ABSTRACT
Microscopy, the gold standard for routine malaria diagnosis and rapid diagnosis tests (RDTs) are required before artemisinin-based combination therapies for malaria case management in Sub-Saharan Africa. Several RDTs have been recommended by World Health Organization (WHO) for use in endemic areas. The aim of this study was to assess the performance of SD Bioline malaria Ag P.f. test and SD Bioline Ag P.f./Pan test, two new RDTs for malaria diagnosis, before their introduction to Mali. A total of 736 patients with clinical malaria were recruited from October to December, 2009 in four field sites of Mali. 75 tests were transported and exposed to usual field conditions before use. The tests were performed on capillary blood. Light microscopic examination of blood smear was performed as the reference. The sensitivity and specificity for detection of Plasmodium falciparum were 99.7% (95% CI, 98.7 to 100) and 73.3% (95% CI, 64.0 to 80.6) for the SD Bioline malaria Ag P.f test, respectively; 99.1% (95% CI, 98 to 99.7) and 73.3% (95%CI, 65.0-80.6) for the SD Bioline Malaria Ag P.f/Pan test, respectively. The sensitivity for the detection of P. falciparum at parasitemia < 100 parasites/µl were 91.7 and 87.5% for SD Bioline Malaria P.f test and Malaria Ag P.f/Pan test, respectively. The sensitivity of Malaria Ag P.f/Pan test for the diagnosis of non-falciparum species was 91.7% and the specificity was 97.8%. Both SD Bioline malaria antigen P.f and SD Bioline malaria antigen P.f/Pan remained positive for Plasmodium species 2 weeks after malaria treatment. The transportation in routine field conditions did not alter the performance of the tests. Both SD Bioline malaria P.f test and malaria Ag P.f/Pan test were adequate for the diagnosis of malaria countrywide in Mali.
Key words: Plasmodium falciparum, SD bioline malaria test, rapid diagnosis tests (RDTs), Mali.
Malaria remains a leading cause of morbidity and mortality, causing 198 million of clinical cases and 584,000 deaths in 2013 (World Health Organization (WHO, 2014; 2015). Approximately 40% of the world’s population is at risk of malaria (WHO, 2006; 2015). The disease is caused by the protozoan parasite of the genus Plasmodium of which five species infect humans: Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium vivax and Plasmodium knowlesi. Children, pregnant women, travelers, refugees and laborers entering endemic areas are considered to be high-risk groups. According to the National Malaria Control Program (NMCP) of Mali, only 10% of malaria cases were confirmed through laboratory testing before treatment in 2009. Morbidity, mortality transmission of malaria and the development of drug resistant parasite can be reduced if infection is promptly diagnosed and adequately treated (http://www.rollbackmalaria.org/ about/about-malaria/what-is-malaria, 2006). Microscopy remains the gold standard for malaria diagnosis. More sensitive methods including polymerase chain reaction (PCR), quantitative buffy coat (QBC) and enzyme-linked immunosorbent assay (ELISA) are available but require qualified technicians, electricity and equipment that are not available in rural settings. Adequate management of malaria cases in rural areas would greatly be improved by the implementation of malaria rapid diagnosis test (RDTs).
Over the past two decades, 86 malaria RDTs have been developed by different companies and approved by World Health Organization (WHO, 2015). These diagnostic tests are fast and easy to perform, and do not require electricity or specific equipment (Bell et al., 2006; Wongsrichanalai, 2001). They are all based on the same principle and use antibodies that detect three groups of Plasmodium antigen including, P. falciparum histidine-rich protein 2 (PfHRP2), P. falciparum lactate dehydrogenase (PfLDH) and Plasmodium Pan-specific antigens (aldolase or pan-Malaria pLDH). While all tests detect P. falciparum antigen, pan-malaria can distinguish a non-falciparum infection from P. falciparum and P. falciparum mixed infections.
SD BIOLINE Malaria Ag P.f® (ref. 05FK50, Standard Diagnostics Inc., Suwon City, South Korea) test is a qualitative test for the detection of histidine-rich protein 2 (HRP-2) antigen of P. falciparum, SD BIOLINE Malaria Ag P.f/Pan® (ref. 05FK60) test is a rapid, qualitative and differential test for the detection of histidine-rich protein 2 (HRP-2) antigen of P. falciparum and common Plasmodium lactate dehydrogenase (pLDH) of Plasmodium species in whole human blood. The ability and reproducibility of RDTs in adequately detecting malaria parasites may vary depending on the antigens used by the manufacturer and a number of biochemical, immunological or genetics parameters. Indeed, prozone effect was shown to be more pronounced with HRP-2 based RDTs than with pan-pLDH or aldolase-based ones (Gillet et al., 2009). Deletion of the hrp2 gene was shown to lead to false negative HRP2-based RDTs (Koita et al., 2012) while persistence of the HRP2 protein and/or presence of gametocytes can cause false positive tests (Ouattara et al., 2011). The aim of this study was to assess the performance of two commercially available immuno-chromatographic malaria rapid diagnosis tests in the field in routine conditions before their introduction in Mali.
Study sites and population
The study was carried out during the malaria transmission season from October to December, 2009 at the primary health care Centers of Bandiagara, Faladje, Kolle and Pongonon (Figure 1). Bandiagara is a town of approximately 14,000 inhabitants located at about 700 km from Bamako (the capital city of Mali) in the center of the country, on a rocky plain. The mean annual rainfall is about 600 mm. Anopheles gambiaesensolato complex are the main malaria vectors. Malaria transmission is highly seasonal, with a peak of up to 60 infective mosquito bites per person per month in August or September. P. falciparum represents 97% of malaria infections, with the remaining 3% due mostly to P. malariae and rare P. ovale infections (Coulibaly et al., 2014). Faladje is a rural village located at about 80 km North-West of Bamako. The village has a Catholic mission health center that serves 23,000 inhabitants living in the village and surrounding areas. Malaria transmission is seasonal (July to October), with a peak in October. Kolle site is a rural area located at about 57 km South-West of Bamako. Falciparum malaria is endemic and seasonal, with parasitemia prevalence ranging from 40 to 50% in the dry season (December to May) and 70 to 85% in the rainy season (June to November). The principal vectors are A. gambiaesensulato (95.5% of the vector population) and Anopheles arabiensis (4.5%). The mean monthly entomologic inoculation rate was 2.8 infectious bites per person, with marked seasonal va-riations (Sogoba et al., 2008). Pongonon is a rural village of 1,400 inhabitants, located in the Dogon country 785 km North-East of Bamako. The climate is Sahelian type, with an annual rainfall from 300 to 600 mm. Malaria is seasonal with transmission peaks between July and October of each year. Patients coming to the respective health centers with fever (or history of fever within the last 24 h) and malaria symptoms were invited to participate to this study.
Ethical aspects and informed consent
The study protocol was reviewed and approved by the Ethics Committee of the Faculty of Medicine, Pharmacy and Odonto-stomatology, University of Science, Techniques and Technologies of Bamako (USTTB), Mali. Permission was also obtained from community and local authorities. All participants included provided written informed consent prior to their inclusion.
Study procedures
Thick and thin blood films were prepared for light microscopy diagnosis at the time of enrollment and immune-chromatographic tests were performed from finger prick according to the manufacturers’ instructions. Thick and thin blood smears were stained in 3% Giemsa for 30 min and examined for parasites density quantifications and parasite species identification. At least 200 consecutive fields were examined for a thick blood smear before a sample was classified as negative (WHO, 2010). Parasitemia was assessed based on the count of 300 leucocytes per smear (with an estimate of 7,500 leukocytes/µl of blood). RDTs were performed concomitantly with blood smears and the results were available within fifteen minutes. RDTs results were considered positive if the internal control and the test band were stained (irrespective of the intensity of the staining). Results were considered negative if only the internal control was stained.
A result was considered invalid if the internal control was not stained. Patients with positive RDT result at the time of enrollment were promptly treated with Artemether-lumefantrine (Coartem®) for uncomplicated malaria cases or Quinine for severe malaria cases. To monitor RDT positivity after treatment with Artemether-lumefantrine, a subset of 25 subjects from Pongonon was followed for 14 days after treatment. Blood smears and RDTs tests were performed concomitantly on days 0, 7 and 14.
Impact of transport conditions onRDTs
In order to assess the impact of transport and field environmental conditions on RDTs performance, 25 SD malaria antigen test from each batch were transported in a container located in the open trunk of a four-wheel drive vehicle from Bamako to Pongonon (14 h drive).
Temperature within and outside the box was recorded every 4 h during the journey. The remaining RDTs were transported inside the air-conditioned cabin of the same vehicle.
Quality assurance and quality control
Microscopists were kept blinded from the RDTs result until the
smear parasite count was provided. An external Microscopist at the Malaria Research and Training Center examined 10% of slides for quality control purposes. Each SD Bioline Malaria P.f or Malaria P.f/Pan kit containing sufficient materials for 25 tests RDTs were kept at room temperature (30 to 35°C) and opened just before use to avoid light and humidity damage.
Data entry and analysis
Data were double entered using Microsoft ACCES and analyzed using Stata Version 10.0. The presence of P. falciparum gametocytes without asexual forms did not define a case of acute malaria. For sensitivity and specificity assessment, RDTs and microscopy results were compared. Sensitivity (Se) was calculated as the proportion of samples with malaria parasites detected using the reference method that gave positive RDT results. The proportion of samples negative by the reference method that showed negative RDT results measured specificity (Sp). Positive and negative predictive values were the proportion of all positive samples that were true positive samples and the proportion of all negative samples that were true negative samples, respectively. Kappa statistics (K), Youden index (Y), likelihood ratios (LR+ and LR-) were calculated and the receiver operating characteristic (ROC) curve compared each test vs. microscopy. P values below 0.05 (two-tailed) were considered statistically significant.
Sample size
The sample size was determined based on 96% sensitivity found for known rapid diagnostic tests in Mali in previous study (Optimal®) (Ouattara et al., 2011). By setting the risk of error at 5%, and precisionat 5%, an estimated malaria prevalence of at least 30% in each site of the sample size would be 197, rounded up to 200 per site and 800 participants for all 4 sites.
Comparisons of RDTs and microscopy
A total 736 participants were enrolled at day 0 with 195, 191, 200 and 150 in Bandiagara, Faladje, Kolle and Pongonon, respectively. Table 1 provided the general and clinical characteristics of the study population. Overall, 595/736 (80.8%); 6/736 (0.8%) and 18/736 (2.4%) samples were positives by microscopy analysis for P. falciparum, P. malaria and mixed infectionof P. falciparum plus P. malaria, respectively. No cases of P. ovale or P. vivax were observed. The discordance rate (positive vs. negative or negative vs. positive) between the reference microscopist and the field readings was 12.5% (data not shown). Invalid results were observed in 6/736 (0.8%) and 4/736 (0.5%) tests of Malaria Antigen P.f test and Malaria Antigen P.f/Pan test, respectively (Table 2).
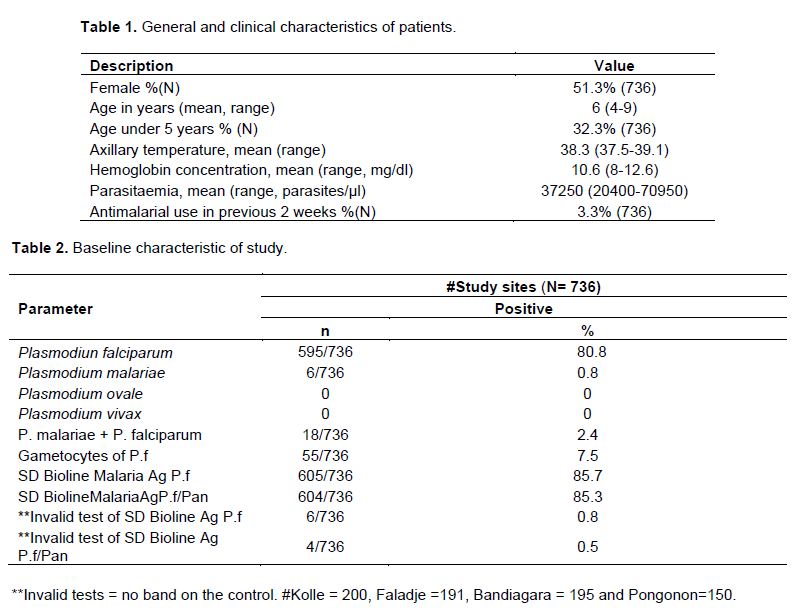
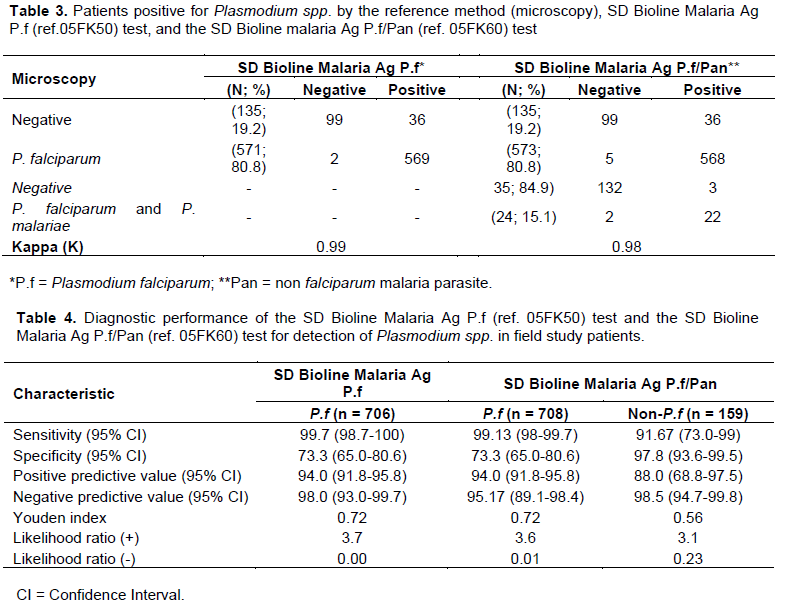
When microscopy was compared to Malaria Antigen P.f test, microscopy examination was positive with P.falciparum only in 571/706 (80.8%) while Malaria Antigen P.f test detected 606/736 (82.3%) such infections. Similarly, when microscopy was compared to the Malaria Antigen P.f/Pan test, 573/708 (80.8%) P. falciparum infections were detected by microscopy vs. 605/736 (82.2%) by the Malaria Antigen P.f/Pan test. In addition, in the later comparison microscopy and the Malaria Antigen P.f/Pan test revealed 24/159 (15.1%) and 25/159 (15.7%) of both P. falciparum and P. malariae infections. The concordance Kappa with microscopy was 0.99 for Malaria Ag P.f test and Malaria Ag P.f/Pan test, and 0.98 for Malaria Ag P.f/Pan test for the detection of non-P. falciparum infections (Table 3).
Both Malaria Antigen P.f and Malaria Antigen P.f/Pan showed sensitivities of 99.7 and 99.1%, respectively for P falciparum. The specificity of both tests for the detection of P. falciparum was 73.3%. For the detection of non-falciparum infections the sensitivity and specificity of the malaria Ag P.f/Pan test were 91.7 and 97.8%, respectively (Table 4). Youden index was 0.72, 0.72 and 0.56 between microscopy and Malaria Ag P.f, Malaria Ag P.f/Pan and Malaria Ag P.f/Pan for non-falciparum infection, respectively. The likelihood ratio (+) was 3.7, 3.6 and 3.1 between microscopy and Malaria Ag P.f, Malaria Ag P.f/Pan and Malaria Ag P.f/Pan for non-falciparum malaria infections, respectively. The likelihood ratio (-) was 0.00, 0.01 and 0.23 between microscopy and Malaria Ag P.f, Malaria Ag P.f/Pan and Malaria Ag P.f/Pan for non-falciparum malaria infections, respectively (Table 4).
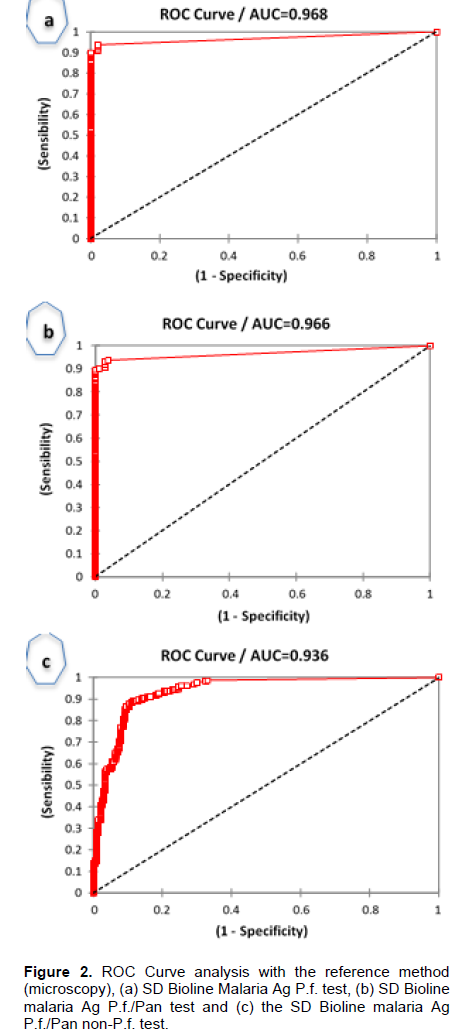
In Figure 2, ROC curve analysis showed AUC = 0.97, 0.97 and 0.94 with the reference method (microscopy) vs.
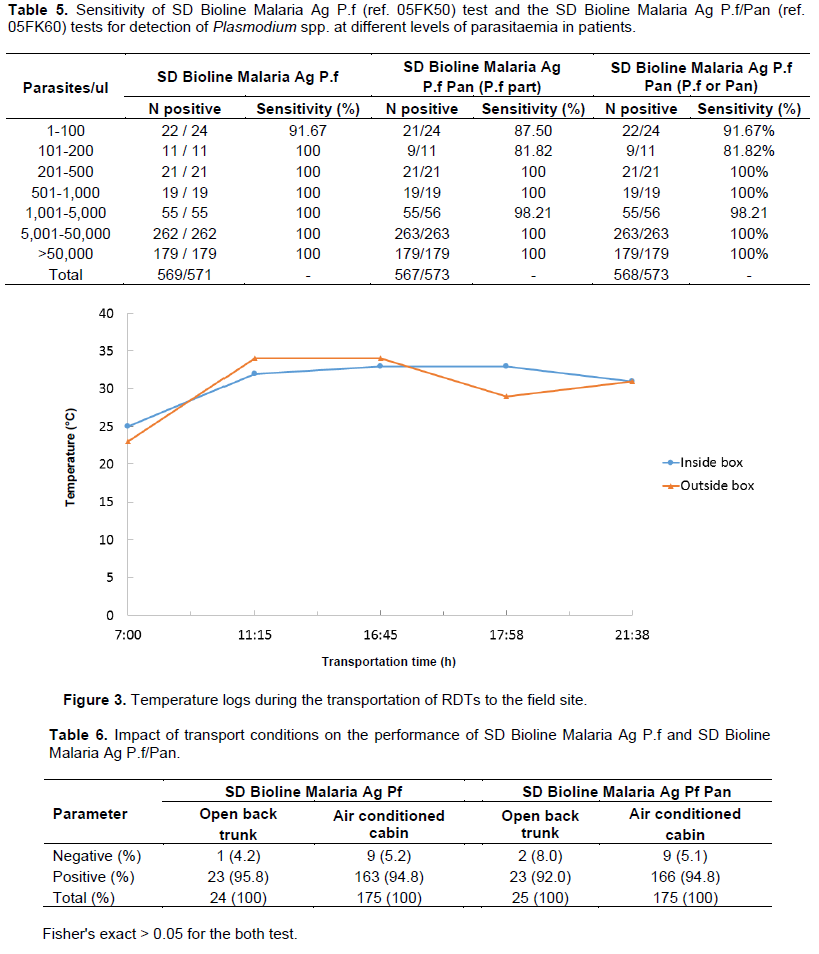
Malaria Ag P.f test, microscopy vs. Malaria Ag P.f/Pan test, and microscopy vs. Malaria Ag P.f/Pan for non-P.falciparum, respectively. With all 25 patients followed for 2 weeks both tests (SD Bioline Malaria Ag P.f and Bioline Malaria Ag P.f/Pan) were positives although microscopy was negative on days 7 and 14. The sensitivity in detecting P. falciparum parasitemia levels < 100 parasites/µl was 22/24 (91.7%) and 21/24 (87.5%) for Malaria Ag P.f and Malaria Ag P.f/Pan tests, respectively. We found one false-negative result of Malaria Ag Pf/Pan tests for P. falciparum malaria at a parasitemia between 1000 to 5000 parasites/µl (Table 5).
RDT performance and transport conditions
It rained all day-long during the journey to Pongonon. Temperatures inside the box containing the RDTs and the outside were similar and varied from 23 to 25°C at the beginning of the journey to 31°C on arrival to Pongonon in the evening, with peaks at 33 and 34°C (Figure 3). The average temperature inside the air-conditioned cabin was 19°C. These temperatures did not alter the performance of both Malaria Antigen P.f and Malaria Antigen P.f/Pan tests transported in the back of the truck (Fisher exact = 0.42) (Table 6).
This study provides the first evaluation of sensitivity and specificity of two commercially available immune-chromatographic assays, the SD Bioline Malaria Ag P.f® (ref. 05FK50) test and the SD Bioline Malaria Ag P.f/Pan® (ref. 05FK60) test (Standard Diagnostics Inc., Suwon City, South Korea) in Mali. The quality of the microscopy readings was certified by an acceptable discordance rate between the reference and the field microscopists.
WHO recommends confirmation of malaria prior to ACT treatment and this is most often done by RDTs (WHO, 2006).
P. falciparum infections. Indeed the sensitivity of these tests was similar to those found in previous studies in Mali (Ouattara et al., 2011; Dolo et al., 2002) and to the results from our unpublished 2009 study with Paracheck® test (a HRP-2 based test) in Bancoumana, Doneguebougou and Bougala-Hameau, Mali where we found a sensitivity of 99.6% during a high malaria transmission season (our unpublished data). Similar high sensitivity was described in China-Myanmar (Liu et al., 2013) and in Ahmedabad in Gujarat State, India (Vyas et al., 2014). The detection sensitivity of Wondfo® rapid diagnostic kit (Pf-HRP2/PAN-pLDH) was similar to our results at 96.5% for P. falciparum (HRP2), 95.0% for P. falciparum (pLDH), and 96.8% for non-falciparum in China and Burma (Wu et al., 2014).
Sensitivity results were superior to reports from Burkina Faso where a sensitivity of 89.9% (95% CI: 89.0-90.8) and a Youden index of 40% was documented using another HRP-2 based test (Paracheck®, Orchid Biomedical Systems, Goa, India). Given the similarity in transmission intensity between Mali and Burkina Faso, these differences may be due to inter-RDTs variabilities. Furthermore, a study in Central African Republic (Bangui) found lower sensitivity than that obtained for SD Bioline Ag P.f [85.4% (95% CI, 80.5 to 90.7%)] and SD Bioline Ag P.f/pan (88.2% (95% CI, 83.2-92.0%)) (Djallé et al., 2014). However, that study used the combined microscopy + PCR as reference, while the current reference was just light microscopy. As was seen in the present data, these tests performed less well in cases of low parasitemia although the sensitivity was >95% at parasitemia > 500 parasites/μl.
The specificity of the tested RDTs was lower than that of the Optimal® (pLDH based test) test and another HRP-2 based assay (Parasight F®)in previous studies in Mali (Ouattara et al., 2011; Dolo et al., 2002). However, the specificity found was similar to results obtained during a more recent unpublished studies with Paracheck® which found a specificity of 70.8% (our unpublished data). These differences may be due to the fact that the referred studies were conducted between 1998 and 2003, that is 6 to 10 years earlier. It should be noted that the obtained specificities were better than that of a recent study conducted in Burkina Faso (50.4% (95% CI: 48.3 to 52.6). The positive predictive value for malaria infection was 77.9% (95% CI: 76.7-79.1) and the negative predictive value was 72.1% (95% CI: 69.7-74.3) in 13 regions of Burkina Faso (Samadoulougou et al., 2014). Specificity results were significantly lower than those found in Madagascar 98.9% (Ratsimbasoa et al., 2008), China-Myanmar (97.8%) (Liu et al., 2013), Gujarat State, India (97.3%) (Vyas et al., 2014) and China-Burma (99.7%) (Wu et al., 2014). The aforementioned studies were all conducted in areas with much lower transmission intensity than West Africa. Indeed, high transmission was recently documented by a survey in Mali, which found parasitemia prevalence above 70% in children (Dicko et al., 2010).it is expected to have better specificity with HRP-2 based test if the study were done in low transmission area such as in Madagascar (Ratsimbasoa et al., 2008) or during low transmission season in areas of high endemicity such as Mali.
A sub-study of both SD Bioline tests showed that they remained positives for Plasmodium species two weeks after malaria treatment, although parasites could not be detected by microcopy. These data confirm previous reports on the persistence of HRP-2 several weeks after the clearance of malaria parasites (Ouattara et al., 2011; Swarthout et al., 2007).
The manufacturer of the tested RDT claims that the tests could withstand temperatures up to 40°C. To verify that, few tests were transported in the open trunk of a vehicle for 14 h and the temperature both inside the container and outside was recorded. Unfortunately, on that day, it rained almost all day long and the maximum temperature to which the tests were exposed to was 34°C. Nevertheless, the performance of both Malaria Antigen P.f and Malaria Antigen P.f/Pan tests were similar to the tests transported in the air-conditioned cabin and outside.
Malaria Antigen P.f and Malaria Antigen P.f/Panperformance were comparable to other RDT brands in use in Mali.
These tests are recommendable for the diagnosis of malaria countrywide in Mali. Additional studies are needed to assess whether the used test strips could serve as sources of malaria parasite DNA for molecular studies.
The authors have not declared any conflict of interest.
We thank all the patients, parents, community leaders and health center staff that participated in the study. Standards Diagnosis (SD) provided the RDTs and partially funded the field studies. Malaria Research and Training Center, University of Science, Techniques and Technologies of Bamako, Mali supported this work. AG was supported by National Institutes of Health, Fogarty International Center.
REFERENCES
|
Bell D, Peeling RW (2006). Evaluation of rapid diagnostic tests: malaria. Nat. Rev. Microbiol. 4:S34-S38.
Crossref
|
|
|
|
Coulibaly D, Travassos MA, Kone AK, Tolo Y, Laurens MB, Traore K, Diarra I, Niangaly A, Daou M, Dembele A, Sissoko M, Guindo B, Douyon R, Guindo A, Kouriba B, Sissoko MS, Sagara I, Plowe CV, Doumbo OK, Thera MA (2014). Stable malaria incidence despite scaling up control strategies in a malaria vaccine-testing site in Mali. Malaria J. 13:374.
Crossref
|
|
|
|
|
Dicko A, Sagara I, Djimdé AA, Touré SO, Traore M, Dama S, Diallo AI, Barry A, Dicko M, Coulibaly OM, Rogier C, Sousa A, Doumbo OK (2010). Molecular markers of resistance to sulphadoxine-pyrimethamine one year after implementation of intermittent preventive treatment of malaria in infants in Mali. Malaria J. 9:9
Crossref
|
|
|
|
|
Djallé D, Gody JC, Moyen JM, Tekpa G, Ipero J, Madji N, Breurec S, Manirakiza A (2014). Performance of Paracheck™-Pf, SD Bioline malaria Ag-Pf and SD Bioline malaria Ag-Pf/pan for diagnosis of Plasmodium falciparum malaria in the Central African Republic. BMC Infect Dis. 14:109
Crossref
|
|
|
|
|
Dolo A, Konaré A, Ouattara A, Thera MA, Poudiougou B, Maiga B, Diallo M, Doumbo OK (2002). Intérêts des nouvelles techniques de diagnostic rapide du paludisme au Mali. Mali Méd. 17(3-4):27-31.
|
|
|
|
|
Gillet P, Mori M, Van Esbroeck M, Van den Ende J, Jacobs J (2009). Assessment of the prozone effect in malaria rapid diagnostic tests. Malaria J. 8:271.
Crossref
|
|
|
|
|
Koita OA, Doumbo OK, Ouattara A, Tall LK, Konaré A, Diakité M, Diallo M, Sagara I, Masinde GL, Doumbo SN, Dolo A, Tounkara A, Traoré I, Krogstad DJ (2012). False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am. J. Trop. Med. Hyg. 86(2):194-198.
Crossref
|
|
|
|
|
Liu H, Li XR, Li CF, Li XL, Wang HY, Nie RH (2013). Field evaluation of SD(BIOLINE) malaria antigen Plasmodium falciparum/Plasmodium vivax rapid test kit. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing ZaZhi. 31(2):160-1
|
|
|
|
|
Ouattara A, Doumbo S, Saye R, Beavogui AH, Traoré B, Djimdé A, Niangaly A, Kayentao K, Diallo M, Doumbo OK, Thera MA (2011). Use of a pLDH-based dipstick in the diagnostic and therapeutic follow-up of malaria patients in Mali. Malaria J. 10:345
Crossref
|
|
|
|
|
Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Ménard D (2008). Evaluation of Two New Immuno-chromatographic Assays for Diagnosis of Malaria. Am. J. Trop. Med. Hyg. 79(5):670-672
|
|
|
|
|
Samadoulougou S, Kirakoya-Samadoulougou F, Sarrassat S, Tinto H, Bakiono F, Nebié I, Robert A (2014). Paracheck® rapid diagnostic test for detecting malaria infection in under five children: a population-based survey in Burkina Faso. Malar J. 13:101
Crossref
|
|
|
|
|
Sogoba N, Vounatsou P, Bagayoko MM, Doumbia S, Dolo G, Gosoniu L, Sékou FT, Thomas AS, Yéya TT (2008). Spatial distribution of the chromosomal forms of Anopheles gambiae in Mali. Malaria J. 7:205.
Crossref
|
|
|
|
|
Swarthout TD, Counihan H, Senga RKK, van den Broek I (2007). Paracheck-Pf accuracy and recently treated Plasmodium falciparum infections: is there a risk of over-diagnosis? Malaria J. 6:58
Crossref
|
|
|
|
|
Vyas S, Puwar B, Patel V, Bhatt G, Kulkarni S, Fancy M (2014). Study on validity of a rapid diagnostic test kit versus light microscopy for malaria diagnosis in Ahmedabad city, India. East Mediterr Health J. 20(4):236-41
|
|
|
|
|
Wongsrichanalai C (2001). Rapid diagnostic techniques for malaria control. Trends Parasitol. 17:307-309.
Crossref
|
|
|
|
|
World Health Organization (2006). RBM Fact Sheet: What is Malaria? Roll Back Malaria Partnership Secretariat, Geneva, Switzerland. Available at:
View
|
|
|
|
|
World Health Organization (2015). Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 6 (2014-2015). December 2015. Geneva, Switzerland. Available at:
View
|
|
|
|
|
World Health Organization (2010). Basic malaria microscopy; 2nd edition: Part 1. Geneva, Switzerland.
|
|
|
|
|
World Health Organization (2014). World Malaria Report 2014. Geneva, Switzerland. Available at:
View
|
|
|
|
|
Wu J, Peng Y, Liu X, Li W, Tang S (2014). Evaluation of wondfo rapid diagnostic kit (Pf-HRP2/PAN-pLDH) for diagnosis of malaria by using nano-gold immunochromatographic assay. Acta Parasitol. 59(2):267-71.
Crossref
|
|