytic effect of the catalyst. The minimum amount of catalyst that is needed to shift the equilibrium to right thereby having more esterification than the hydrolysis was investigated with 4:1 methanol to oil ratio at 60°C as done for the catalyst selection. The amount of catalyst was varied as shown in Figure 4, from 0.25 to 1.5 g. 1 g of the catalyst was found to show greatest conversion efficient.
Effect of methanol to oil molar ratio
The change in acid value of neem oil with respect to change in alcohol-to-oil molar ratio in the range of 4:1 to 11:1 over the period of 2 h was studied and the result is shown in Figure 5. It can be understood that the higher the amount of methanol in relation to the oil, the more the reaction equilibrium is shifted to the right thereby favoring the formation of more esters. At methanol to oil ratio of 9:1, 86% conversion efficiency was obtained.
Base catalyzed transesterification
Besides the presence of FFA and moisture, as well as the agitation intensity, the base-catalyzed transesteri-fication is also affected by the type and concentration of catalyst, the molar ratio of methanol to oil, reaction time and the reaction temperature. Molar ratio is defined as the number of moles of alcohol to number of moles of vegetable oil. Theoretically, transesterification reaction requires 3 moles of alcohol for each mole. However, practically, molar ratio should be higher than stoichiometric ratio (Sathya and Manivannan, 2013). The higher molar ratio is required to complete the reaction at higher rate. In lower molar ratio it takes longer time to complete .The most often used molar ratio of methanol to oil and KOH amount are 6:1 and 1%, respectively (Panneerselvam et al., 2011). This was found to be optimum in the present experiments leading to a satisfactory conversion of triglycerides to methyl esters (Table 1).
In the present investigation, magnetic mesoporous silica SBA-15-diphylamine was found to be a promising strong solid base catalyst for the esterification of neem oil (NO) with methanol by significantly reducing the FFA value. The currently utilized process has many advantages such as operational simplicity, less time, low cost, high yield, no saponification and reusability of the catalyst, which makes it hold greater potential for the green process.
Overall, the developed magnetic mesoporous silica SBA-15 seems to be an attractive, effective and economical catalyst for esterification of FFA that can be explored for large scale commercial applications.
The authors have not declared any conflict of interests.
The authors are grateful to Nanotechnology Research Centre, SRM University for carrying out SEM analysis.
REFERENCES
|
Anya UA, Chioma NN, Obinna O (2012). Optimized reduction of free fatty acid content on neem seed oil, for biodiesel production. J. Basic Appl. Chem. 2(4):21-28.
|
|
|
|
Atadashi IM, Aroua MK, Abdul-Aziz AR, Sulaiman NM (2012). Production of biodiesel using high free fatty acid feedstock. Renew. Sustain. Ener. Rev. 16:3275-3285.
Crossref
|
|
|
|
|
Balat M, Balat H (2010). Progress in biodiesel processing. Appl. Ener. 87:1815-1835.
Crossref
|
|
|
|
|
Chung KH, Chang DR, Park BG (2008). Removal of free fatty acid in waste frying oil by esterification with methanol on zeolite catalysts. Bioresour. Technol. 99:7438-7443.
Crossref
|
|
|
|
|
Helen IN, Nicholas AZ, Thomas AF, Edward TS, Wenbin L (2010). Mesoporous silica-supported diaryammonium catalysts for esterification of free fatty acids in greases. J. Am. Oil Chem. Soc. 87:445-452.
Crossref
|
|
|
|
|
Heroor SH, Rahul SD (2013). Production of biofuel from crude neem oil and its performance. Int. J. Environ. Eng. Man. 4:425-432.
|
|
|
|
|
Jiang ST, Zhang FJ, Pan LJ (2010). Sodium phosphate as a solid catalyst for biodiesel preparation. Braz. J. Chem. Eng. 27(1):137-144.
Crossref
|
|
|
|
|
Marchetti JM, Miguel VU, Errazu AF (2007). Heterogeneous esterification of oil with high amount of free fatty acids. Fuel 86:906-910.
Crossref
|
|
|
|
|
Mishra AK, Singh N, Sharma VP (1995). Use of neem oil as mosquito
|
|
|
|
|
Muthu H, SathyaSelvabala V, Varathachary TK, Kirupha SD, Nandagopal J, Subramanian S (2010). Synthesis of biodiesel from neem oil using sulfated zirconia via tranesterification. Braz. J. Chem. Eng. 27(4):601-608.
|
|
|
|
|
Panigrahi N, Mohanty MK, Pradhan AK (2012). Non-edible karanja biodiesel-A sustainable fuel for C.I. engine. Int. J. Eng. Res. App. 2(6):853-860.
|
|
|
|
|
Panneerselvam P, Morad N, Tan AK (2011). Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel (II) from aqueous solution. J. Hazard. Mat. 186:160-168.
Crossref
|
|
|
|
|
Sathya T, Manivannan A (2013). Biodiesel production from neem oil using two step transesterification. Int. J. Eng. Res. Appl. 3(3):488-492.
|
|
|
|
|
Sathyaselvabala V, Thiruvengadaravi KV, Dinesh KS, Vijayalakshmi P, Sivanesan S (2010). Removal of free fatty acid in Azadirachta indica (Neem) seed oil using phosphoric acid modified mordenite for biodiesel production. Bioresour. Technol. 101(15):5897-5902.
Crossref
|
|
|
|
|
Shieh CJ, Liao HF, Lee CC (2003). Optimization of lipase-catalyzed biodiesel by response surface methodology. Bioresour. Technol. 88(2):103-106.
Crossref
|
|
|
|
|
Wenlei X, Xiaoming H (2006). Synthesis of Biodiesel from Soybean Oil using Heterogeneous KF/ZnO Catalyst. Catal. Lett. 107(1-2):53-59.
Crossref
|
|
|
|
|
Woodford JJ (2013). Hierarchical nanoporous solid base catalysts for biodiesel synthesis (Doctoral dissertation, Cardiff University).
View
|
|