ABSTRACT
Knowledge of mosquito ecology is a prerequisite for efficient implementation of vector control strategies. This one-year study was carried out during the 2013 rainy season and the 2014 dry season throughout the flooded areas of the suburbs of Dakar with the aim to characterize and map anopheline larval habitats. In both seasons, all water bodies that were encountered within the study departments were geo-located and their features (type of water body, size, turbidity and distance to human dwellings) recorded. The presence of anopheline and/or culicine larvae and predators was assessed. A total of 908 and 278 potential larval habitats were visited during the rainy season and the dry season, respectively. A significant positive association was found between the rainy season and the presence of anopheline larval sites, which consisted of ponds, puddles, ravines, drain channels, streams and canals. Anopheline larvae were more likely to be found in clear water bodies located within 10 m to human dwellings. During the dry season, only puddles were likely to host anopheline larvae. Anopheline larval habitats were significantly more frequent in the Department of Rufisque during the rainy season (univariate analysis, P = 0.006) and in the Department of Guediawaye during the dry season (multivariate analysis, P = 0.036). The malaria vector identified was Anopheles arabiensis. Data gathered in this study will guide larval control programmes in urban settings prone to flooding.
Key words: Anopheline, larval habitat, flood, suburb of Dakar, Senegal.
Despite a decade of control efforts and a 54% decrease in malaria deaths (between 2000 and 2013), malaria remains a serious public health problem across sub-Saharan Africa (World Health Organization (WHO), 2014). In 2013, 198 million malaria cases leading to 584,000 deaths were recorded in the continent. Malaria transmission is heterogeneous in nature (Bousema et al., 2012; Opondo et al., 2016). Among the multiple factors creating this heterogeneity is urbanization which leads to new control challenges (Dongus et al., 2009; Donnelly et al., 2005; Klinkenberg et al., 2008). Many African cities face rapid and uncontrolled urbanization that is worsening the socio-economic and environmental conditions (RGPHAE, 2013; World Urbanization Prospects (WUP), 2014). Although urban areas are normally characterized by low malaria burden (Robert et al., 2003), unplanned urbanization can lead to the creation of new larval habitats that can potentially produce more vectors and hence contribute to malaria transmission (Dongus et al., 2009).
The suburbs of Dakar, the capital city of Senegal is an area where malaria transmission persists all year long within some localities (Diallo et al., 2012; Programme National de Lutte contre le Paludisme (PNLP), 2013) despite the mass distribution of insecticide-treated nets (ITNs), as well as, introduction of artemisinin-based combination therapy (ACT). The non-structured urbanization and recurrent flooding observed in these areas facilitate the proliferation of natural and/or man-made anopheline larval habitats. Dakar’s suburbs are facing huge environmental issues due to the presence of such spontaneous and irregular habitats in these floodplains and they have led to critical sanitation problems throughout the year. This situation has allowed the proliferation of malaria and arbovirus vectors. The development of anopheline immature stages usually occurs in fresh or brackish non-polluted water, with or without vegetation, exposed or not to the sun (Carnevale and Robert, 2009). However, recent studies showed that Anophelese gambiae sensu lato can adapt to extreme conditions and may grow in a wide variety of man-made larval sites, sometimes polluted by industrial, agricultural or domestic runoffs. Some anopheline mosquitoes have thus diversified their larval sites, from previously clear, unpolluted waters to include more polluted stagnant water sources (Djouaka et al., 2007; Gunathilaka et al., 2013, 2014; Gunathilaka and Karunaraj, 2015; Keating et al., 2004).
The use of ITNs and indoor residual spraying (IRS) are the principal vector control strategies deployed to stop the spread of malaria transmission (Bhatt et al., 2015). In addition to ITNs and IRS, mosquito larval source management (LSM) has been recommended as a complementary tool for vector control strategies (WHO, 2013) where it has been successful in the control and elimination of certain vectors in Brazil (Soper and Wilson, 1943), in the United States of America (Carlson, 2006; Gadawski, 1989) and throughout Europe (Becker, 2010).
Fillinger and Lindsay (2011) provided evidence of the efficiency of the LSM for malaria control in Africa based on trials in areas where all larval habitats were well defined and accessible. These results give evidence that LSM can be used to complement and strengthen core vector control interventions to further reduce malaria transmission in an urban area such as Dakar and its suburbs (Fillinger and Lindsay, 2011; RBM, 2011; WHO, 2013). However, an accurate knowledge of malaria vector larval ecology is a prerequisite before implementing any effective LSM strategy.
Few studies have investigated the dynamics of anopheline larval sites throughout the suburbs of Dakar (Awono-Ambene and Robert, 1999; Gadiaga et al., 2011; Robert et al., 1998) and none, to our knowledge, has assessed the consequence of the recurrent flooding on the larval habitats of malaria vector species. Therefore, the aim of this study was to map and to characterize anopheline larval habitats in the suburbs of Dakar.
Study area
The study was carried out in the Dakar region of Senegal located at the westernmost part of the Cape-Verde peninsula (14°40'20"N, 17°25'22"W). Dakar belongs to the Sudano-Sahelian domain and is characterized by specific eco-geographical areas called “Niayes”, where the presence of sand dune depressions and a flushing water table make them prone to flooding during the rainy season. The region essentially has two seasons: a hot, rainy season from July to October (with average temperatures ranging from 24 to 30°C) and a mild cold, dry season from November to June (with average temperatures ranging from 19 to 25°C). The annual rainfall was 566 mm in 2013 (ANAMS, 2013). The Dakar region is the most populated area of Senegal with a density of 5,404 inhabitants per km² (RGPHAE, 2013).
Selection of study sites
The region is divided into four departments; Dakar, Guediawaye, Pikine and Rufisque and this study was undertaken in all except Dakar. Flooding is common in particular localities within the departments during the rainy season. Thus, in each of them, both flooded and non-flooded areas have been investigated. These three departments with different population densities have experienced dramatic changes in demography as well as unplanned and uncontrolled urban expansion. The department of Guediawaye is 17.8 km² with a population density of 18,541 inhabitants per km². The department of Pikine with 14,472 inhabitants per km² has a surface area of 80.9 km². Rufisque is the largest department (379.6 km²) with the lowest population density, at 1,292 inhabitants per km². However, within Rufisque department, only the urban area was investigated as its population density (10,511 inhabitants per km²) is comparable to those of Pikine and Guediawaye(ANAT, 2016; RGPHAE, 2013).
Investigation and characterization of water bodies
The study was conducted during the 2013 rainy season from July to October and the 2014 dry season from March to June. Within each department, all water bodies encountered were geo-located using a Garmin eTrex® GPS (Legend H), listed then characterized according to type, size, depth, turbidity and exposure to sunlight. The distance between each encountered water body and the nearest human dwelling and the presence/absence of predators (tadpoles and fish) were also recorded. Each water body was inspected once per season and was mapped using ArcGIS version 9.1 software.
Larval collection, sampling and identification
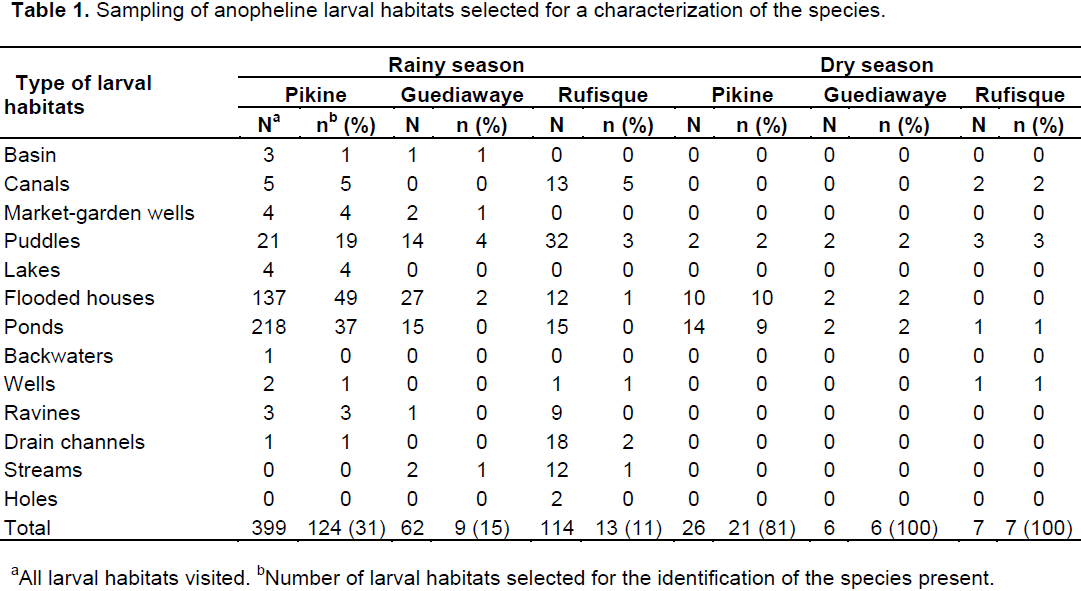
The surface and edges of water bodies were checked at different places for the presence of mosquito larvae. The immature stages of mosquito populations from positive water bodies of each study area were collected using the standard dipping method (Service, 1993). The anopheline larvae were separated from the culicines (Carnevale and Robert, 2009). The anopheline larvae were then transferred to the laboratory in buckets filled water from the site where the larvae were collected. Anopheles were identified to species level from a random selection of 146 and 34 anopheline larval habitats from the three departments during 2013 rainy season and 2014 dry season, respectively (Table 1). Larvae from the selected larval habitats were reared to obtain adults. These were pooled according to the type of larval habitat, the department of origin and the season, and allowed to emerge. A total of 2447 adult were obtained. Half (1226/2447), distributed according to the type of larval habitat, to the department and to the season were identified morphologically (Diagne et al., 1994). In each stratum composing 1226 anopheles morphologically identified, a random sample proportional to the size (Loonis, 2009) of the original population was made in order to have a total of 638 specimens for molecular identification of the species (Wilkins et al., 2006).
Data analysis
All statistical analyses were performed using STATA 11 software (Stata Corp-LP®). Logistic regression was used to investigate the effect of geographic (department) and bio-ecological factors (season, type, water turbidity, depth, proximity to human dwellings, size, sunlight and the presence of predatory species) on presence/absence of anopheline larvae.
For each variable, the modalities poorly represented (<5) or those in which the absence of anopheline larvae was noted, were not included in the regression analyses. Variables with a p-value strictly lower than 0.2 for the simple regression analyses were integrated in multiple regression analyses. A stepwise logistic regression analysis was done and variables removed sequentially based on Akaike information criterion (AIC) till the best model was achieved. The significance level was fixed at α = 0.05 in the final model. Proportion of anopheline and/or culicine larvae per season was assessed using Chi-square tests.
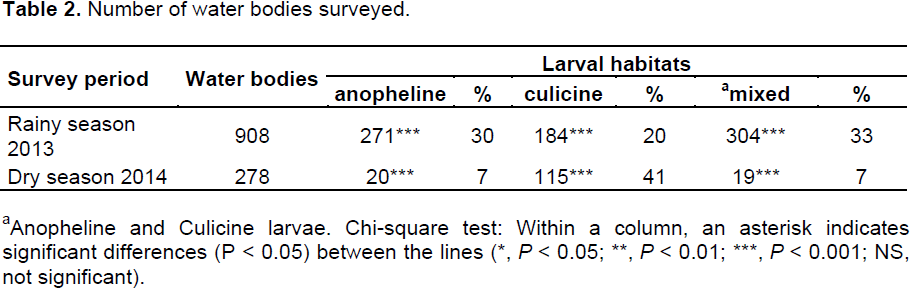
Water body survey
During the whole survey, a total of 908 water bodies, consisting of 13 types of water bodies, which varied in size, depth and exposure to the sun, were identified (Supp.S1). Of the overall 908 that were present during the rainy season, only 278 were encountered during the dry season. Eighty three percent (759/908) of water bodies were positive for mosquito larvae during the rainy season versus 55% (154/278) throughout the dry season. Positive water bodies were composed of anopheline, culicine or mixed (anopheline and culicine) larval habitats. In the rainy season, anopheline larvae (anopheline exclusively and mixed) colonized a higher proportion of water bodies (63%) than the culicine larvae(culicine exclusively and mixed) (53%) (χ2= 17.17; P < 0.001). An opposite trend was observed during the dry season where culicine larval sites (culicine exclusively and mixed) were more frequent (48%) (χ2 = 75.73; P < 0.001) compared to those sites harbouring anopheline larvae (anopheline exclusively and mixed) (14%) (Table 2). The anopheline larval sites were 13 times more frequent during the rainy season (odds ratio [OR] = 12.99; P < 0.001) than the dry season (Table 3). The presence of anophelines larvae did not have an effect on the presence of culicines larvae in both the rainy (OR = 0.91; P = 0.487) and dry season (OR = 1.02; P = 0.944) (Tables 3 and 4).
Characterization of anopheline larval habitats
During the survey, thirteen types of water bodies were identified corresponding to the basins, market-garden wells, canals, puddles, lakes, flooded houses, ponds, backwaters, wells, ravines, drains channels, streams and holes. Overall, ponds, flooded houses, puddles and canals were the most frequent water bodies encountered during the survey. During the rainy season, all holes (2/2) and backwaters (1/1) were colonized by anopheline larvae and 86% (19/22) of drain channels, 81% (13/16) of ravines, 75% (67/89) of puddles, 75% (6/8) of market-garden wells, 74% (14/19) of streams, 62% (176/286) of flooded houses, 61% (248/404) of ponds and 60% (18/30) of canals were also positive for anopheline larvae (Table 3).
The univariate analysis showed that during the rainy season, flooded houses (OR = 3.6; P = 0.037), ponds (OR = 3.58; P = 0.037), puddles (OR = 6.85; P = 0.003), ravines (OR = 9.75; P = 0.010), drain channels (OR =14.25; P = 0.002) and streams (OR = 6.3; P = 0.021) were more likely to be colonized by anopheline larvae. Furthermore, in the multivariate analysis, ponds (adjusted OR [AOR] = 3.68; P = 0.044), puddles (AOR = 6.6; P = 0.006), ravines (AOR = 7.18; P = 0.031), drain channels (AOR = 13.87; P = 0.004), streams (AOR = 5.55; P = 0.040) and canals (AOR = 5; P = 0.032) were all positively associated with the presence of anopheline larvae (Table 3). During the dry season, anopheline larvae were scarce and only found in 47% (7/15) of puddles, 25% (1/4) of wells, 16% (17/106) of ponds and 12% (12/100) of flooded houses (Table 4). The presence of anopheline larvae was significantly associated with the puddles in univariate (OR = 9.18; P = 0.014) and multivariate (AOR = 19.48; P = 0.005) analyses during this season (Table 4).
The majority of water bodies (92%) were within 10 m of human dwellings. During the rainy season, 64% (536/832) of these water bodies hosted anopheline larvae. The regression analysis showed that water bodies within 10 m of houses were positively associated with the presence of anopheline larvae both in the univariate analysis (OR = 1.72, P = 0.025) and the final multivariate model (AOR = 1.80, P = 0.029) (Table 3).
There was a negative association between water turbidity (Figure 1) and presence of anopheline larvae both in univariate (OR = 0.17, P < 0.001) and multivariate (AOR = 0.16, P < 0.001) analyses. Presence of predators (tadpoles) also had a negative correlation with presence of anopheline larvae (univariate analysis, OR = 0.71, P = 0.014) (Table 3).

Both size and depth of the bodies of water appeared to influence the presence of anopheline larvae. During the rainy season, medium (100 to 500 m²) and large (>500 m2) water bodies harboured anopheline larvae less frequently than water bodies of a smaller size (<100 m²) (OR = 0.59, P = 0.003) (Table 3). At the same time, the deepest water bodies were less favorable for the presence of anopheline larvae (OR = 0.66, P = 0.004) (Table 3). Conversely, in the dry season, no association was found between anopheline larvae presence and size or depth of water bodies (OR = 0.90, P = 0.778) (Table 4). During this dry season, proximity to human dwelling, turbidity of water, sunlight and the presence of predators (tadpoles and fish) also were not associated with the presence of anopheline larvae.
Spatial and temporal distribution of anopheline larval habitats
During the rainy season, most of the water bodies were found within the Pikine department, followed by Rufisque and Guediawaye (Figure 2) but a higher proportion of the water bodies in Rufisque (114/158) and Guediawaye (62/88) contained anopheline larvae as compared to Pikine(399/662) (Table 3). During this season, water bodies encountered in the department of Rufisque were more likely to host anopheline larvae (OR = 1.71, P = 0.006) than the others (Table 3).
However, during the dry season, anopheline larval habitats were more likely to be found in the Guediawaye department (AOR = 3.15, P = 0.034) where the highest positivity rate for anopheline larval habitats was obtained (26%) (Figure 3).
Molecular identification of An. gambiae s.l. species by PCR
All 638 An. gambiae s.l. specimens from 13 types of larval habitats across the three departments were identified molecularly as An. arabiensis (Figure 4).
This study is part of a larger program that seeks to generate data to guide the first LSM in Dakar to further reduce malaria transmission in this area. For this first phase, an area of 107 km² of the 122 km² contained within three of the four departments of the Dakar Region were monitored for one year. This provides a snapshot of the distribution and frequency of anopheline larval sites and allows for planning a potential larval source management intervention.
Our results show that the probability of finding anopheline larval habitats was highest during the rainy season compared to the dry season. Indeed, the daily amounts of rainfall recorded during this period combined with the shallow water table of the study areas caused flooding and consequently long standing water and thus potential malaria vector habitats were created. The presence of stagnant rainwater increased the diversity of anopheline larval sites in rainy season. During this season, anopheline larvae were found in all types of water bodies in all the departments surveyed. Water bodies that had the greatest role in the development of anopheline larvae were ponds, puddles, ravines, drainage channels, streams and canals.
The presence of immature stages of anopheline in puddles has already been reported (Gadiaga et al., 2011; Gillies, 1987; Huang et al., 2006; Miller et al., 2007; Ndenga et al., 2011). For ponds, ravines, drain channels, streams and canals their importance could be explained by the rainfall which reduced the concentration in organics substances allowing the favorable conditions for anopheline larval growth (Chirebvu and Chimbari, 2015; Holstein, 1954). This could explain why in this present study, such a diverse range of habitats were observed.
Human mediated factors like poor garbage disposal that subsequently leads to blockage of water flow in drain channels, ravines and streams may also have contributed to the creation of larval habitat similarly that were observed elsewhere (Castro et al., 2010; Keating et al., 2003). Moreover, the absence of predators (tadpoles and fish) in the majority of these types of water bodies made them ideal sites for anopheline immatures.
Most of the water bodies identified and surveyed during the rainy season dried up in the dry season, leading to a significant decrease of potential larval sites as had been shown previously (Simard et al., 2000; Yaro et al., 2012) confirming the importance of the season on the larval habitat distribution. Among the types of water bodies that remained during this period, only the puddles were positively associated with the presence of anopheline larvae. Puddles were created from the resurgence of water from the shallow groundwater table following establishment of a pipeline system.
Although previous studies often reported market-garden wells as the most common anopheline larval habitats (Robert et al., 1998; Trape et al., 1992), only a few were found in the study area. According to Robert et al. (1998) about 5000 market-garden wells were recorded in 1996. Their scarcity nowadays could be a consequence of rapid urbanization (ONU-HABITAT, 2008; RGPHAE, 2013) leading to the disappearance of the majority of the market-garden wells. However, since this study only surveyed residential areas, we might have missed out other wells that located throughout less populated and rural settings.
During the rainy season, the probability of finding anopheline larvae increases when water bodies were located within 10 m of human habitations. The study sites are located within areas that formerly contained water (marshes, ponds) but dried during the droughts of the 1970s (Faye et al., 1995; Ndao, 2012) and as a consequence the population move in and built houses. However, persistent increases of rainfall amounts are now accompanied by increased flooding in a populated area. This localization of the mosquito larval sites, including anopheline larval habitats near human dwellings brought the oviposition site in the same vicinity of the blood meal source, limiting mosquito dispersal (Manga et al., 1993; Njan nloga, 1993) and increasing the risk of malaria transmission (Bogh et al., 2007; Salem, 1994; Thomas and Lindsay, 2000; Trape et al., 1992).
During our survey, anopheline preference for clear water was observed as already described in Sub-Saharan Africa (Bates, 1949; Gimnig et al., 2001; Minakawa et al., 1999; Munga et al., 2005; Mwangangi et al., 2010). However, during the survey, anopheline larvae were also found in the turbid water similarly to a previous study in Ethiopia, where An. arabiensis was occasionally found in turbid water (Ye-Ebiyo et al., 2003). Another study in Cameroon, recently reported the development of both An. coluzzi and An. gambiae in urban polluted waters (Kamdem et al., 2012). The capacity of anopheline vector species to colonize polluted water in urban areas (Awolola et al., 2007; Chinery, 1984; Kamdem et al., 2012; Sattler et al., 2005) may have an impact on malaria epidemiology within these areas (Keating et al., 2003; Macintyre et al., 2002). Nevertheless, the notion of turbid water may not involve water pollution. Turbidity may be due to materials that can serve as food particles for anopheline and thus contribute to their development (Sattler et al., 2005), but the turbidity may also be caused by matter, such as decaying vegetation, that would impede larval growth (Awolola et al., 2007; Muirhead-Thomson, 1951).
Consistent with previous studies, smaller water bodies identified here were more likely to host An. gambiae s.l. larvae (Holstein, 1954; Muirhead-Thomson, 1951; Ndenga et al., 2011), although not consistent with the findings of Majambere et al. (2008) that showed that anophelines were more likely to colonize large water bodies.
In our study areas anopheline larvae were less frequently encountered in the deep water as previously observed by Clements (Clements, 1992), who explain this observation by the fact that anopheline larvae are surface feeders. However, when in low food conditions at the water surface An. gambiae s.l. larvae can increase their diving frequency (Phelan and Roitberg, 2013), which can affect larval mortality as seen in deep water bodies (Tsila et al., 2015; Tuno et al., 2004).
Larvae predators (tadpole and fish) are known to have great impact on mosquito larvae presence and density (Depinay et al., 2004; Gouagna et al., 2012; Kweka et al., 2011; McCrae, 1984; Munga et al., 2005; Munga et al., 2006). This study showed negative association between the presence of tadpoles and anopheline larvae during the rainy season, while no impact was found in the dry season. No association was found between the presence of fish and anopheline larvae in our study. This could be explained by the availability of others food sources for fish in these water bodies (Garcia-Berthou, 1999; Kumar and Hwang, 2006; Specziar, 2004). Thorough studies are needed to assess the role of these larvivorous fish, previously introduced in this area for a biological control (Awono-ambene, 1999). However the presence of these potential larvivorous species may influence the choice of oviposition site of the gravid female in order to safeguard the survival of her offspring (Depinay et al., 2004; Gouagna et al., 2012; Kweka et al., 2011; McCrae, 1984; Munga et al., 2005; Munga et al., 2006).The geographical distribution of anopheline larval habitats was heterogeneous across the study areas and the season. During the rainy season, the probability of encountering immature stages of malaria vectors was more important in the Rufisque department. Within this department, the majority of types of water bodies found were those more likely to host anopheline larvae. As explained earlier, most of the anopheline sites were temporary as a most of the sites in Rufisque. In addition, the absence of tadpoles within most of larval sites was often noted in Rufisque department. During the dry season, the scarcity of water bodies was noted and anopheline larvae were associated with the resurgence of groundwater as the result of human activities on shallow water table. Larvae, although found at low numbers, were also observed at the edges of permanent water bodies. Anopheles arabiensis was the only member of the An. gambiae complex and the only malaria vector that was identified in the study areas and is consistent with previous work within the same region (Awono-Ambene and Robert, 1999; Robert et al., 1998). However, An. melas was recently reported in the Niayes area but in very low numbers (Gadiaga et al., 2011).
The Niayes, a coastal marine area, located between inter-dune depressions is characterized by the presence of several ponds and lakes, whose edges are comparatively clear and sunlit and can act as preferential larval habitats for An. arabiensis (Carnevale and Robert, 2009; Rajendran and Reuben, 1991). The predominance of this vector species can also be explained by its high adaptability resulting in colonization of a wide spectrum of water bodies (Pages et al., 2007).
The study showed a wide distribution of anopheline larval habitats across the flooded area of the Dakar suburbs. Although the presence of anopheline larvae in the water bodies does not always mean high larval and adult productivity, abundance of anopheline larval habitats during the period of malaria transmission could influence the production of adult vectors and therefore, the malaria transmission. This study provides baseline database for future implementation of larval source management to accelerate malaria pre-elimination accordingly to the Senegal National Malaria Control Program goals. Further studies are needed to develop effective control strategies against these immature stages of mosquitoes. Regular monitoring of these larval sites would allow evaluate their productivity and to identify periods of risk.
The authors declare that they have no conflicts of interest.
The authors thank the municipal officers of the study area, their leaders and the Service National d’Hygiene of Dakar for allowing and facilitating the field work. We thank all staff of the Laboratory of Vector and Parasite Ecology, the Research Unit of Emerging infectious and Tropical diseases in Dakar and the National Malaria Control Program Senegal who provided assistance for field surveys, laboratory work or data analysis. This work was funded in part by the United States Agency for International Development.
REFERENCES
|
ANAMS (2013). Agence Nationale de la Météorologie, ANAMS, Sénégal.
|
|
|
|
ANAT (2016). Agence Nationale de l'Aménagement du Territoire, ANAT, Sénégal.
|
|
|
|
|
Awolola TS, Oduola AO, Obansa JB, Chukwurar NJ, Unyimadu JP (2007). Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. J. Vector Borne Dis. 44:241-244.
|
|
|
|
|
Awono-ambene HP (1999). Gamétocytélnie de Plasmodium falciparum chez des patients en accès palustre traités avec un antimalarique, et sporogonie chez des vecteurs du conlplexe Anopheles gambiae. Thèse de Doctorat de troisième cycle, Université Cheikh Anta Diop de Dakar, Sénégal.
|
|
|
|
|
Awono-Ambene HP, Robert V (1999). Survival and emergence of immature Anopheles arabiensis mosquitoes in market-gardener wells in Dakar, Senegal. Parasite 6:179-184.
Crossref
|
|
|
|
|
Bates M (1949). The natural history of mosquitoes. New York: The Macmillan Company.
|
|
|
|
|
Becker N (2010). The Rhine Larviciding Program and Its Application to Vector Control. In: Vector Biology, Ecology and Control Springer Netherlands pp. 209-219.
Crossref
|
|
|
|
|
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CL, Smith DL, Hay SI, Cibulskis RE, Gething PW (2015). The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207-211.
Crossref
|
|
|
|
|
Bogh C, Lindsay SW, Clarke SE, Dean A, Jawara M, Pinder M, Thomas CJ (2007). High spatial resolution mapping of malaria transmission risk in the Gambia, West Africa, using LANDSAT TM satellite imagery. Am. J. Trop. Med. Hyg. 76:875-81.
|
|
|
|
|
Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, Ghani A, Drakeley C, Gosling R (2012). Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 9:e1001165.
Crossref
|
|
|
|
|
Carlson DB (2006). Source reduction in Florida's salt marshes: management to reduce pesticide use and enhance the resource. J Am Mosq Control Assoc. 22:534-537.
Crossref
|
|
|
|
|
Carnevale P, Robert V (2009). Les anophèles Biologie, transmission du Plasmodium et lutte antivectorielle. IRD Éditions Institut de Recherche pour le Developpement, Marseille.
|
|
|
|
|
Castro MC, Kanamori S, Kannady K, Mkude S, Killeen GF, Fillinger U (2010). The importance of drains for the larval development of lymphatic filariasis and malaria vectors in Dar es Salaam, United Republic of Tanzania. PLoS Negl. Trop. Dis. 4:e693.
Crossref
|
|
|
|
|
Chinery WA (1984). Effects of ecological changes on the malaria vectors Anopheles funestus and the Anopheles gambiae complex of mosquitoes in Accra, Ghana. J. Trop. Med. Hyg. 87:75-81.
|
|
|
|
|
Chirebvu E, Chimbari MJ (2015). Characteristics of Anopheles arabiensis larval habitats in Tubu village, Botswana. J. Vector Ecol. 40:129-138.
Crossref
|
|
|
|
|
Clements AN (1992). The Biology of Mosquitoes, Volume I: Development, Nutrition and Reproduction. Chapman & Hall, New York.
|
|
|
|
|
Depinay JM, Mbogo CM, Killeen G, Knols B, Beier J, Carlson J, Dushoff J, Billingsley P, Mwambi H, Githure J, Toure AM, McKenzie FE (2004). A simulation model of African Anopheles ecology and population dynamics for the analysis of malaria transmission. Malar. J. 3:29.
Crossref
|
|
|
|
|
Diagne N, Fontenille D, Konate L, Faye O, Lamizana MT, Legros F, Molez JF, Trape JF (1994). [Anopheles of Senegal. An annotated and illustrated list]. Bull. Soc. Pathol. Exot. 87:267-277.
|
|
|
|
|
Diallo A, Ndam NT, Moussiliou A, Dos Santos S, Ndonky A, Borderon M, Oliveau S, Lalou R, Le Hesran JY (2012). Asymptomatic carriage of plasmodium in urban Dakar: the risk of malaria should not be underestimated. PLoS One 7:e31100.
Crossref
|
|
|
|
|
Djouaka RF, Bakare AA, Bankole HS, Doannio JM, Kossou H, Akogbeto MC (2007). Quantification of the efficiency of treatment of Anopheles gambiae breeding sites with petroleum products by local communities in areas of insecticide resistance in the Republic of Benin. Malar. J. 6:56.
Crossref
|
|
|
|
|
Dongus S, Nyika D, Kannady K, Mtasiwa D, Mshinda H, Gosoniu L, Drescher AW, Fillinger U, Tanner M, Killeen GF, Castro MC (2009). Urban agriculture and Anopheles habitats in Dar es Salaam, Tanzania. Geospat Health 3:189-210.
Crossref
|
|
|
|
|
Donnelly MJ, McCall PJ, Lengeler C, Bates I, D'Alessandro U, Barnish G, Konradsen F, Klinkenberg E, Townson H, Trape JF, Hastings IM, Mutero C (2005). Malaria and urbanization in sub-Saharan Africa. Malar. J. 4:12.
Crossref
|
|
|
|
|
Faye O, Gaye O, Fontenille D, Hebrard G, Konate L, Sy N, Herve JP, Toure Y, Diallo S, Molez JF (1995). [Drought and malaria decrease in the Niayes area of Senegal]. Sante 5:299-305.
|
|
|
|
|
Fillinger U, Lindsay SW (2011). Larval source management for malaria control in Africa: myths and reality. Malar. J. 10:353.
Crossref
|
|
|
|
|
Gadawski R (1989). Annual report on mosquito surveillance and control in Winnipeg. Winnipeg: Insect Control Branch, Parks & Recreation Department.
|
|
|
|
|
Gadiaga L, Machault V, Pages F, Gaye A, Jarjaval F, Godefroy L, Cisse B, Lacaux JP, Sokhna C, Trape JF, Rogier C (2011). Conditions of malaria transmission in Dakar from 2007 to 2010. Malar. J. 10:312.
Crossref
|
|
|
|
|
Garcia-Berthou E (1999). Food of introduced mosquitofish: ontogenetic diet shift and prey selection. J. Fish Biol. 55:135-147.
Crossref
|
|
|
|
|
Gillies MT, Coetzee M (1987). A supplement to the anophelinae of Africa south of the Sahara (Afrotropical region). The South African Institut for Medical Research No. 55, Johannesburg.
|
|
|
|
|
Gimnig JE, Ombok M, Kamau L, Hawley WA (2001). Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J. Med. Entomol. 38:282-288.
Crossref
|
|
|
|
|
Gouagna LC, Rakotondranary M, Boyer S, Lemperiere G, Dehecq JS, Fontenille D (2012). Abiotic and biotic factors associated with the presence of Anopheles arabiensis immatures and their abundance in naturally occurring and man-made aquatic habitats. Parasit. Vectors 5:96.
Crossref
|
|
|
|
|
Gunathilaka N, Karunaraj P (2015). Identification of sibling species status of Anopheles culicifacies breeding in polluted water bodies in Trincomalee district of Sri Lanka. Malar. J. 14:214.
Crossref
|
|
|
|
|
Gunathilaka N, Fernando T, Hapugoda M, Wickremasinghe R, Wijeyerathne P, Abeyewickreme W (2013). Anopheles culicifacies breeding in polluted water bodies in Trincomalee District of Sri Lanka. Malar. J. 12:285.
Crossref
|
|
|
|
|
Gunathilaka PADHNRF, Fernando MAST, Hapugoda MD, Wijeyerathne P, Wickremasinghe AR, Abeyewickreme W (2014). Breeding habitat diversity and species composition of Anopheles mosquitoes in trincomalee district, Sri Lanka.
|
|
|
|
|
Holstein MH (1954). Biology of Anopheles gambiae: research in French West Africa. World Health Organization: Geneva.
|
|
|
|
|
Huang J, Walker ED, Vulule J, Miller JR (2006). Daily temperature profiles in and around Western Kenyan larval habitats of Anopheles gambiae as related to egg mortality. Malar. J. 5:87.
Crossref
|
|
|
|
|
Kamdem C, Tene Fossog B, Simard F, Etouna J, Ndo C, Kengne P, Bousses P, Etoa FX, Awono-Ambene P, Fontenille D, Antonio-Nkondjio C, Besansky NJ, Costantini C (2012). Anthropogenic habitat disturbance and ecological divergence between incipient species of the malaria mosquito Anopheles gambiae. PLoS One 7:e39453.
Crossref
|
|
|
|
|
Keating J, MacIntyre K, Mbogo C, Githeko A, Regens JL, Swalm C, Ndenga B, Steinberg LJ, Kibe L, Githure JI, Beier JC (2003). A geographic sampling strategy for studying relationships between human activity and malaria vectors in urban Africa. Am. J. Trop. Med. Hyg. 68:357-365.
|
|
|
|
|
Keating J, Macintyre K, Mbogo CM, Githure JI, Beier JC (2004). Characterization of potential larval habitats for Anopheles mosquitoes in relation to urban land-use in Malindi, Kenya. Int. J. Health Geogr. 3:9.
Crossref
|
|
|
|
|
Klinkenberg E, McCall P, Wilson MD, Amerasinghe FP, Donnelly MJ (2008). Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar. J. 7:151.
Crossref
|
|
|
|
|
Kumar R, Hwang, JS (2006). Larvicidal efficiency of aquatic predators: a perspective for mosquito biocontrol. Zool. Stud. 45:447-466.
|
|
|
|
|
Kweka EJ, Zhou G, Gilbreath TM, 3rd, Afrane Y, Nyindo M, Githeko AK, Yan G (2011). Predation efficiency of Anopheles gambiae larvae by aquatic predators in western Kenya highlands. Parasit. Vectors 4:128.
Crossref
|
|
|
|
|
Loonis V (2009). L'échantillonnage de la théorie à la pratique, Courrier des statistiques n° 126.
|
|
|
|
|
Macintyre K, Keating J, Sosler S, Kibe L, Mbogo CM, Githeko AK, Beier JC (2002). Examining the determinants of mosquito-avoidance practices in two Kenyan cities. Malar. J. 1:14.
Crossref
|
|
|
Majambere S, Fillinger U, Sayer DR, Green C, Lindsay SW (2008). Spatial distribution of mosquito larvae and the potential for targeted larval control in The Gambia. Am. J. Trop. Med. Hyg. 79:19-27.
|
|
|
|
Manga L, Fondjo E, Carnevale P, Robert V (1993). Importance of low dispersion of Anopheles gambiae (Diptera: Culicidae) on malaria transmission in hilly towns in south Cameroon. J. Med. Entomol. 30:936-938.
Crossref
|
|
|
|
|
McCrae AW (1984). Oviposition by African malaria vector mosquitoes. II. Effects of site tone, water type and conspecific immatures on target selection by freshwater Anopheles gambiae Giles, sensu lato. Ann. Trop. Med. Parasitol. 78:307-318.
|
|
|
|
|
Miller JR, Huang J, Vulule J, Walker ED (2007). Life on the edge: African malaria mosquito (Anopheles gambiae s. l.) larvae are amphibious. Naturwissenschaften 94:195-199.
Crossref
|
|
|
|
|
Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G (1999). Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. Am. J. Trop. Med. Hyg. 61:1010-1016.
|
|
|
|
|
Muirhead-Thomson RC (1951). Mosquito Behaviour in Relation to Malaria Transmission and Control in the Tropics. London: Edward Arnold & Co.
|
|
|
|
|
Munga S, Minakawa N, Zhou G, Barrack OO, Githeko AK, Yan G (2005). Oviposition site preference and egg hatchability of Anopheles gambiae: effects of land cover types. J. Med. Entomol. 42:993-997.
Crossref
|
|
|
|
|
Munga S, Minakawa N, Zhou G, Barrack OO, Githeko AK, Yan G (2006). Effects of larval competitors and predators on oviposition site selection of Anopheles gambiae sensu stricto. J. Med. Entomol. 43:221-224.
Crossref
|
|
|
|
|
Mwangangi JM, Shililu J, Muturi EJ, Muriu S, Jacob B, Kabiru EW, Mbogo CM, Githure J, Novak RJ (2010). Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar. J. 9:228.
Crossref
|
|
|
|
|
Ndao M (2012). Dynamiques et gestion environnementales de 1970 à 2010 des zones humides au Sénégal : étude de l'occupation du sol par télédétection des Niayes avec Djiddah Thiaroye Kao (à Dakar), Mboro (à Thiés et Saint-Louis); Thèse de Doctorat unique.
|
|
|
|
|
Ndenga BA, Simbauni JA, Mbugi JP, Githeko AK, Fillinger U (2011). Productivity of malaria vectors from different habitat types in the western Kenya highlands. PLoS One 6:e19473.
Crossref
|
|
|
|
|
Njan nloga A, Robert V, Toto JC, Carnevale P (1993). La durée du cycle gonotrophique d'Anopheles moucheti varie de trois à quatre jours en fonction de la proximité par rapport aux gîtes de ponte. Bull. liais. doc. - OCEAC V01.26W2
|
|
|
|
|
ONU-HABITAT (2008). Sénégal: Profil urbain de Dakar, Programme des Nations Unies pour les Établissements Humains (ONU-HABITAT).
|
|
|
|
|
Opondo KO, Weetman D, Jawara M, Diatta M, Fofana A, Crombe F, Mwesigwa J, D'Alessandro U, Donnelly MJ (2016). Does insecticide resistance contribute to heterogeneities in malaria transmission in The Gambia? Malar. J. 15:166.
Crossref
|
|
|
|
|
Pages F, Orlandi-Pradines E, Corbel V (2007). [Vectors of malaria: biology, diversity, prevention, and individual protection]. Med. Mal Infect 37:153-161.
Crossref
|
|
|
|
|
Phelan C, Roitberg BD (2013). Effects of food, water depth, and temperature on diving activity of larval Anopheles gambiae sensu stricto: evidence for diving to forage. J. Vector Ecol. 38:301-306.
Crossref
|
|
|
|
|
PNLP (2013). Rapport statistique (2010-2013), Programme National de Lutte Contre le Paludisme, PNLP, Sénégal.
|
|
|
|
|
Rajendran R, Reuben R (1991). Evaluation of the water fern Azolla microphylla for mosquito population management in the rice-land agro-ecosystem of south India. Med. Vet. Entomol. 5:299-310.
Crossref
|
|
|
|
|
RBM (2011). Vector Control Working Group. Report of the 6th Annual Meeting of the Roll Back Malaria Partnership, Geneva, Switzerland.
|
|
|
|
|
RGPHAE (2013). Agence Nationale de la Statistique et de la Démographie, Rapport définitif- Recensement général de la population et de l'habitat de l'agriculture et de l'élevage (RGPHAE), Sénégal.
|
|
|
|
|
Robert V, Awono-Ambene HP, Thioulouse J (1998). Ecology of larval mosquitoes, with special reference to Anopheles arabiensis (Diptera: Culcidae) in market-garden wells in urban Dakar, Senegal. J. Med. Entomol. 35:948-955.
Crossref
|
|
|
|
|
Salem G, Legros F, Lefebvre-Zante E, Ndiaye G, Bouganali H, Ndiaye P, Badji A, Trape JF (1994). Espace urbain et risque anophélien à Pikine (Sénégal). Cahiers santé 4p.
|
|
|
|
|
Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, Lengeler C (2005). Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar. J. 4:4.
Crossref
|
|
|
|
|
Service MW (1993). Mosquito ecology: Field Sampling Methods. Vector biology and control, Liverpool of Tropical Medicine, second edition. 988p.
|
|
|
|
|
Simard F, Lehmann T, Lemasson JJ, Diatta M, Fontenille D (2000). Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Mol. Biol. 9:467-479.
Crossref
|
|
|
|
|
Soper FL, Wilson DB (1943). Anopheles gambiae in Brazil, 1930 to 1940. New York city: The Rockefeller Foundation.
|
|
|
|
|
Specziar A (2004). Life history pattern and feeding ecology of the introduced eastern mosquitofish, Gambusia holbrooki, in a thermal spa under temperate climate, of Lake Heviz, Hungary Hydrobiologia 522:249-260.
Crossref
|
|
|
|
|
Thomas CJ, Lindsay SW (2000). Local-scale variation in malaria infection amongst rural Gambian children estimated by satellite remote sensing. Trans R Soc. Trop. Med. Hyg. 94:159-63.
Crossref
|
|
|
|
|
Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, Salem G (1992). Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am. J. Trop. Med. Hyg. 47:181-9.
|
|
|
|
|
Tsila HG, Foko Dadji GA, Messi J, Tamesse JL, Wabo Pone J (2015). Effect of the larval habitat depth on the fitness of the malaria-vector mosquito, Anopheles gambiae s. s. J. Parasitol. Vector Biol. 7(7):151-155.
|
|
|
|
|
Tuno N, Miki K, Minakawa N, Githeko A, Yan G, Takagi M (2004). Diving ability of Anopheles gambiae (Diptera: Culicidae) larvae. J. Med. Entomol. 41:810-812.
Crossref
|
|
|
|
|
WHO (2013). Larval source management, A supplementary measure for malaria vector control, An Operational Manual - World Health Organization, WHO, Geneva, Switzerland.
|
|
|
|
|
WHO (2014). Global Malaria Program, World malaria report, WHO, Geneva, Switzerland.
|
|
|
|
|
Wilkins EE, Howell PI, Benedict MQ (2006). IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar. J. 5:125.
Crossref
|
|
|
|
|
WUP (2014). World Urbanization Prospects (WUP), United Nations, The 2014 Revision. UN. New York
|
|
|
|
|
Yaro AS, Traore AI, Huestis DL, Adamou A, Timbine S, Kassogue Y, Diallo M, Dao A, Traore SF, Lehmann T (2012). Dry season reproductive depression of Anopheles gambiae in the Sahel. J. Insect Physiol. 58:1050-9.
Crossref
|
|
|
|
|
Ye-Ebiyo Y, Pollack RJ, Kiszewski A, Spielman A (2003). Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. Am. J. Trop. Med. Hyg. 68:748-752.
|
|