ABSTRACT
Tomato (Solanum esculentum Mill.) fruits were collected from farmers and retailers in Toke Kutaye District, Ethiopia during main-season (October to December, 2012) and off-season (December, 2012 to March, 2013) with an objective to identify microbial organisms that cause post-harvest rot of tomato. Diseased tissues were cultured in Potato Dextros Agar media for fungal and in Nutrient media for bacterial identification. Identification of isolated microbes were made based on cultural characteristics, microscopic examinations and biochemical test. A total of nine microorganisms comprising five bacteria and four fungi were isolated from the infected tomato fruits. The identified bacteria include: Erwinia carotovora, Clavibacter spp., Xanthomonas campestris, Ralstonia solanacearum and Pseudomonas aeruginosa. Among the isolated bacterial species, X. anthomonas campestris exhibited the highest frequency of occurrence (42.4%) followed by Ralstonia solanacearum (19.55%). Fungal pathogens such as Alternaria spp., Fusarium spp., Penicilium spp. and Rhizophus spp. were isolated from the infected tomato fruit samples. The average frequency of occurrence of Alternaria spp. was 37.5%, and Fusarium spp. and Rhizophus spp., had the frequency of occurrence of 25%. The pathogenicity test revealed that none of these pathogens could initiate the rot symptoms when inoculation was made on unwounded tomato fruits, indicate that these microorganisms fail to penetrate directly through the waxy skin of tomato. However, small wounds created during the post-harvest handling enabled them to infect fruit tissues. Hence, careful handling of the produce is important to minimize bruising and injury to the tissue and to further spread of the disease.
Key words: Tomato, post-harvest rot, fungal pathogens, bacterial pathogens.
Tomato (Solanum esculentum Mill.) is one of the most important vegetable crops grown in Ethiopia. It is produced in altitudes ranging between 700 and 2000 m.a.s.l. characterized by warm, dry and cooler night which are favorable for optimum growth and development of tomato (Lemma, 2002). In the rainy season also it is possible to cultivate, but needs intensive pest management (MoARD, 2009). The total area under tomato production in Ethiopia is about 51,698 ha with an annual estimated production of 230,000 tons (CSA, 2006). Tomato is the most profitable crop providing a higher returns to small farmers compared with other vegetable crops. The national average of tomato fruit yield in Ethiopia is 125 q/ha, whereas yield up to 400 q/ha can be recorded on research plots (Lemma, 2002).
Despite the remarkable progress made in increasing food production at the global level, approximately half of the population in the third world does not have access to adequate food supplies. There are many reasons for this, one of which is food losses occurring in the post-harvest and marketing system (FAO, 2002). Tomato is one of a perishable vegetable with a short shelf-life and high susceptibility to diseases (CSA, 2006). In developing countries, the post-harvest losses of fruits and vegetables oscilate between 20 and 50% (Okezie, 1998).
Post-harvest tomato fruits is affected by different types of diseases, like gray mold (Botrytis cinerea), Rhizopus rot (Rhizopus stolanifer), Anthracnose (Colletotrichum coccoides, Colletotrichum gloeosporioides, Colletotrichum dematium), early blight (Alternaria solani), bacterial soft rot and hollow stem (Erwinia carotovora pv. carotovora), Phoma rot (Phoma spp.), Southern blight (Sclerotium rolfsii) bacterial wilt (Pseudomonas solanacearum), Fusarium rot (Fusarium oxysporum f. sp. lycopersici) and some viral diseases.
In Toke Kutaye district of Ethiopia tomato is extensively cultivated in large volume. Of the total 1600 ha farm land, 1585.7 ha is used for tomato production both in the main/rainy cropping season and off-season. Despite high production of tomato in this district, farmers do not obtain the desired return from their produce because it is lost through improper handling. The current work envisaged at isolating and identifying fungal and bacterial pathogens responsible for post-harvest rot of tomato fruits for undertaking appropriate management.
Collection of tomato fruits
Diseased/infected tomato fruits were collected from farmers as well as retailers in Toke Kutaye district, West Shoa Zone of Oromia Regional State, Ethiopia both in main-season (October to December, 2012) and off-season (December 2012 to March 2013). In the laboratory, the tomato fruits were exposed in open trays to allow further decaying of the fruits.
Preparation of culture media
Nutrient Agar medium (NA) was prepared by diluting 28 g of nutrient agar in 1000 ml of distilled water. Similarly, Potato Dextrose Agar medium (PDA) was pepared by diluting 39 g of PDA agar in 1000 ml of distilled water. Both the NA and PDA were further autoclaved for 20 min at 120 lb pressure. To restrict bacterial growth, PDA was amended with 100 mg/L streptomycin, then poured into Petri plates.
Isolation of bacteria and fungal isolates
The bacterial isolation technique employed by Chiejina (2008) was used in our experiment. Thin sections of 2 mm diameter were cut from the periphery of diseased tomato fruit and sterilized in 70% alcohol, and rinsed into sterilized distilled water with three changes. Finally, the samples were macerated by pistel and mortal, then one loop full of the suspension was taken and streaking on NA medium and incubated in inverted position for 24 h. For isolation of fungal pathogens, small portion of the deteriorating parts of the fruits (showing softening, rotting and discoloration) were cut and disinfected using 70% alcohol, and washed with three changes of sterilized distilled water, and then transfered to Potato Dextrose Agar medium. Finally, the culture plates were incubated at a temperature of 25±2°C for a week.
Identification of bacterial isolates
After 24 h, the bacterial colonies showing different morphological characters were picked up from the Nutrient Agar plates and were re-streaked several lines on pre-steralized NA plates to obtain pure cultures of the isolates. There after, the isolated bacterial strains from the tomato samples were subjected to different biochemical tests for identification (Holt et al., 1994).
Gram staining reaction
The Gram-reaction was performed following the procedure developed by Schaad (1988). Thinly spread bacterial smear was prepared on a clean slide, dried in air and fixed by heating. The dried smear was flooded with crystal violet solution for one minute and washed in tap water for few seconds. It was again flooded with iodine solution for one minute and washed and blot-dried. It was then decolorized with 95% ethyl alcohol by applying drop by drop until no more color flows fromthe smear, and washed and blot dried. Finally slides were counter stained for about 10 seconds with safranin, washed and examined under microscope using oil immersion objective. Isolates that appeared pink, Gram negative bacteria.
Biochemical tests
Catalase test
Catalyst test was carried out by mixing a loop full of a fresh bacterial culture with 2 drops of solution of (3%H2O2) on the microscope slide according to method described by He et al. (1993). Presence of bubble indicated catalase positive responses.
Oxidase test
This test was conducted following the method outlined by Schaad (1988). For this of 0.1 g N,N,N’,N’-Tetra methyl-p-phenylene di amine di hydrochloride [C6H4[N(CH3)2.2HCL] was dissolved in 10 ml of distilled water in order to prepare 1% solution. A fresh culture of bacteria colony from media was taken by using a wooden stick and mixed with the prepared solution on the Whatman filter paper. Isolates, which developed a blue or deep purple color within 30 seconds, were considered positive for oxidase test.
Tween 80 hydrolysis test
For this, 10 g of Peptone, 5 g of NaCl, 0.1 g CaCl2, 2H2O and 15 g of Agar were mixed with 1000 ml of distilled water in Erlenmeyer flask and heated to dissolve completely. And 10 ml Tween 80 was autoclaved separately then and added to the medium then poured. From a fresh broth culture, a loop full was taken and transferred on the agar medium by using spot inoculation method and incubated at 30°C for upto seven days. An opaque zone of crystals formed around a colony was considered as positive reaction for hydrolysis of Tween 80 (Sands, 1990).
Starch hydrolysis test
For this, 5 g of 2% soluble starch added in nutrient agar medium was melted and poured into the sterile Petri dishes and solidified. Then after by using sterile technique, it made single streaks inoculation of each bacterium at the center of its plate and incubated at 30°C until heavy growth occurred an inverted position then flooded the surface of the plates with iodine solution with a dropper for 30 s. Finally, if a clear zone around a colony was recorded as positive reaction (Sands, 1990).
Identification of fungal isolates
After a week, the cultures growing on PDA plates with different morphology were counted separately and sub culturing was carried out by taking a portion of the growing edge of the colony in a separate PDA plate. For identification of the fungal pathogens, portion of the colonies were picked up with the help of a pair of needles and mounted on a clean slide with lactophenol cotton blue stain. The slide was gently heated with a sprit lamp so as to facilitate the staining and removal of air bubbles if any. The excess stain was removed with the help of tissue paper and the slides were observed under a compound microscope. Identification of fungi was made based on the growth patterns, color of mycelia and microscopic examinations of vegetative and reproductive structures according to Ellis (1976) and Barnett and Hunter (1999).
Percentage of bacteria and fungal occurrence
The procedure adopted by Ukeh et al. (2012) was used to determine the percentage of occurrence of different bacterial and fungal isolates in the culture. Isolations were made for different diseased tomato fruits separately, and the frequency of occurrence for each of the isolates were recorded, and expressed as percentage.
Percentage of occurrence = X/N x 100%
where X= Total number of each organisms in all the fruits, N= Total number of the entire organisms in all the fruits screened.
Pathogenicity test
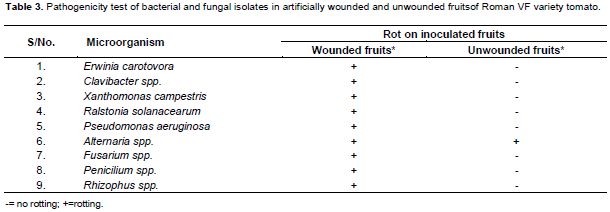
The pathogenicity tests were carried out using the techniques of Okigbo et al. (2009) to confirm the ability of various isolated microorganisms to infect and cause deterioration/rot of apparently healthy tomato fruits. Fresh tomato fruits, visibly free of any physical damage and disease symptoms, were used in this study. Fruits were washed and surface-disinfected before inoculation with pathogens by soaking in 70% alcohol for about 5 min, and rinsed with three changes of sterile distilled water, and drained off a sterile filter paper. 1 ml of bacterial solution with a cell concentration of 1x10-8 was used to inoculate tomato fruits with and without wound. In the case of unwounded tomato, fruit was immersed in 1 ml of bacterial suspension for 5 min. The fruits which were dipped in sterile distilled water treated as control. For fungal inoculation, a razor blade was used to cut the fruits, and then the cultures of the isolates were introduced into the open cut and replaced with the core. In control, 1 ml of distilled sterilized water was introduced into the wounded fruit and replaced with core. Petroleum jelly was used to cover the core to avoid development of any organisms outside. These were kept in the incubator for 5 days as mentioned above at different temperatures for bacteria (30°C) and fungi (25±2°C). On establishment of disease symptoms, samples from infected fruits were taken and cultured on Nutrient Agar and PDA media. Pure cultures were identified according to Ellis (1976) and Barnett and Hunter (1999) for fungi and Schaad (1980) and Holt et al. (1994) for bacteria. The symptoms were identical to those of infected tomatoes from which the first cultures were identified. For bacteria, different biochemical tests, and for fungal, morphological characteristics of conidia and mycelia of the fungi were used, and in the reisolated fruits confirmed Koch’s postulates.
Bacterial pathogens causing decay
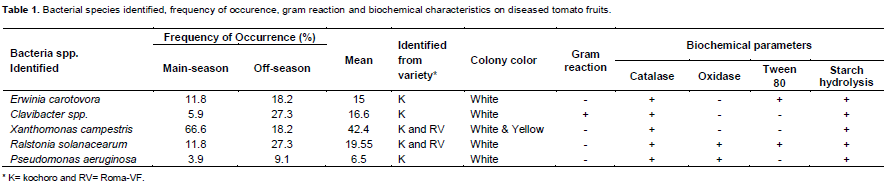
From the tomato samples isolated, the following five post-harvest rot caused by bacteria were isolated. They were Erwinia carotovora, Clavibacter spp., Xanthomonas campestris, Ralstonia solanacearum and Pseudomonas aeruginosa. In the main cropping season, among the bacterial species Xanthomonas campestris exhibited the highest frequency of occurrence (66.6%), followed by E. carotovora and R. solanacearum with similar frequency of occurrence of 11.8%. P. aeruginosa and Clavibacter spp. showed 5.9 and 3.9% frequency, respectively.
Similarly in the off-season, similar thesame type of bacterial species were identified, but with different percentage frequency of occurrence as observed in the main season. The most frequently observed bacterial species were Clavibacter spp and R. solanacearum with identical percentage frequency of 27.3% (Table 1); followed by E. carotovora and X. campestris bacterial species with similar frequency of occurrence of 18.2%. P. aeruginosa occured at 9.1% frequency. The pathogenicity test revealed that none of these pathogenes intiate the fruit rot symptoms when inoculation was made on unwounded fruits (Table 3).
Fungal pathogens causing decay
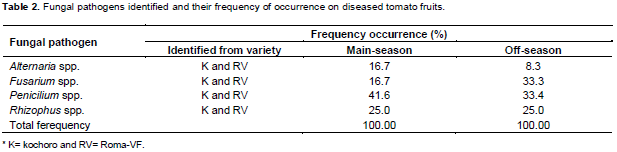
The investigation revealed that four fungal pathogens namely, Alternaria spp., Fusarium spp., Penicilium spp. and Rhizophus spp., were isolated from tomato fruit samples that were collected both in the main and off cropping seasons. The percentage frequency of occurence of Alternaria spp. was 16.7 and 8.3% for the main and off-seasons, respectively.
The Fusarium spp., showed frequency of occurrence of 16.7 and 33.3% for the main and off-seasons, respectively (Table 2). Fusarium spp., colonies were characterised by fast growth having bright pink color with aerial mycelium (Plate 1). The species of Fusarium produced both macro- and microconidia. Alternaria colonies had fast growth and was black or greyish in color (Plate 2). Penicilium in both seasons showed the highest percentage frequency of occurence 41.6% in the main season and 33.4% in the off cropping seasons. Rhizophus showed 25% frequency of occurrence in both the seasons (Table 2). The pathogenicity test revealed that none of these pathogens barring Alternaria spp. intiate the rot symptoms when inoculation was made on unwounded tomato fruits (Table 3).
Precise identification of the pathogens causing post-harvest rot disease is central to the formulation of an appropriate disease management strategy. In the current investigation, a total of nine microorganisms, comprising five bacteria and four fungi, were isolated from diseased tomato fruits. The pathogenicity test conclusively confirmed that, the identified bacterial pathogens viz., E. carotovora, Clavibacter spp., X. campestris, R. solanacearum and Pseudomonas spp., and the fungal pathogens namely Alternaria spp., Fusarium spp., Penicilium spp. and Rhizophus spp. were the causal agents of the tomato fruit rot. Jerry et al. (2009) reported that, the most common and aggressive bacteria are strains of Erwinia carotovora sub spp. carotovora. Certain species of Pseudomonas, Xanthomonas and Bacillus can also cause a soft rot of tomatoes.
Several fungal pathogens cause detrimental diseases leading to substantial yield losses worldwide (El-Katatny and Emam, 2012). The main post-harvest pathogens that have been reported include Alternaria alternata (Feng and Zheng, 2007) and Rhizopus stolonifer (Stevens et al., 1997). Our results corrolate with the finding of these reports.
The number of post-harvest rot causing bacteria that were identified in our study were greater than fungal pathogens for these bacterial soft rots are very important post-harvest diseases in the study area. The pathogenicity test elucidated that none of the identified bacteria intiate the rot symptoms when inoculation was made on unwounded tomato fruits. Similarly, the pathogenicity test for identified fungal pathogens using artificial inoculation of the unwounded tomatoes have not developed disease symptoms barring the Alternaria spp. Because most of rot causing bacteria and fungi cannot penetrate directly through the waxy skin of tomato fruit. However, even small wounds, even the size of abrasion from sand particles, enable the bacteria to infect fruit tissues. The post-harvest spoilage of tomato fruits was reported to be caused by microbial infection which might have gained entry through stomata openings, growth cracks or surface injuries. Infection of fruit and vegetables by post-harvest pathogens can occur before, during or after harvest. In the present case, these pathogens could have gained entry through injuries caused by rough handling, poor transport and storage facilities. Careful handling of the produce is warranted to avoid bruising and injury to the tissue and further spread of disease.
The authors have not declared any conflict of interest.
The authors greatly acknowledge Professor Cherukuri, Department of Plant Sciences, College of Agriculture and Veterinary Sciences, Ambo University for editing the manuscript.
REFERENCES
|
Barnett HL, Hunter BB (1999). Illustrated Genera of Imperfect Fungi. (4th edition). The American Phytopathological Society. St. Paul, Minnessota,USA. P. 218. |
|
|
|
Central Statistical Authority (CSA) (2006). Agricultural sample survey. Report on area and production of crops. Statistical bulletin 1(361). Addis Ababa, Ethiopia. |
|
|
|
Chiejina NV (2008). Microflora of some salad vegetables. Bio-Res. pp. 392-395. |
|
|
|
El-Katatny M, Emam AS (2012). Control of postharvest tomato rot by spore suspension and antifungal metabolites of Trichoderma harzianum: J. Microbiol. Biotechnol. Food Sci. 1(6):1505-1528.
|
|
|
|
Ellis MB (1976). Dematiaceous Hypomycetes. Common wealth Mycological Institute, Kew, Surrey, England P. 62. |
|
|
|
FAO (2002). |
|
|
Feng W, Zheng XD (2007). Essential oils to control Alternaria alternate in vitro and in vivo. Food Control. 18(9):1126-1130 (Abstract).
Crossref |
|
|
|
|
He LY, Sequeria L, Kelman A (1983). Characteristics of strains of Pseudomonas solanacearum from China. Plant Dis. 67:1357-1361.
Crossref |
|
|
|
|
|
Holt JG, Krieg NR, Sneath PHA, Staley TT, Williams ST (1994). Bergey's Manual for determinative Bacteriology, 9th edition, Academic Press, London. pp. 125-324. |
|
|
|
Jerry A, Bartz SA, Sargent, Michael M (2009). Guide to identifying and controlling postharvest tomato diseases in Florida. |
|
|
|
Lemma D (2002). Tomato research experience and production prospects. Research report No. 43. Ethiopian Agricultural Research Organization. Addis Ababa, Ethiopia, pp. 20-28. |
|
|
|
MoARD (2009). Improved horticultural crop technologies. Fruit nursery management and production practices. pp. 1-16. |
|
|
|
Okezie BO (1998). World food security: the role of postharvest technology. Food Technol. 52:64-69. |
|
|
|
Okigbo RN, Ramesh P, Achus CT (2009). Postharvest deterioration of cassava and its control using extracts of Azadirachta indica and Aframomum melegueta. E- J. Chem. 6(4):1274-1280. |
|
|
|
Sands DC (1990). Phusiological criteria-Determinative test. In: Klement Z., Rudolph K.,Sands. D.C. (eds).Methods in Phytobacteriol. AkademiaiKiado, Budapest, Hungary. pp. 137-143. |
|
|
|
Schaad NW (1988). Initial identification of common genera. In: Laboratory Guide for Identification of Plant Pathogenic Bacteria. N.W. Schaad (ed.), American Phytopathol.Society, St. Paul., MN, USA. pp. 1-15. |
|
|
Stevens C, Khan VA, Lu JY, Wilson CL, Pusey PL, Igwegbe C K., Kabwe K, Mafolo Y, Liu J, Chalutz, E, Droby S (1997). Integration of ultraviolet (UV- C) light with yeast treatment for control of post-harvest storage rots of fruits and vegetables. Biological Control: 10: 98-103.
Crossref |
|
|
|
|
Ukeh JA, Chiejina NV (2012). Preliminary Investigations of the Cause of Post-harvest Fungal Rot of Tomato. Journal of Pharmacy and Biological Sciences. 4(5):36-39.
Crossref |
|
|
|
|