ABSTRACT
Distilled water, acetone and ethanol (polar solvent) extracts from 20 g/100 ml and 30 g/100 ml levels of extraction of Calpurnia aurea and Milletia ferruginea were tested as protectant against maize weevils in maize grains under laboratory condition. They were applied at a rate of 10 and 15% (w/v) in admixture bioassays from both of the aforementioned extraction levels. Parental weevil’s mortality, F1 progeny emergence, percent protection, grain damage and weight loss were the parameters measured. All polar solvent extracts of the tested plants applied at a rate of 10 and 15 ml from the two extraction levels induced significant (p≤0.05) toxicity effect against weevils than solvent treated grains at all dates after treatment. Besides, significantly (p≤0.05) higher mortality of parental weevils were recorded in all polar solvent extracts (>75%) of the tested botanicals applied at 15 ml dosages from 30 g/100 ml extraction levels than those applied at a rate of 10 ml following 96 h treatment application. Furthermore, all the polar solvent extracts applied at rates of 10 and 15% also induced good degree of protection of maize grains (≥78%) against F1 progeny emergence, percent grain damage (≤1.33) and weight loss (≤0.28) by maize weevils than negative control in about 2 months storage period (56 days). Consequently, the solvent extracts of C. aurea and M. ferruginea were potent and therefore, they can be used in management of maize weevils in stored maize under subsistence farmer’s storage conditions.
Key words: Calpurnia aurea, Milletia ferruginea, Sitophilus zeamais, botanicals, polar solvent extracts, stored maize.
Maize is the major staple food crop in Africa that contributes significantly to the agricultural sector (Tefera et al., 2011). It is also one of the major cereal crops grown for its food, feed, firewood and construction values (Sori, 2014). Of the cereal crops, it ranks second to tef in area coverage and first in total production nationally (Gemu et al., 2013). But, post-harvest insect pest’s grains have been indicted as major problems to food and income security of resource poor farmers in sub-Saharan Africa including Ethiopia due to their heavy losses of grains (Tadesse, 1991, 1997; Abebe et al., 2009). The most economically important of these storage insect pests are Coleopterous weevils (Getu and Abate, 1999). Different management strategies such as synthetic insecticides, botanicals and cultural practices have been used to control weevils in particular and storage pests in general, of which synthetic insecticides were the most commonly used farmers in Africa including Ethiopia (Mvumi et al., 1995; Mvumi and Stathers, 2003). However, health and environmental concerns have been associated repeated usage of synthetic pesticides over the years (Ofuya and Longe, 2009). This and other facts mentioned earlier indicates the presence of urgent need for searching cheap, environmentally sound and effective management options such as botanicals for reducing weevils losses in the aforementioned countries. Birbira, Milletia ferruginea (Hochst.) Baker and Calpurnia aurea (Ait.) Benth plants may have protective role of stored maize against weevils. The former one is a large shady tree which grows up to a length of 35 m and is endemic to Ethiopia and widely grown at the elevation between 1,000 and 2,500 m above sea level (Jembere, 2002; Getu, 2014).
It has been commonly used in traditional medicine. According to MacLachlan (2001), the roots and seeds of this plant are also used as insecticides and pesticides in many parts of the world, and rotenone are responsible for their toxicity. The later one is a small, multiâ€stemmed tree, 3 to 4 m tall plant. It is widely distributed in Ethiopia. It is widely grown in high land areas (Birhanu and Asale, 2015) and is easily cultivated (Germishuizen and Meyer, 2003). The plant has been commonly used in traditional medicine to treat diverse medical conditions and parasitic infestation, in humans and animals in African including Ethiopia (Watt and Breyerâ€Brandwyk, 1962). Its leaves and powdered roots are used to destroy lice and to relieve itches and they contain tannins, flavonoids, terpenoids, saponins, steroids, glycosides, alkaloids (Nega et al., 2016). Considering that the former plant is reported to contain rotenone and the later one to contain tannins, flavonoids, terpenoids, saponins, steroids, glycosides, and alkaloids, the present study was initiated with the following objectives: (1) to evaluate the toxicity potency of solvent extracts leaves of C. aurea and M. ferruginea against the most economically important storage insect pest of maize, maize weevil (Sitophilus zeamais) under laboratory conditions and (2) to determine the possibility of using these plants for management of insect pests stored maize by poor framers at national level and elsewhere with similar pest problem.
The study period
The study was conducted in between 1, October to 30, June of 2016/2017 in the Insect Science Laboratory of Zoological Science Department, Addis Ababa University of Ethiopia.
The test insect’s culture
S. zeamais adults were collected from maize grains stored in various farmers traditional storage facilities of major maize producing localities Shashogo and Sankura districts of Southern Ethiopia and brought to the Laboratory of Addis Ababa University, Faculty of Life Science, Insect Science Insectary of Zoological Science Department of Ethiopia. These test insects were cultured at 27 ± 3°C and 55 to 70% RH (Jembere et al., 1995; Zewde and Jembere, 2010). Shone variety of maize grains were also obtained from farmer’s storages of the aforementioned districts. It was the most commonly grown hybrid in the region and considered to be susceptible to insect infestation. The grains were kept at -20 ± 2°C for 2 weeks to kill any infesting insects, cleared of broken kernels and debris and then graded manually according to size and similar sized grains were selected for the experiment (Gemechu et al., 2013). Following the methods by Zewde and Jembere (2010), fifteen pairs of the adult of the test insects were placed in 12, 1-L glass jars containing 250 g seeds. The jars were then covered with nylon mesh and held in a place with rubber bands to allow ventilation and to prevent the escape of the experimental insects. The parent of the test insects were sieved out after an oviposition time of 13 days. Then, the seeds were kept under laboratory condition until F1 progeny emergence. The F1 progeny, which emerged after 30 days, were sieved out and used for the experiment.
Description of the tested botanicals and their preparation
Plant materials (that is leaves) used for the study were collected from natural habitats of Hadiya zone, Southern Ethiopia and the identities of the plants were confirmed into C. aurea and M. ferruginea species at the National Herbarium of Life Science Faculty of Addis Ababa University.
Admixture bioassay with botanicals leaves solvent extracts
Disinfested shone variety of maize grains (100 g) were placed in 1 L glass jars and treated with the water, acetone and ethanol extracts of the test botanicals at a rate of 10 and 15 ml from the two extraction levels; 20 g/100 ml and 30 g/100 ml following similar procedures by Zewde and Jembere (2010). The jar contents were shaken thoroughly for 5 min to ensure uniform distribution of the solution over grain surface. Then, the treated grains were kept for 24 h for acetone extracts and 36 h for ethanol extracts to allow complete evaporation of solvents before conducting of bioassay based on their property. Malathion 5% dust at the recommended dose rate of 0.05 g per 100 g of maize grains (positive control) and solvent treated grains (negative control) served as comparison and control tests respectively (Arannilewa et al., 2006). Then after, 20, three to seven day old unsexed experimental insects were introduced to the treated and untreated grains in each of the glass jars. The jars were covered with nylon mesh and held in place with rubber bands. Then, treated grains and controls were then kept under same experimental condition indicated in insect capture section. All treatments of solvent extracts were arranged in Completely Randomized Design (CRD) in three replications. Mortality was evaluated 24, 48, 72 and 96 h after the beginning of exposure following similar procedures by Gebreselassie and Getu (2009) and Zewde and Jembere (2010). All live insects were also sieved and discarded after 13 days of introduction.
F1 Progeny assessment bioassay
The treated grains and controls were also kept until emergence of F1 progeny under same experimental condition indicated in insect capture section. Then the numbers of F1 progeny produced by the experimental insects were counted. Counting were stopped after 56 days from the day of introduction to avoid overlapping of generation following similar procedures Gebreselassie and Getu (2009) and Zewde and Jembere (2010).
Damage and weight loss assessment assay
Two days after the last F1 count of 56 days, samples of 30 grains were taken randomly from each jar and the number of damaged (grains with characteristic hole) and undamaged grains were counted and weighed. Damaged grains were expressed as a percentage of the total number of seeds in each replicate. Percentage weight losses were calculated by count and weight method following similar procedures earlier researchers (FAO, 1985; Gebreselassie and Getu, 2009; Zewde and Jembere, 2010) as follow:
Loss in weight (%) = [UNd – Dnu / U (Nd + Nu)] × 100
where U = weight of undamaged grain, D = weight of damaged grain, Nd = number of damaged grain, and Nu = number of undamaged grain.
Following similar procedures by Gebreselassie and Getu (2009) also percent protection or inhibition in F1 progeny emergence (% IR) was calculated using the following formula:
IR (%) = (Cn-Tn) × 100 / Cn
where Cn is the number of newly emerged insects in the untreated (control) jar and Tn is the number of insects in the treated jar.
Data analysis
Data on parental adult mortality, F1progeny emergence, and grain damage and weight loss were managed with the Microsoft Excel version 2013 and then were subjected to analysis of variance (ANOVA) of SPSS Version 16. Data of the former one was analyzed using appropriate statistical method, Univariate analysis, but data of the later ones were analyzed by one-way ANOVA. Significant differences between means of different treatments and time of exposure were separated using Tukey's studentized (HSD) test at 5% confidence interval. Difference among means were stated significant when p<0.05 and highly significant when p<0.01. Standard errors (±SE) are given the following means in Tables and as T-shaped beams in figures. Correlation between the treatments and the efficacy measuring parameters like weight loss and others were determined using Pearson’s correlation of SPSS program of version 16.
C. aurea and M. ferruginea leaf solvent extracts on the mortality of maize weevil
All polar solvent extracts (Distilled water, acetone and ethanol) the C. aurea and M. ferruginea applied at the rates of 10 and 15 ml of the two tested levels of extraction (20 g/100 ml and 30 g/100 ml) caused significantly (p< 0.05) higher mortality of maize weevils at all dates after treatment than the negative control. Besides, their efficacy was increased with increased dosage, extraction level and exposure time after treatment application. Polar solvent extracts of the tested plants leaves applied at rates of 10 and 15 ml from the aforementioned two levels of extraction also induced significantly (p < 0.05) higher toxicity effect against weevils following 72 (≥ 55%) and 96 h (≥ 60%) post treatment exposure than prior to them (Figures 1 and 2). The efficacy of the tested plants leaves solvent extracts in weevil’s morality was also varied (p < 0.05) significantly with the type of solvent used for extraction; the highest being occurred in ethanol exacts, followed by in acetone and in distilled water extracts in general. Significantly (p < 0.05) higher mortality (>75%) of parental S. zeamais adults were also recorded in all polar extracts of the tested botanicals applied at 15 ml dosages from 30 g/100 ml extraction levels than those applied at a rate of 10 ml following 96 h treatment application, the maximum of which occurred in ethanol extract (>80%), followed by in acetone and in distilled water extracts (between 75 and 80%) in general (Figures 1 and 2).
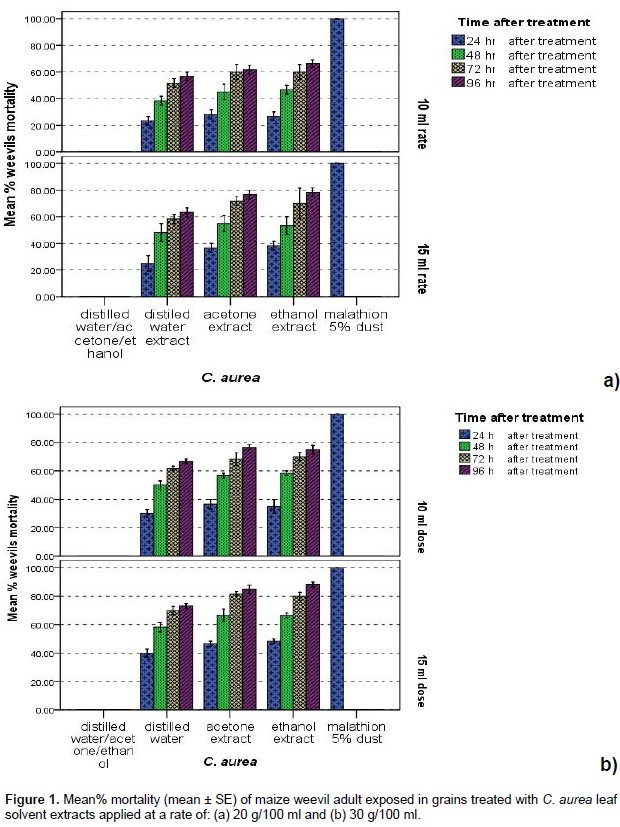
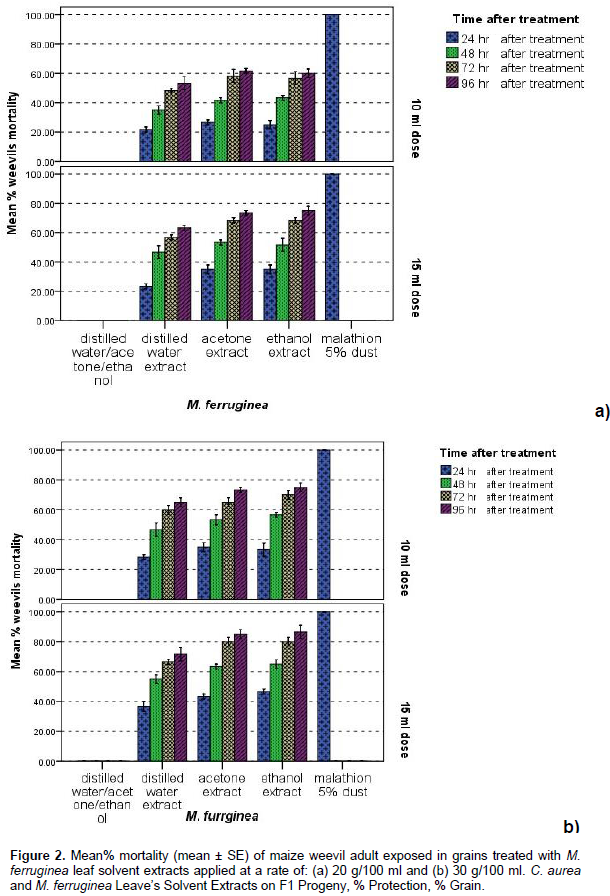
Damage and mean percentage weight loss
The number of F1 progeny produced, percentage grain damage and weight loss caused by S. zeamais in all treatments of botanicals solvent extracts were significantly (p < 0.05) lower as compared to negative control (each solvent treated grains). All polar solvent extracts treatments of the two tested botanicals applied at all rates (10 and 15% of solvent extracts) induced more than 73% inhibition in F1 progeny production and significantly (p < 0.05) higher reduction in grain damage (≤4.33) and weight loss (≤0.58) of maize grain by S. zeamais than negative control. However, 100% F1 progeny production inhibition, as well as no grain damage and weight loss of maize were observed in polar solvent extract treatments of the tested botanicals applied at a rate of 10% from 30 g/100 ml extraction levels and in those applied at 15% from both 20 g/100 ml and 30 g/100 ml extraction levels likewise that of the positive control (Tables 1 and 2). The correlations among the treatments solvent extracts of the tested plants leaves applied at different dosage and the efficacy parameters measured were found to be highly significant. The correlations between the various treatments of solvent extracts of the tested plants leaves and the various parameters measured (the number of F1 progeny emerged, percentage grain damage and weight loss) were negative. However, they were strongly positive between F1 progeny produced and percent grain damage and weight loss of all treatments of the tested plants solvent extracts (Tables 3 and 4).
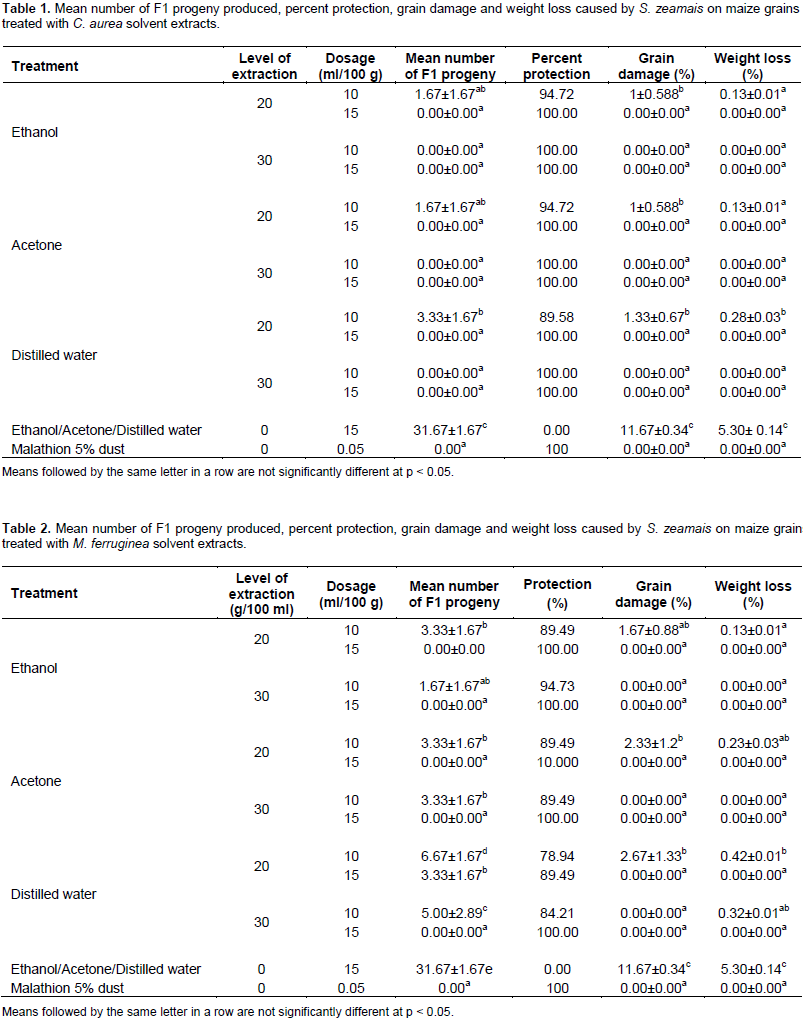
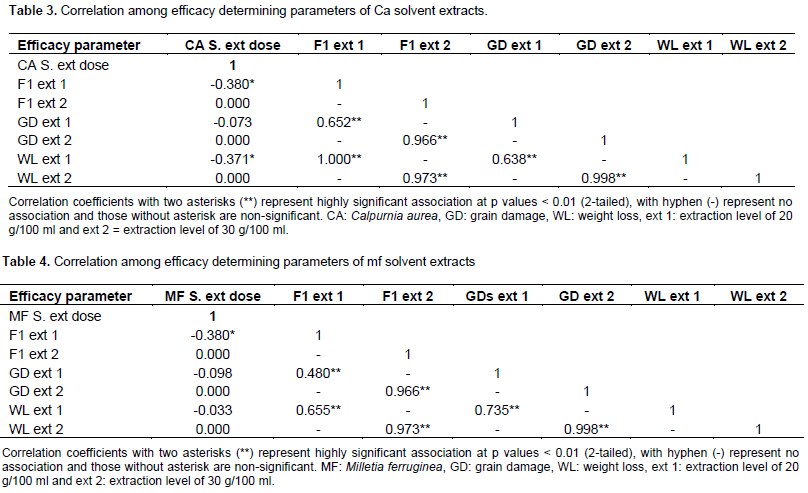
The current study revealed that the percentage of adult weevil’s mortality was increased (p≤0.001) significantly with increased dosage (concentration), extraction level, and exposure time after treatment for both tested botanicals in general in the bioassay tested. This result is in line with the findings of Gebreselassie and Getu (2009), Zewde and Jembere (2010), Qwarse (2015) and Gebre-Egziabiher (2016) in which mortality effect of botanicals were indicated to be dose and exposure time dependent. The present study also revealed that all polar solvent extracts (distilled water, ethanol and acetone extracts) of the tested botanicals leaves at tested rates (10 and 15 ml) from tested extraction levels induced significant toxic effect against S. zeamais than negative control. This suggests the presence of more polar solvent soluble phytochemicals in leaves of C. aurea and M. ferruginea which are responsible higher weevil’s mortality and as most of them probably might be polar since like dissolves like. Amante (2016) also suggested that the active ingredients in the leaf extract of the plant reside in the polar fractions indicating that the active principles are polar in nature after he studied castor bean plant against ectoparasites of animals. Jembere et al. (2005) also indicated that high Zabrotes subfasciatus mortality was caused by M. ferruginea water extract that probably might be due to the presence high water soluble chemicals in the seeds of it. Getu (2014) also indicated that the polar solvent extracts (acetone and water) of M. ferruginea seeds caused significantly high toxicity to Z. subfasciatus 48 h after treatment. Furthermore, Blum and Bekele (2002) also reported that C. aurea has been used as a natural pesticide to improve grain storage. It was also indicated that C. aurea possess potent activities (louscidal and acaricidal effects) against ectoparasites of animals (Amante, 2016).
In the current study, all polar solvent extract treatments (distilled water, ethanol and acetone extracts) of the two tested botanicals also caused significantly higher mortality of adult S. zeamais than negative control at all dates after treatment. They also induced significantly higher inhibition in F1 progeny emergence, as well as significantly higher reduction in grain damage and weight loss than negative control in about 2 months storage period. This higher efficacy the crude extracts may be attributed due to either the toxic or repellant effects of phytochemicals in the tested plants or starvation and interference with respiration due to suffocation of maize weevils. This result thus, suggests the potency of both the solvent extracts of the tested plants in protecting maize grains against weevils. Toxicity caused by crude extracts of the two botanicals tested against maize weevils in the current study was also in accordance to the result of pervious researchers (Kasa and Tadese, 1996; Jembere et al., 2005; Zewde and Jembere 2010; Gebreselassie and Getu, 2009; Getu, 2014). In the present study, the efficacy of the tested botanicals in weevil’s morality also varied (P≤0.05) significantly with the type of solvent used for extraction; the highest being occurred in ethanol exacts, followed by in acetone and in distilled water extracts. This result also agrees with finding of Jembere (2002) in which the water extracts of M. ferruginea was indicated to be the 3rd effective, 3 days after post treatment exposure, following acetone and ethanol extracts against maize weevils. In similar manner, Fredrick (2012) also indicated that the deleterious effects of the plant extracts against the maize weevil varied with the type of solvent extract applied. The aforementioned highest efficacy in ethanol might also be probably due to its broad solubility properties of organic compounds of the tested botanicals. In accordance with this, it was also shown that ethanol is an accepted solvent for contact application because of its broad solubility properties and low toxicity, among the various solvents (water, ethanol, acetone, petroleum ether and others) that have been used in the preparation of plants extracts for testing their toxicity to insect pests (Amoh, 2010). Similarly, Koffi et al. (2010) also showed that ethanol extract were found to be more effective than aqueous extracts of the same plant in general, as a result of higher solubility of organic compounds in ethanol.
In conclusion, all the tested polar solvent extracts (distilled water, ethanol and acetone extracts) of C. aurea and M. ferruginea were potent in protecting maize grains against maize weevils attack at a rate of 10 and 15%. This in turn confirmed the presence of possibility to exploit the potential of C. aurea and M. ferruginea in the management of S. zeamais under substance farmer’s storage conditions. Thus, solvent extracts, particularly water extracts of C. aurea and M. ferruginea which is cheap relatively can be used for management of maize weevils in stored maize under subsistence farmer’s storage conditions in Ethiopia and elsewhere with similar pest problems. However, their effect on human being, natural enemies and cost effectiveness in farmer’s storage conditions need further study before wide implementation of the outcomes this study.
The authors have not declared any conflict of interests.
The authors sincerely like to thanks Arba Minch University and Zoology Department of Addis Ababa University for providing them with financial support to conduct the study.
REFERENCES
|
Abebe F, Tefera T Mugo S, Beyene Y, Vidal S (2009). Resistance of maize varieties to the maize weevil Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). Afr. J. Biotechnol. 8:5937-5943.
Crossref
|
|
|
|
Amante M (2016). In vitro louscidal and acaricidal activities of alkaloid of Calpurnia aurea and fractions of Ricinus communis extracts against Linognathus ovillus and Amblyomma variegatum. M.Sc. Thesis, Addis Ababa University, College of Veterinary Medicine and Agriculture, Department of Pathology and Parasitology P 90.
|
|
|
|
Amoh BA (2010). Efficacy of ethanolic extract of Thevetia peruviana (Pers.) K. schum (milk bush) root in the control of major insect pests of cowpea. M.Sc. thesis, Kwame Nkrumah University, Ghana.
|
|
|
|
Arannilewa ST, Ekrakene T, Akinneye JO (2006). Laboratory evaluation of four medicinal plants against the weevil, Sitophilus zeamais (Motsch). Afr. J. Biotechnol. 5:2032-2036.
|
|
|
|
Birhanu A, Asale AG (2015). Larvicidal activity of solvent extractions from some selected indigenous plants against the Mediterranean fruit fly larvae Ceratitis Capitata identified from coffee berry (Diptera: Tephritidae) in Jimma Zone, Southwestern Ethiopia. Journal of Applied Sciences Agricultural, 10:78-85.
|
|
|
|
Blum A, Bekele A (2002). Storing grains as a survival strategy of small farmers in Ethiopia. J. Int. Agric. Ext. Ed. 9:77-83.
|
|
|
|
Food and Agriculture Organization (FAO) (1985). Prevention of post-harvest food losses: A training manual, FAO, Training Series No. 11, Rome, Italy.
|
|
|
|
Fredrick I (2012). The potential of bio-active protectants of maize grains against Sitophilus zeamais in western Kenya. M.Sc. Thesis, Kenyatta University.
|
|
|
|
Gebreselassie A, Getu E (2009). Evaluation of botanical plants powders against Zabrotes subfasciatus (Boheman) (Coleoptera: Bruchidae) in stored haricot beans under laboratory condition. Afr. J. Agric. Res. 4:1073-1079.
|
|
|
|
Gebre-Egziabiher KG (2016). Evaluation of some botanicals and sorghum varieties and landraces for the management of maize weevil, Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). M.Sc. thesis. Haramaya University.
|
|
|
|
Gemechu F, Santiago DR, Sori W (2013). Laboratory evaluation of cotton (Gossypium hirsutum) and Ethiopian mustard (Brassica cariata) seed oils as grain protectants against maize weevil, Sitophilus zeamais M. (Coleoptera: Curculionidae). African Journal of Agricultural Research, 8(32):4374-4379.
|
|
|
|
Gemu M, Getu E, Yosuf A, Tadess T (2013.) Management of Sitophilus zeamais Motshulsky (Coleoptera: Ciurculionidae) and Sitotroga cerealella (Olivier) (Lepidoptera: Gelechidae) using locally available inert materials in southern Ethiopia. Greener J. Agric. Sci. 3:508-515.
Crossref
|
|
|
|
Germishuizen G, Meyer NL (2003). Plants of southern Africa: An annotated checklist. Strelitzia 14, National Botanical Institute, Pretoria 6:1231.
View
|
|
|
|
Getu E (2014). Bio-efficacy of products derived from Milletia ferruginea (Hochst) Baker against the bean bruchid Zabrotes subfasciatus (bruchidae: coleoptera) in stored bean in Ethiopia. Afr. J. Agric. Res. 9:2819-2826.
Crossref
|
|
|
|
Getu E, Abate T (1999). Management of maize stem borer using sowing date at Arsi-Negele. Pest Manage. J. Ethiop. 3:47-51.
|
|
|
|
Jembere B (2002). Evaluation of the toxicity potential of Millettia ferruginea (Hochst) Baker against Sitophilus zeamais (Motsch.). Int. J. Pest Manage. 48:29-32.
Crossref
|
|
|
|
Jembere B, Getahun D, Negash M, Seyoum E (2005). Toxicity of Birbira (Milletia ferruginea) seed crude extracts to some insect pests as compared to other botanical and synthetic insecticides. In: 11th NAPRECA (Natural Products and Drug Delivery) symposium book of proceeding, Astanarivo, Madagaskar pp. 88-96.
|
|
|
|
Jembere B, Obeng-Ofori D, Hassanali A (1995). Products derived from the leaves of Ocimum kilimandsharicum (Labiate) as post-harvest grain protectants against the infestation of three major stored product insect pests. Bull. Entomol. Res. 85:361-367.
Crossref
|
|
|
|
Kasa A, Tadese A (1996). Evaluation of some botanicals against the maize weevil, Sitophilus zeamais Motsch on stored sorghum at Bako. In: Bekele E, Abdulahi A, Yemane A (Eds.). Proceedings of the Third Annual Conference of the Crop Protection Society of Ethiopia. May 18-19, 1995, Addis Ababa, Ethiopia pp. 120-126.
|
|
|
|
Koffi E, Sea T, Dodehe Y, Soro S (2010). Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. J. Anim. Plant Sci. 5:550-558.
|
|
|
|
MacLachlan M (2001). Manual of Highland Ethiopian Trees (English/Amharic version). SIM Forestry Study Project. Addis Ababa: Benawee Printing Press
|
|
|
|
Mvumi BM, Giga DP, Chiuswa DV (1995). The maize (Zea mays L.) post-production practices of smallholder farmers in Zimbabwe: findings from surveys. J. App. Sci. S. Afr. 4:115-130.
Crossref
|
|
|
|
Mvumi BM, Stathers TE (2003). Challenges of grain protection in sub-Saharan Africa: the case of diatomaceous earths. Food Africa internet based Forum, 31 March- 11 April 2003,
View
|
|
|
|
Nega HM, Gnanasekaran N, Melaku U, Daniel S (2016). Phytochemical screening and assessment of in vitro antioxidant activities of Calpurnia aurea seeds and leaves. International Journal of Pharmacy and Pharmaceutical Sciences, 2:1-12.
|
|
|
|
Ofuya TI, Longe OO (2009). Investigation into fumigant effect of Eugenia aromatica dust against Callosobrunchus maculatus (Fabricius). International Journal of Crop Science,1(1):44-49.
|
|
|
|
Qwarse M (2015). Assessment of bioactivity of selected plants against pests and microbes from agro-pastoral communities in Mbulu district. M.Sc. thesis. Open University of Tanzania. P 150.
|
|
|
|
Sori W (2014). Effect of selected botanicals and local seed storage practices on maize insect pests and health of maize seeds in Jimma Zone. Singapore Journal of Scientific Research, 4(2):19-28.
Crossref
|
|
|
|
Tadesse T (1991). The Biology, significance and control of the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: curculionidae) in maize. M.Sc. Thesis, Alemaya University of Agriculture, Ethiopia.
|
|
|
|
Tadesse T (1997). Arthropods associated with stored maize and farmers' management practices in the Bako area, Western Ethiopia. Pest Management Journal Ethiopian, 1:19-27.
|
|
|
|
Tefera T, Mugo S, Likhayo P (2011). Effects of insect population density and storage time on grain damage and weight loss in maize due to the maize weevil, Sitophilus zeamais and the larger grain borer, Prostephanus truncates. African Journal of Agricultural Research, 6:2249-2254.
|
|
|
|
Watt JM, Breyer-Brandwijk MG (1962). The medicinal and poisonous plants of the Southern and Eastern Africa 2nd ed. Livingstone, Livingstone Ltd., Edinburgh. Health Fitness 1457p.
|
|
|
|
Zewde DK, Jembere B (2010). Evaluation of orange peel citrus sinensis (L) as a source of repellent, toxicant and protectant against Zabrotes subfasciatus (Coleoptera: bruchidae). Momona Ethiopian Journal of Science, 2(1):61-75.
Crossref
|