ABSTRACT
This study was carried out to reveal microbial contaminants on some selected ready-to-eat fruits and vegetables (eggplant, apple, tiger nut, bitter kola, cola nut, date and carrot) vended at motor parks, busy roads and local markets in Akure and Ado Ekiti metropolis (South Western Nigeria). It also evaluated the effectiveness of polyethylene packaging in controlling microbial contamination of these foods using product shelf life indices as criteria. Bacteria contaminants were isolated from samples and characterized using standard microbiological methods. Samples were also disinfected and stored in 1, 4, 7, 10 and 13 µm thick polyethylene bags at 29°C and 84% RH for 10 days. Result showed that cola nut, carrot, eggplants and tiger nut sold at all wholesale and retail points studied (100%) were contaminated. Apple and bitter kola at wholesale points were however free of microbial contaminants as was also observed for apple at two of its retail points (20%) and bitter kola at only one of the ten retail points studied (10%). Staphylococcus spp. was the most frequently isolated, followed by Klebsiela spp., others include Proteus, Bacillus, Pseudomonas, Serratia and Streptococcus species. Storage of bitter kola, eggplant and date in 7, 10 and 13 µm thick polyethylene films extended shelf life for ten days in ambient temperature of the tropics, while 1 and 4 µm were best for carrot under the same condition. Contrariwise, cola nuts stored in the different polyethylene thicknesses studied became discoloured during storage. Packaging of bitter kola, eggplant, date and carrot in polyethylene prevented microbial contamination and extended shelf life during retailing.
Key words: Ready-to-eat, microbial contaminants, polyethylene bags, storage.
Diverse plant parts consumed by man including leaves, roots, tubers, flowers, fruits and nuts are generally described as fruits and vegetables. The ready-to-eat fruits and vegetables are moreover, those plant parts that are eaten raw, without necessarily having to cut, peel, rinse or further processed (Kalia and Gupta, 2006). Despite the benefits of fresh fruits and vegetables to healthy living, their contamination with pathogenic bacteria such
as Clostridium perfringes, Escherichia coli (both enterotoxigenic and enterohemorrhagic species), Staphylococcus aureus, Salmonella typhi, Lysteria monocytogenes, Yesinia enterocolitia, Campylobacter jejuni and Campylobacter coli has been reported in different regions of the world (Beuchat, 1996; De Reova, 1998; Carmo et al., 2004; Bukar et al., 2010; Eni et al., 2010; Beuchat et al., 2013; US FDA, 2015; Denis et al., 2016). Reports on disease outbreaks caused by consumption of bacterial contaminated fruits and vegetables and those contaminated with viruses such as hepatitis A virus, Calicivirus and Norwalk-like virus or protozoan contaminants such as Cryptosoporidium parvum, Cylosporacaye tanensis and Giardia laniblia are creating burden to consumers (US FDA, 2000; ANON 2000; ANON, 2002). Gomez-Govea et al. (2012) reported coliforms of about 105cfu g‑1 on tomatoes and parsley in Monterrey, Mexico. Mangoes contaminated with Salmonella species were also reported to cause disease outbreaks in the US (US FDA, 2000; ANON 2002). Eni et al. (2010) isolated S. aureus, Klebsiella spp., Salmonella spp. and E. coli at loads ranging between 9.0 × 105 and 3.0 × 107cfu ml‑1 from carrot, runner bean, cucumber, fresh cut pineapple, green pepper, cabbage, spring onions, lettuce, water melon and apple in Sango-Ota, Nigeria. The 2014 joint FAO/IAEA program-NAFA report revealed that foodborne illnesses occur in every country in ever increasing frequency and many of these illnesses are now being attributed to fruits and vegetables imported from developed and developing countries.
Microbial contamination of fruits and vegetables may occur during production in open fields, greenhouses and hydroponic systems. Level of contamination is also affected by the location of the edible parts during growth; products that developed in the soil and those located at the soil surface for example are exposed to greater contaminants than those at the aerial parts of the plant. Wash tanks, irrigation water and water used in the application of fungicides and insecticides are other source of microbial contamination (ANON, 2002; De Lapeyre de Bellaire et al., 2010; Beuchat, 1996). Furthermore, manure or other composted organic matter and municipal sludge, droppings of wild and domestic animals are important sources of microbial contaminants on fruits and vegetables. Harvesting equipment, transport vehicles and handling activities that enhance contact with the soil, fecal materials or insects when products are in the hands of wholesalers and retailers are also sources of contamination (Burnette and Beuchat, 2001). Furthermore, globalization of food market transfers microbial contaminants via fruits to the different regions of the world. Imported fruits and vegetable are now seen as vehicle of pathogenic microbes in international markets (ANON, 2002). Microbes can also adhere onto the surface of fruits, invade or penetrate fruit surface and multiply within the tissue (Montville and Matthews, 2008; Abadias et al., 2008).
Packaging of fruits and vegetables in polyethylene bags prevents microbial contamination during retailing after commodities have been washed and appropriately disinfected. Sealed polyethylene bags however modifies the atmosphere around fruits and vegetables; it reduces O2 and increases CO2 as well as relative humidity levels (Bastiaanse et al., 2010; Lassois and de Lapeyre de Bellaire, 2014; Mahajan et al., 2014). These changes occur as a result of fruit’s respiration and transpiration activities; since the fruits and vegetables are still living even after harvest. Fruits and vegetables respond to CO2 and O2 levels differently, for example, banana ripen unevenly and becomes damaged, when stored under CO2 levels above 7 to 12% or O2 levels below 1 to 2% (Thompson, 1998). Appropriate equilibrium concentration of reduced O2 and increased CO2 however slows metabolic activities down and extends shelf life of fruits and vegetables, some combinations (high CO2 >15% and low O2 < 1%) are even fungi toxic (Al Zaemey et al., 1994; Caleb et al., 2013; Troncoso-Rojas and Tiznado-Hernandez, 2014; Errampalli, 2014). Storage of persimmon and Penicillium expansum inoculated apple fruits in modified atmosphere, created by sealing in low-density polyethylene bags have been reported to delay development of black-rot disease in the case of persimmon and controlled progress of fruit decay in the inoculated apple (Ben-Arie et al., 1991; Suzaki et al., 2008). Also modified atmosphere of 1 to 2% O2 and 12 to 14% CO2 caused a 20% reduction in crow rot disease on banana (Bastiaanse et al., 2010). Levels of O2 and CO2 concentrations in modified atmosphere created by polyethylene bags depend on produce weight, its respiration rate, temperature of the environment, surface area, perforations and thickness of the polyethylene, permeability of gases to the film used in packaging and composition of air outside the polyethylene bag (Thompson, 1998; Mahajan et al., 2014). Humidity of the microenvironment of fruits packed in polyethylene, on the other hand, is influenced by respiration and transpiration activities of the produce as well as the water vapour permeability of the polyethylene (Mahajan et al., 2014). Most of the water molecules produced as a result of respiration and transpiration remain within the polyethylene bags causing condensation; a factor critical in microbial growth and decay of produce (Linke and Geyer, 2013). Permeability of polyethylene depends on film thickness and the quality of the polyethylene used (Thompson, 1998). Respiration rate of produce is however the only important factor in the determination of appropriate thickness for packaging since all other factors can be kept constant during the storage of a particular commodity (Mahajan et al., 2014). Most of the reports on modified atmosphere storage are however in combination with low temperature storage and tropical fruits develop chilling injuries when stored in these low temperature ranges (Troncoso-Rojas and Tiznado-Hernandez, 2014; Mahajan et al., 2014). This study is therefore aimed at revealing the microbial contaminants on retailed fruits and vegetables in the study areas and determines the effectiveness of packaging in different thicknesses of polyethylene films on shelf life at the ambient temperature of the tropics.
Collection and preparation of samples
Ready-to-eat fruits and vegetables including cola nut (Cola nitida), bitter kola (Garcinia kola), apple (Malus domestica), date (Phoenix dactylifera), carrot (Daucus carota), white eggplant (Solanum aethiopicum) and tiger nut (Cyperus esculentus) were studied. Samples were obtained from hawkers and retailing spots in ten motor parks; five in Akure metropolis, Akure South local government area of Ondo state and five in Ado metropolis, Ado local government area of Ekiti State both in the South Western part of Nigeria. Those in Akure metropolis included the motor parks of Ilesha, Benin, Oja Oba, FUTA North gate and old garage, samples were also collected from Ijigbo, Shasha, Oja Oba, Basiri and Ilesa motor parks in Ado metropolis. Furthermore, samples were also obtained from the wholesalers at the main markets of Akure or Ado Ekiti to trace the sources of contamination. Samples were collected into sterile plastic containers with lid and transferred to the laboratory. Mature and disease free fruits and vegetables of uniform sizes and colour were selected aseptically and used for the study.
Microbial analysis
Microbial load on each produce was enumerated by plating 1 ml of 10-2 and 10-4 dilutions of the wash waters on the nutrient agar and the colonies counted after 24 h incubation at 37°C.
Polyethylene packaging
Cola nut, apple, date, carrot, eggplant fruits, bitter kola and tiger nut samples were washed under running potable water and further disinfected by washing in dilute solution of sodium hypochlorite (1:9 of commercial JIK: water) before rinsing with sterile water. Samples were allowed to drained at room temperature (25±1â°C) and packed in polyethylene bags made from 1, 4, 7, 10 and 13 µm thick films (measured with micrometer screw gauge 543 to 471B MITUTOYO Instrument, Japan) in quadruplets. Three replicates of each polyethylene thickness were set up. Polyethylene bags were made by sealing film with impulse sealing machine. Polyethylene films were cut with sterile blade and thereafter sealed into pouches left with an open end. Bags were disinfected in hot water at 60°C for 30 min before use. Open ends of the polyethylene bags were sealed after packaging of the samples. Samples packed in polyethylene bags were labelled and stored for 10 days at 25±1°C and 84±2% relative humidity. Disease severity was ranked daily by modifying the method described by Aborisade and Ojo (2009). Briefly, 1 represented no mould growth/healthy appearance; 2 = appearance of mould or any other form of disease development; 3 = mould growth on up to 25% of fruit surface or any other form of disease development on the same portion; 4 = mould growth on up to 50% fruit surface or any other form of disease development on the same portion and 5 represented fruits and vegetables that were completely covered with mould or totally diseased. For the colour development during storage 1 represented normal colour, 2 = trace of colour change, 3 = less dicoloured portion than normal coloured portion, 4 = more dicoloured portion than normal coloured portion, 5 = totally dicoloured produce. Similarly, texture change during harvest was monitored as wrinkling or shriveling by grading 1 as normal appearance, 2 = appearance of wrinkling or shriveling, 3 = less wrinkled portion than normal portion, 4 = more wrinkled portion than normal portion and 5 = totally wrinkled produce appearance.
Data obtained were subjected to analysis of variance and where significant, the means were compared with New Duncan’s multiple range test at P≤0.05.
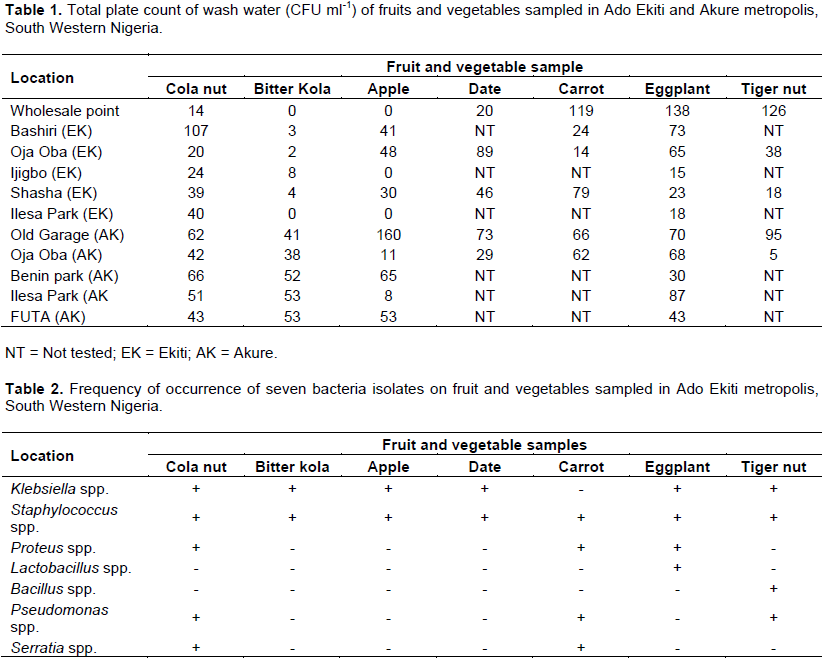
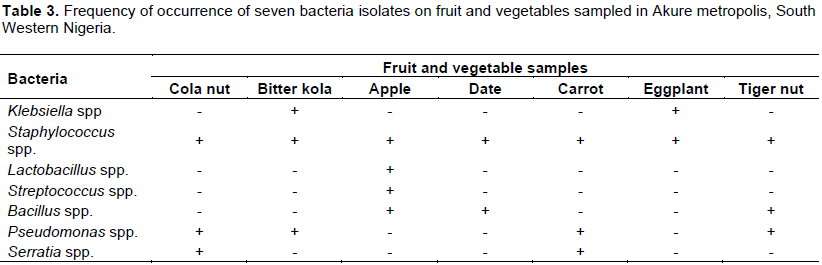
Bitter kola and apple samples obtained from the wholesale points were free of microbial contamination while cola nut, date, carrot, eggplant fruit and tiger nuts obtained from the wholesaler were contaminated (Table 1). Highest microbial load of 138 cfu ml-1 was obtained on eggplant fruits from the wholesalers, this value was closely followed by 126 and 119 cfu ml-1 obtained from the wash waters of the tiger nut and carrot respectively. The least contamination on samples from the wholesalers was 14 cfu ml-1which occurred in cola nut; the date had a microbial load of 20 cfu ml-1at the wholesale points. Among the retailed fruits and vegetable bitter kola and apple obtained from Ilesa park and the apple from Ijigbo park all in Ado Ekiti metropolis were free of microbial contamination. Apple retailed at Old garage in Akure metropolis however had the highest contamination with a microbial load of 160 cfu ml-1 followed by cola nut retailed at Basiri in Ado Ekiti metropolis with a microbial load of 107cfu ml-1 (Table 1). Of all the produce tested, bitter kola had the least contamination in all the retail outlets studied in Ado Ekiti metropolis. Retailed fruits were generally more contaminated in Akure metropolis than in Ado metropolis in almost all cases in this study. Microbial contamination was higher on cola nut, bitter kola, apple and date at the retail points studied in the two metropolis than at their wholesale points. This was however reversed in the case of carrot, eggplant and tiger nuts which were more contaminated at the wholesale points than at their retail points in the two metropolis studied (Table 1). Microbial contamination of these produce however did not follow a particular trend at the retail points in the two metropolis studied. Frequency of isolation of Staphylococcus spp. was highest in this study, it occurred on all the produce tested in the two study areas (Tables 2 and 3). Klebsiela spp. was also isolated on all produce in Ado metropolis except on carrot while Pseudomonas spp. was isolated on cola nut, carrot and tiger nut from the retail points in Ado Ekiti and Akure metropolis and only on biter kola in Akure but not in Ado Ekiti (Tables 2 and 3). Serratia spp. contaminated cola nut and carrot in the Ado Ekiti and Akure study areas (Tables 2 and 3).
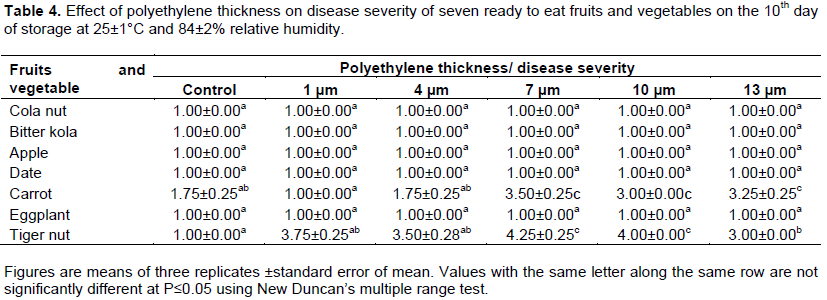
Cola nut, bitter kola, apple, date and eggplant fruit packed in 1, 4, 7, 10 and 13 µm polyethylene bags and their unpacked control remained disease free throughout the 10 days of storage (Table 4). Only 1 µm thick polyethylene bag however protected carrot for the same period when its control became diseased at mean 1.75.
Rot ranged between 1.75 and 3.50 on all the other carrot tubers packed in 4, 7, 10 and 13µm polyethylene bags on the 10th day of storage (Table 4). None of the polyethylene thicknesses protected tiger nut, the nuts became diseased at means between 3.00 and 4.25 on the 10th day of storage. Unpacked tiger nuts however remained disease free (Table 4).
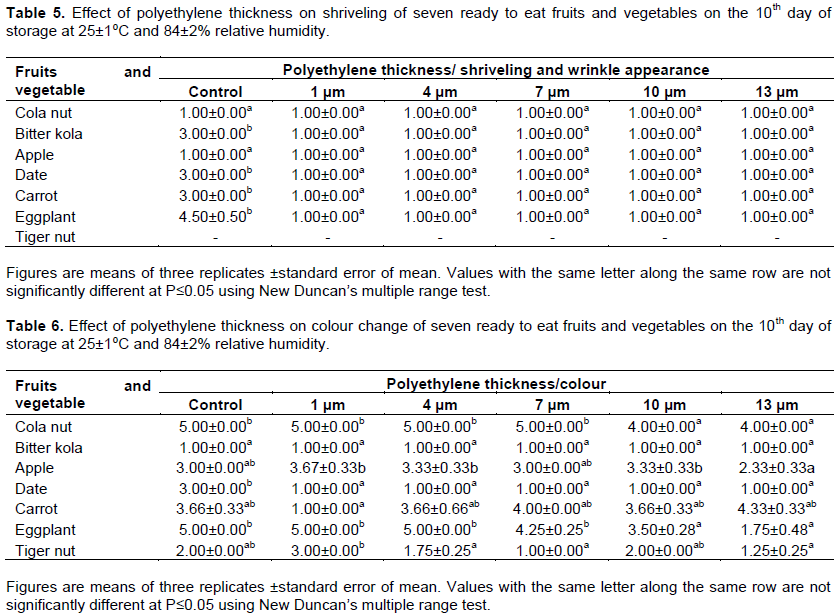
All polyethylene thicknesses protected all the produce tested from shriveling and wrinkling during storage, they all remained at mean 1.00 on the 10th day of storage (Table 5). Unpacked bitter kola, date, carrot and eggplant fruits were howevershriveledatmeansbetween3.00 and 4.50 on the same day. Shriveling was not observed on the unpacked cola nut and apple; they remained at mean 1.00 as those kept in polyethylene bags on the 10th day of storage (Table 5). Cola nut stored in the different polyethylene thicknesses turned green at means 4.00 and 5.00 on the 10th day of storage (Table 6). Colour change was not observed on bitter kola stored in all thicknesses of polyethylene tried, nuts remained at mean 1.00 as at the start of experiment. The colour of apples fruits stored in 13 µm thick polyethylene bags was best when compared with those packed in 1, 4, 7 and 10 µm polyethylene thicknesses on the 10th day of storage (Table 6). These fruits had the least colour change at mean 2.33 compared with values between 3.00 and 3.67 obtained for other apple fruits stored in 1, 4, 7 and 10 µm polyethylene thicknesses and the unpacked control (Table 6). Dates stored in the different polyethylene thicknesses were at mean 1.00, they were better than the unpacked control at mean 3.00. The result showed that the thicker the polyethylene film used in packaging, the lower the ripening in eggplant fruits; eggplant fruits stored in 13 µm thick polyethylene ripened least at mean 1.75 compared with the 5.00 of the control and those stored in 1, and 4 µm thick polyethylene bags (Table 6). Those packed with 7 and 10 µm thick polyethylene films ripened at means 4.25 and 3.50 respectively. Summarily, result obtained from the disease severity, wrinkling and colour change studies showed that bitter kola and date packed in 1, 4, 7, 10 and 13 µm thick polyethylene were better than the control. Apart from the protection against microbial contamination during the period of retailing, these polyethylene thicknesses protected bitter kola and date from disease development, they also prevented shriveling and maintained the colour throughout the 10 days of storage. Apples were also protected by the 13 µm thick polyethylene bags from disease development, shriveling and ripening. This was also observed in carrot packed in 1 µm thick polyethylene bags. Tiger nuts left unpacked during storage were better than those sealed in the polyethylene bags in this study, disease severity was between 3.00 and 4.25 on the tiger nuts packed in the polyethylene bags while those left opened on the tray remained wholesome at mean 1.00 (Table 4). Carrot packed in 1 µm thick polyethylene bags were better than the control and those packed in 4, 7, 10 and 13 µm thicknesses which were diseased at means between 1.75 and 3.5 on the 10th day of storage (Table 4).
Packaging of bitter kola, apple, date, carrot and eggplant fruits in polyethylene to prevent microbial contamination during retailing effectively protected the tested produce from disease development, shriveling, colour change and senescence in ambient temperature of the tropics in this study. Effectiveness was however thickness and produce dependent; 1, 4, 7, 10 and 13 µm thick polyethylene bags were effective on bitter kola and date, while only 1 µm was effective on carrot. These produce remained wholesome and were better than the unpacked control for ten days at 25±1°C temperature. Furthermore, packaging of eggplant fruits and apple in 13 µm thick polyethylene bags prevented disease development and shriveling, it also significantly reduced ripening when compared with the control. Polyethylene has lower water vapour permeability relative to transpiration rates of fresh fruits and vegetables (Linke and Geyer, 2013). This caused the trapping of most of the water molecules evaporated from the produce thereby enhancing the water vapour pressure within the package which must have prevented shriveling of the stored produce in this present study. Polyethylene is described as one of the cheapest and most widely used packaging material in the food and beverage industries (Brown, 1992). Packaging of these produce in polyethylene bags is a good replacement for the current practice of packing fruits and vegetables vended at motor parks, busy streets and highway in used paper, dry leaves, open trays and wheel barrow in the area of study. Packaging in polyethylene prevents recontamination of produce during retailing after washing and disinfection. All produce purchased and tested in this study were contaminated with microorganisms at the retail points, Staphylococcus spp. which is pathogenic according to the report of Lowy (1998) and Weems (2010) was isolated from all the produce tested. Other disease causing organisms like Klebsiella, Pseudomonas, Proteus and Bacillus species were also frequently isolated. This finding is also alluded to in the report of Eni et al. (2010), Adebolu and Ifesan (2001), Uzeh et al. (2009), and Bukar et al. (2010) on microbial contamination of fresh fruits and vegetables in Nigeria. Unfortunately, people on transit or travelers on Nigeria roads most of the time are compelled to purchase these ready-to-eat fresh produce and eat immediately. Thorough washing is limited because of the vehicle constraint or unavailability of water or both, forcing people to eat these produce as purchased. Cola nut being a stimulant for example is usually consumed by many to ward off sleep when on long journeys. Lack of adequate disease surveillance has been the reason why lots of the diseases associated with the consumption of these produce have not been properly reported in the study area.
Furthermore, results obtained from the study showed that microbial loads on cola nut, bitter kola, apple and date significantly increased on retail samples over the wholesales’. There were as much as 7-fold increase in microbial contamination on cola nut sampled during retailing over those sampled at wholesale points. A100-fold increase was also recorded on bitter kola and apple while 4-fold increase was recorded on date. This showed that microbial contamination of the studied produce occurred majorly during retailing.
The absence of microbial contaminants on bitter cola obtained at the wholesale point might be because of the antimicrobial properties of the bitter kola which is very high immediately the nuts are removed and cleaned-off the disintegrated pod (Okigbo and Mmeka, 2008; Akinnibosun and Itedjere, 2013). This process is usually carried out by the farmers before sale to the wholesalers. In the case of apple being an imported commodity, the freedom of fruits surface from contamination is expectedly due to the handling immediately after harvest which involves thorough washing and disinfection before packaging in paper board boxes received by the wholesalers from the exporting country. Carrot, eggplant fruits and tiger nuts purchased from the wholesalers were however heavily contaminated in this study; and may be as a result of the location of the commodity during growth for example the carrot being a root tuber was located in the soil during the period of development. Eggplant fruits on the other hand are usually harvested manually in Nigeria, they are conveyed in baskets overhead and poured on bear flour in farmers’ markets where wholesalers purchase and sell directly to the retailers. The wholesalers may also stock the fruits without washing until the retailers pick them up. The observed reduction in the microbial load on the retailed fruits over the wholesale samples is as a result of the washing of the fruits by the retailers before sale to attract consumers. The inoculum on fruit surface after such washing as observed in this study is the left over resulting from the heavily contaminated wash tanks or possibly poor quality water used for the so called washing. These observations are in agreement with the report of Beuchat (1996) and that of Ray and Bhunia (2007) that the microorganisms present on fruits and vegetables are a direct reflection of the sanitary quality of the harvesting, transportation, storage and processing of produce. Many studies in Nigeria and elsewhere have isolated bacteria contaminants on surfaces of fruits and vegetables (Beuchat, 1996; De Reova, 1998; Carmo et al., 2004; Bukar et al., 2010; Eni et al., 2010; Beuchat et al., 2013), this study however established that the contaminants on produce retailed at the motor parks where consumers are constrained to eat without washing are majorly from the handling at the retail points. This will help to establish the risk profile and identify the element of hazard relevant to risk management decisions. Importation of fruits and vegetable from outside of the European community has been identified as one of the factors contributing to risk encountered when fresh fruits and vegetables are eaten raw (ANON 2002). Knowledge of the major source of contamination of the produce studied will help forestall the possible transfer of the inoculum with these produce to other regions of the world.
The study also showed that there was disparity in the microbial loads on produce obtained from the different locations, meaning that handling by the individual vendors significantly affected microbial contamination of the studied produce. Similar observation was made by Eni et al. (2010) who reported on the microbial contaminations of fruits in Ota, Ogun State Nigeria.
Solution to the problem of microbial contamination on bitter kola, apple, date, carrot and eggplant fruits retailed at motor parks and busy roads in Akure and Ado Ekiti, South Western Nigeria may be by packaging of these produce in polyethylene bags immediately after washing and disinfection. This will extensively reduce, if not totally remove microbial contamination on these ready- to- eat fruits and vegetables. Bitter kola and date remained wholesome for 10 days when packed in 1, 4, 7, 10 or 13 µm polyethylene films similarly, carrot should be packed in 1 µm thick polyethylene while eggplant fruits and apple may be packed in 13 µm thick polyethylene films. Thicker polyethylene should however be tied on cola nuts as the 13 µm thickness resulted to better colour than the lower thicknesses but the level of discolouration was still high.
REFERENCES
|
Abadias M, Usail J, Anguera A, Solsona C, Vinas I (2008). Microbiology of fresh minimally-processed Fruits and vegetables, and sprouts from retail Establishments. Int. J. Food Microbiol. 123:121-129.
Crossref
|
|
|
|
Adebolu TT, Ifesan BO (2001). Bacteriological quality of vegetables used in salads. Niger. J. Microbiol. 15(1):81-85.
|
|
|
|
|
Akinnibosun FI, Itedjere E (2013). Evaluation of the antibacterial properties and synergistic effect of Garcinia Kola Heckel (Family: Guttiferae) seed extract and honey on some bacteria. Afr. J. Microbiol. Res. 7(3):174-180.
|
|
|
|
|
Al Zaemey AB, Magan N, Thompson AK (1994). In vitro studies of the effect of environmental conditions on the anthracnose pathogen of banana, Colletotrichum musae. Int. Biodeter. Biodegrad. 33:369-381.
Crossref
|
|
|
|
|
ANON (2000). Pathogen and produce: Foodborne Outbreak Incidents. European Chilled Food Federation. Presented to the EC Scientific Committee for Food. March, 2000.
|
|
|
|
|
ANON (2002). Risk Profile on the microbiological Contamination of Fruits and Vegetables Eaten Raw. Report of the EC Scientific Committee on Food. April, 2002.
|
|
|
|
|
Bastiaanse H, de Lapeyre de Bellaire L, Lassois L, Misson C, Jijakli MH (2010). Integrated Control of Crown Rot of Banana with Candida oleophila strain O, Calcium Chloride and Modified Atmosphere Packaging. Biol. Control 53:100-107.
Crossref
|
|
|
|
|
Beuchat LR (1996). Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59(2):204-216.
Crossref
|
|
|
|
|
Beuchat LR, Komitopoulou E, Beckers H, Betts RP, Bourdichon F, Fanning S, Joosten HM, Ter- Kuile BH (2013). Low-water activity foods: increased Concern as vehicles of foodborne pathogens. J. Food Prot. 76(1):150-172.
Crossref
|
|
|
|
|
Ben-Arie R, Klein J, Zutkhi Y (1991). Long-term storage of persimmons. Postharvest Biol. Technol. 4:169-179.
Crossref
|
|
|
|
|
Brown WE (1992). Properties of plastics used in Food Packaging in Plastics in food packaging, Ed. W. E. Brown Marcel Dekker, Inc., Ney York P 105.
|
|
|
|
|
Bukar A, Uba A, Oyeyi TI (2010). Occurrence of some pathogenic bacteria in some minimally and fully processed ready to eat foods in Kano metropolis, Nigeria. Afr. J. Food Sci. 4(2):032-036.
|
|
|
|
|
Burnette AB, Beuchat LR (2001). Human Pathogen associated with raw produce and unpasteurized juices, and some reasons related to difficulties in decontamination. J. Ind. Microbiol. Biotechnol. 25:281-287.
Crossref
|
|
|
|
|
Caleb OJ, Mahajan PV, At-Said FA, Opara UL (2013). Modified Atmosphere Packing Technology of Fresh and Fresh-cut produce and the Microbial consequences. Food Bioprocess Technol. 6:303-329.
Crossref
|
|
|
|
|
Carmo LS, Cummings C, Linardi VR, Souza JM, Sena MJ, Santos DA, Shupp JW, Pereira RK, Jett M (2004). A case study of massive Staphylococcal food poisoning incident. Foodborne Pathog. Dis. 1:241-246.
Crossref
|
|
|
|
|
De Lapeyre de Bellaire L, Lassois L, Jijakli HM, Chillet M (2010). Preharvest Factors Involved in Postharvest Disease Development and Integrated Control Methods- Crown Rot of Bananas. Plant Disease 94(6):648-658.
Crossref
|
|
|
|
|
Denis N, Zhang H, Leroux A, Trudel R, Bietlot H (2016). Prevalance and Trends of Bacterial Contamination in Fruits and Vegetables Sold at Retail in Canada. Food Control. 67:225-234.
Crossref
|
|
|
|
|
De Reova C (1998). Microbial safety evaluations and recommendations on fresh produce. Food Control 9(6):321-347.
Crossref
|
|
|
|
|
Eni AO, Oluwawemitan IA, Solomon OU (2010). Microbial quality of fruits and vegetables sold in Sango Ota. Nigeria. Afr. J. Food Sci. 4(2):291-296.
|
|
|
|
|
Errampalli D (2014). Penicillium expansum (Blue mould) In: Postharvest Decay control strategies ed. S. Bautista-Banos Academic Press, USA pp. 205-206.
|
|
|
|
|
FAO/IAEA (2014). Contaminants and Residue. Report of the Joint Food and Agriculture/ International Atomic Energy Agency program- Nuclear Techniques in Food and Agriculture.
|
|
|
|
|
Gomez-Govea M, Solis-Soto L, Heredia N, Garcia S, Moreno G, Tovar O, Isunza G (2012). Analysis of microbial contamination levels of fruits and vegetables at retail in Monterrey, Mexico. Food Agric. Environ. 10:151-156.
|
|
|
|
|
Kalia A, Gupta RP (2006). Fruit microbiology. In Ed. Hui YHJ, Cano MP, Gusok W, Sidhu JW, Sinha NK. Hand book of fruits and fruit processing. 1st Edition. Blackwell publishing pp. 3-28.
Crossref
|
|
|
|
|
Lassois L, de Lapeyere de Bellaire (2014). Crown rot Disease of Banana. In: Postharvest Decay control strategies ed. S. Bautista-Banos Academic Press, USA pp. 103-130.
|
|
|
|
|
Linke M, Geyer M (2013). Condensation dynamics in plastic film packaging for fruits and vegetables. J. Food Eng. 116:144-154.
Crossref
|
|
|
|
|
Lowy FD (1998). Staphylococcus aureus infections. New England J. Med. 339:520-532.
Crossref
|
|
|
|
|
Mahajan PV, Caleb OJ, Singh Z, Watkins CB Geyer M (2014). Postharvest treatment of fresh produce. Phil. Trans. R. Soc. A. 372. 20130309.
Crossref
|
|
|
|
|
Montville TJ, Matthews KR (2008). Food Microbiology. An introduction, 2nd Ed. American Society of Microbiology (ASM) Press.
|
|
|
|
|
Okigbo RN, Mmeka EC (2008). Antimicrobial effects of three tropical plant extracts on Staphylococcus aureus, Escherichia coli and Candida Albicans. Afr. J. Tradit. Complement Altern. Med. 5(3):226-229.
Crossref
|
|
|
|
|
Ray B, Bhunia AK (2007). Fundamental Food Microbiology. 4thedn., CRC Press, USA, P 492.
|
|
|
|
|
Suzaki K, Ito T, Kanematsu S (2008). Occurrence and prevention of blue mould disease in apple. Mycotoxins 58:137-141.
Crossref
|
|
|
|
|
Thompson AK (1998). Controlled Atmosphere Storage of Fruits and Vegetables. Wallingford. CABI 278 p.
|
|
|
|
|
Troncoso-Rojas R, Tiznado-Hernandez ME (2014). Alternaria alternate (Black Rot, Black Spot) In: Postharvest Decay control strategies ed. S. Bautista-Banos Academic Press, USA pp. 170-171.
|
|
|
|
|
US FDA (2000). Experience with microbial Hazards in fresh produce. Lee Ane Jackson. Center for Food safety and Applied Nutrition Food and Drug Administration. Presented to the EC Scientific committee on food, March, 2000.
|
|
|
|
|
US FDA (2015). Outbreaks associated with fresh and fresh cut produce.
View
|
|
|
|
|
Uzeh RE, Alade FA, Bankole M (2009). The microbial quality of pre-packed mixed vegetable salad in some retail outlets in Lagos, Nigeria. Afr. J. Food Sci. 3(9):270-272.
|
|
|
|
|
Weems JJ (2010). The many faces of Staphylococcus aureus infections. Postgraduate Med. 110(4):24-36.
Crossref
|
|