ABSTRACT
The present study was done in October 2008 and March 2009 to explore the seasonal effects on soil physico-chemical properties and fungal population found at different depth (that is, 5, 10 and 15 cm) as well as on plant parts. Results indicate that in both seasons, organic carbon, nitrogen, phosphorus and potassium was higher in the top 5 cm followed by lower depths (10 and 15 cm). Fungal species isolated from different soil depths indicate a decline in number of fungal species with an increase in soil depth, except in the rhizospheric region where the number of fungal species was higher due to highest microbial activity in this region and higher nutrient contents (due to degradation of organic compound into simpler one). In case of isolation from plant parts, it indicates that the population of fungal sp. was higher in root region compared to shoot region.
Key words: fungal species, physic-chemical properties, organic soil.
One of the major drawbacks to agricultural production is fungal plant disease incidence. Nearly 25% of loss in yield in developing countries is due to fungal diseases (Bowyer, 1999). To reduce the disease incidence and increase production of food for continuing increasing world population, control measures need to be taken (Gohel et al., 2006). A major problem of many crops is Fusarium wilt caused by Formae speciales of the soil-borne fungus Fusarium oxysporum (Raquel et al., 2000). Leguminous crops occupy an important position among food crops. Pulse crop belongs to this group and contributes 10 to 13 million tones of grains annually from 22 to 23 million hectares of cultivable land in India (Khanna and Gupta, 1988). The chief limitation factors accountable for squat yield and ambiguity in the yield of pulse crops are pest and pathogens (Grewal, 1988; Tiyagi, 1991). Fusarium wilt disease of pulses appears to be widespread throughout Asia. In many of the Asian countries, fusarium wilt occurs on heavy black soils having a neutral to alkaline pH. Leguminous crops occupy an important position among food crops. Pigeonpea (Cajanus cajan L.) is an important pulse crop widely grown in India. Its natural drought resistance and a cheap source of protein make this a valuable crop. Its woody stem residue is used as a solid fuel. Wilt disease caused by Fusarium udum (Butler) is the most important disease of the crop (Kannaiyan et al., 1984). The soil consists of four major parts- mineral materials, organic matter, water and air (Buckman and Brady, 1960). Besides these, another important component is the microbial community. Interaction of soil physical and chemical characteristics with microbial population is an integral of carbon, nitrogen, phosphorus, sulfur and water cycles in the soil (Doran and Parkin, 1996). All these factors influence greatly the microbial spore germination and favor the growth of fungi in soil. Temperature, soil type, water retentive nature of the soil and nutrient availability has been found to affect fusarium population (Rael et al., 2012). Hence, these factors influence the soil fungistatis, a widespread phenomenon in soil (Lockwood, 1977). The organic matter and moisture content favors the growth of fungi and perhaps the higher fungistatic activity due to higher fungal population in rainy season. Soil pH is the most important factor which governs the availability of nutrients in soil. It can increase or decrease the solubility of phosphates and of the hydroxides of metal ions. Increase in soil temperature and soil water increases phosphate availability. Soil pH is an important factor in controlling nitrogen availability from soil organic matter. The present study was done in the Kalli Paschim village, Lucknow, India. The main objective of the study is to determine seasonal effect on soil characteristics at different depths namely, 5, 10 and 15 cm depths and also the fungal diversity in soil at the same depth along with rhizospheric region and from plant samples from the pigeonpea crop field.
Collection of soil samples
Soil and plant samples were collected from the site in October 2008 and March 2009 seasons. Samples were used for physico-chemical characteristics of soil as well as the isolation of fungal organisms from both soils as well as plant parts. Samples were collected in sterile zipper polyethylene bags and stored at 4°C before processing.
Physicochemical characterization of soil samples
Physicochemical parameters include organic carbon/nitrogen, pH, water content and temperature etc. Microbial population density generally decreases with depth as a function of the availability of organic carbon and molecular oxygen, parameters which typically decrease with depth. Temperature of the soil samples was recorded on the spot. Soil samples were dried at 60°C for 72 h, powdered in pestle and mortar and filtered through 2 mm sieve and the sieved soil moisture content, pH, % organic carbon and % organic nitrogen were measured according to standard procedure as next explained.
Soil moisture content
Moisture content was determined by weighing the field fresh soil samples separately followed by drying the soil in an electric oven and then reweighing. The loss in weight is expressed as percentage of oven dried weight. The samples were dried in an oven at 105°C for 24 h at constant weight then cooled in desiccators and then moisture content was calculated by the formula.
Soil pH
20 gm soil was taken in 100-ml beaker to which 50 ml distilled water (1:2.5(2.5w/v) was added and vortexing for 5 min at 120 rpm then pH was measured by digital pH meter (Orion720A+).
Electrical conductivity
20 gm of soil was shaken immediately with 50 ml distilled water in a beaker for one hour and allowed to stand overnight. Alternatively, the clear extract, after pH determination, was used for electrical conductivity.
The conductivity of the supernatant liquid was determined with the help of salt (conductivity) bridge meter. A 0.01 M KCl solution was used for standardization of conductivity meter before taking the sample reading.
Organic carbon and organic matter
Soil (0.1 g) was mixed with 10 ml 1 N K2Cr2O7 and 20 ml of H2SO4. The flask was allowed to stand for 30 min. Then 5 ml of concentration orthophosphoric acid and 25 ml distilled water was added and allowed to cool. Thereafter, two drops of diphenyl amine indicator was added. Then contents were titrated with 0.5 N ferrous ammonium sulfate. At the end point solution turned into green color representing the percentage of organic carbon present in a soil sample. A blank without soil run by the same procedure (Walkley and Black, 1934). For calculating organic matter, the van bemmelen factor of 1.724 is used because organic matter contains 58% carbon.
Nitrogen estimation (alkaline permagnate method)
In an 800-ml dry kjeldhal flask, 20 gm of soil was taken. To this, 20 ml of water was added followed by 100 ml each of the 0.32% KMnO4 and 2.5% NaOH solution. To prevent frothing and bumping, two drops of liquid paraffin was added. The contents were distilled in a collected assembly at a steady rate and the NH3 liberate collected in a 250-ml conical flask containing 20 ml of 2% boric acid (mixed with methyl red and bromocresol green). By the absorption of ammonia, the pink color turns green. About 125 ml of distillate collects and titrate with 0.02 N H2SO4 and again turns into pink at the end point. Blank was also run without soil followed the same procedure (Subbiah and Asifa, 1956).
Available phosphorus
The soil of 2.5 gm was mixed with 20 ml 2% solution of sodium bicarbonate (NaHCO3) and a spoon of charcoal was shaken for 30 min. After shaking, samples were filtered and 5 ml of this aliquot was mixed with 1 ml composite reagent (5 ml of 3% ammonium molybedate, 12.5 ml of conc. H2SO4, 5 ml of ascorbic acid and 25 ml of potassium antimony tartrate). In case of blank (control), distilled water was taken in place of aliquot. After 5 min, blue color was developed. The optical density was measured by UV Spectrophotometer at 885 nm wavelength. Various dilutions at the interval of 0.1 mg P/L was prepared from standard phosphate solution (K2HPO4) of 10 ppm (Olsen et al., 1954).
Available potassium
Five gram of soil was shaken on an electrical shaker with 20 ml 2% ammonium acetate for 30 min and filtered immediately through dry filter paper. The volume of filtrate was maintained up to 20 ml by adding distilled water. Potassium concentration in the extract was determined with the help of flame photometer. After standardization, calibration was done by solution of different concentration (10, 15 and 40 ppm) of potassium (Jackson, 1958).
Isolation of fungi from soil by serial dilution agar plating
Soil samples were collected from different depths, that is, 5, 10 and 15 cm. Ten gram soil was added to sterilize distilled water to make 10 ml. Serial dilutions of 10-1, 10-2 and 10-3 was prepared by pipetting 1 ml volume into 9 ml sterile distilled water for additional dilution. Finally X10-3 serial dilution soil: water suspension was prepared. 1 ml of this suspension was taken out and inoculated into nutrient plates. The plates were incubated at 25±2°C for 7 days and after the incubation period was over the plates were identified (Figure 1).
The infected root, stem and fruit samples were cut into small segments, washed thoroughly under tap water followed by distilled water. The samples were surface disinfected by putting them into 2% sodium hypochloride solution for 5 min. The samples were washed two times with distilled water and were blotted on sterilize filter paper for drying of adhered water with samples.
The sections of root stem and fruit samples were placed on autoclaved Czapek -Dox agar plates and were incubated at 25±2?C for 7 days. After incubation period was over, the colonies appeared were identified with the help of literatures available (Murumkar and Chavan, 1985; Saxena and Singh, 1987) (Figure 2).
On the basis of above experiments it was observed that the first sampling of the pigeon pea crop field which was done in September 2008 showed that moisture content was 8.81% at 5 cm, 10.68% at 10 cm and 9.43% at 15 cm depth while sampling done in March 2009 showed that percentage moisture content at 5 cm was 5.45%, 9.86% at 10 cm and 8.72% at 15 cm (Figure 3). The R2 value was found to be 0.8 and 0.9 for first and second sampling respectively. pH ranges of 6 .9 to 7.1 was observed at all depths in both sampling periods (September 2008 and March 2009). There was not such variation.
From Figure 4, electrical conductivity ranges from 0.16 to 0.21 mmho at all the depths, that is, 5, 10 and 15 cm depth in September 2008 sampling period while E.C. ranges from 0.15 to 0.16 mmho in March 2009 at all respective depths.
In September 2008 the percentage organic carbon was found to be 0.58% at 5 cm depth, 0.51% at 10 cm depth, and 0.36% at 15 cm depth; while in March 2009, percentage organic carbon was 0.50, 0.45 and 0.26% at 5, 10 and 15 cm depth respectively (Figure 5). The R2 value was calculated as 0.955 and 0.913 for first and second sampling respectively.
The percentage organic matter was 1.0% at 5 cm depth, 0.88% at 10 cm depth and 0.61% at 15 cm depth in September 2008, and in March 2009 it was found to be 0.87% at 5 cm, 0.77% at 10 cm, and 0.45% at 15 cm depths (Figure 6). The R2 value of both sampling seasons was found to be 0.955 and 0.912 respectively.
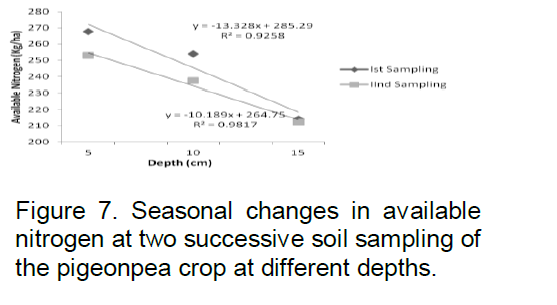
Available nitrogen in sampling period September 2008 was found to be 263.42 kg/ha at 5 cm depth, 254.02 kg/ha at 10 cm and 214.29 kg/ha at 15 cm depth respectively; while in March 2009 its amount was 252.96 kg/ha at 5 cm depth, 237.4 kg/ha at 10 cm depth and 212.20 kg/ha at 15 cm (Figure 7). The correlation coefficient R2 value was 0.925 and 0.981 at first and second sampling periods, respectively.
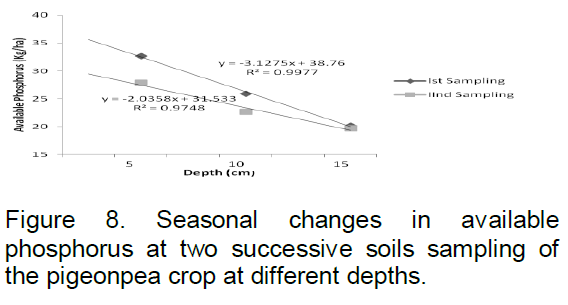
Available phosphorus calculated in September 2008 at 5 cm depth was 32.68 kg/ha, 25.91 kg/ha at 10 cm and 20.17 kg/ha at 15 cm depth. In March 2009, available phosphorus level was estimated as 27.84 kg/ha at 5 cm, 22.63 kg/ha at 10 cm and 19.70 kg/ha at 15 cm depth (Figure 8). The R2 value was found to be 0.997 and 0.974 at first and second sampling periods respectively.
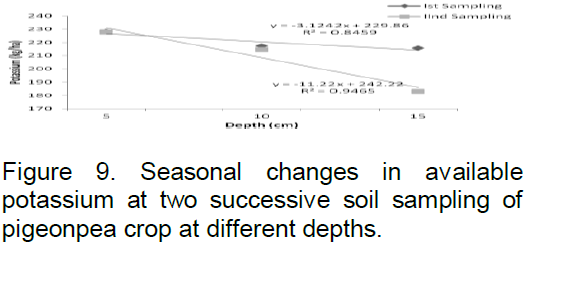
Available potassium level was found to be 228.27 kg/ha at 5 cm, 217.41 kg/ha at 10 cm and 215.78 kg/ha at 15 cm depth in September 2008; while in March 2009 its level was 227.92 kg/ha at 5 cm, 214.72 kg/ha at 10 cm, 183.04 kg/ha at 15 cm depth of soil (Figure 9). The R2 value was found to be 0.845 for first sampling period and 0.946 for second sampling period.
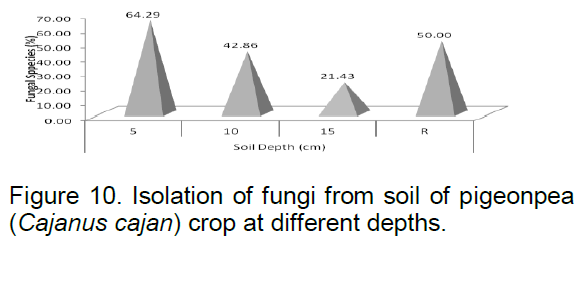
During isolation of fungal species from soil of pigeon pea crop field, result showed that out of 14 fungal species (Figure 10) isolated from different depth of soil of pigeon pea crop field, nine fungal species viz. Aspergillus dutrenticus, Aspergillus fumigatus, Aspergillus niger, Chaetomium globosum, Fusarium sp., Fusarium udum, Penicillium citrinum, Penicillium chrysogenum and Rhizactonia solani were found at 5 cm; six fungal species viz,. Alternaria tenius, A. dutrenticus, Aspergillus flavus, Aspergillus sydowii, F. udum and Rhizopus nigricans at 10 cm depth; three fungal species viz., A. dutrenticus, A. niger and F. udum at 15 cm depth and seven fungal species viz., A. flavus, A. fumigatus, A. niger, Aspergillus terreus, F. udum , P. citrinum and R. nigricans in rhizospheric region. The number of fungal species decreased with an increase in depth except in rhizospheric region where the number of fungal species was found higher.
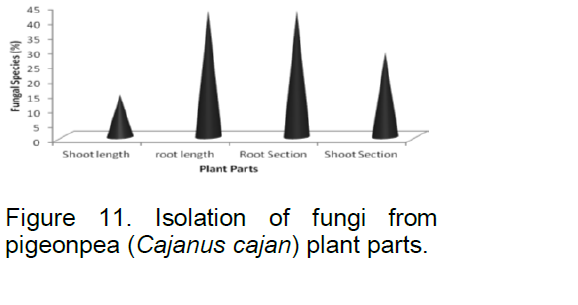
During isolation of fungal species from pigeon pea plant parts, result showed that out of seven fungal species isolated from pigeon pea plant parts, three fungal species viz., Aspergillus sulfurius, Penicillium germinicum, P. citrinum and A. niger, F. udum, Nigrosporium spharica were isolated from root length and root section followed by two from shoot section viz., A. niger and A. flavus and one, that is, F. udum isolated from shoot length (Figure 11). The result indicated that the population of fungal soap was higher in root region compared to shoot region.
From above study, it was postulated that the upper horizon contained more organic matter, nitrogen, phosphorus and other elements which in turn favors the growth of fungi in soil. The organic matter and nitrogen favored the growth of fungi in soil. In rainy season, the number of fungal colonies was more in comparison to summer due to higher moisture content which favored the germination of spores. The increased moisture content which may be attributed to the high microbial activity of soil rich in organic matter agreed with the findings of other workers (Chacko and Lockwood, 1996; Chin, 1967; Kruger, 1969; Wyk and Baard, 1971). The microbial activity was higher in monsoon season followed by winter while lower in summer season. The organic matter and nitrogen favored the growth of soil. The upper horizon contained more organic matter, nitrogen, phosphorus and other elements which in turn favors the growth of fungi in soil (Dwivedi and Dwivedi, 1972).
If such a variety of pigeon pea which was sown in winter and harvested in summer developed, that will be beneficial towards crop productivity as well as reduce the chances towards disease development. The interrelationship between changes in physical, chemical and biological characteristics of soil was further illustrated in Abbott and Murphy (2003). When one factor was changed, there may be a consequential effect on other factors and the final outcome was that the habitat of soil organisms was changed in several different ways following every type of disturbance (Abbott and Abbott, 1989). Organic matter and moisture content is essential to ensure the presence of an active microbial population in the soil. Physicochemical properties of the soils affect the density and diversity of microbes in the soil (Rohilla and Salar, 2012).
The present study thus revealed that the microbial population of soil is responsible for governing the nature of fungal population which is governed by ecological factors and the nutrient level of soil. The similarity of mycoflora of different regions may be attributed to the fact that soil environment is physically more buffered than the sub-aerial environment. The reduction in number of fungi with increasing depth is due to reduction in organic matter and oxygen level of soil and increased carbon dioxide concentration.
The authors have not declared any conflict of interests.
REFERENCES
|
Abbott LK, Abbott IJ (2000). Effects of agricultural practices on the soil biological environment for plant growth. Soil management for sustainable agriculture (Ed. Robertson GA). Western Australian Department of Agriculture. |
|
|
|
Abbott LK, Murphy MV (2003). Soil biological fertility: A key to sustainable land use in agriculture. Kluwer Academic Publishers, Dordrecht, The Netherlands, P. 264. |
|
|
Bowyer P (1999). Plant disease caused by fungi: phytopathogenecity. Molecular Fungal Biology, (R.P.Oliver, M.Schweizer). Cambridge University Press, Cambridge.
Crossref |
|
|
|
Buckman HO, Brady NC (1960). The nature and properties of soils. Sixth Edition Macmillan, New York, P. 567. |
|
|
|
Chacko CI, Lockwood JL (1966). A quantitative method for assaying soil fungistasis. Phytopathology. 56:576-577. |
|
|
|
Chin SHF (1967). Differences in Fungistasis in some Saskatchavan soils with special reference to Cochliobolus sativus, Phytopathology. 57:224-227. |
|
|
|
Doran JW, Parkin TB (1996). Quantitative indicators of soil quality. A minimum data set, J. N. Jones, a. j. (Eds.) Method for assessing soil quality. Soil Science Society of America, Madison, WI, pp. 25-37. |
|
|
|
Dwivedi R, Dwivedi RS (1972). Rhizosphere microflora of coriander with emphasis on fungistasis. Ann. Inst. Pasteur. 122:455-461. |
|
|
|
Gohel V, Singh A, Maisuria V, Phadnis A, Chhatpar, HS (2006). Bioprospecting and antifungal potential of chinolytic microorganisms. Afr. J. Biotechnol. 5(2):54-72. |
|
|
|
Grewal JS (1988). Disease of pulse crops-An overview. Indian Phytopathology 41:1-14. |
|
|
|
Jackson ML (1967). Soil Chemical analysis. Prentice Hall of India, Pvt. Ltd., New Delhi, P. 498. |
|
|
Jackson RM (1958). An investigation of fungistasis in Nigerian soil. J. Gen. Microbiol.18:248-258.
Crossref |
|
|
Kannaiyan J, Nene YL, Reddy MV, Rayan JG, Raju TN (1984). Prevalence of pigeon pea diseases and associated crop losses in Asia, Africa and the America. Tropical Pest Management, 30:62-71.
Crossref |
|
|
|
Khanna SS, Gupta MP (1988). Raising production of pulses. Yojna 32:4-8. |
|
|
|
Kruger W (1969). untersuchunger uber spacelotheca reiliana. I Die Beinflussung der sporenkeimung in Boden. Phytopathol. Z. 64:201-212. |
|
|
Lockwood JL (1977). Fungistasis in Soil.Soil. Biol. Rev.52:1-43.
Crossref |
|
|
|
Murumkar CV, Chavan PD (1985). Physiological changes in chickpea leaves infected by Fusarium wilt. Biovigyanam 11:118-121. |
|
|
|
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954). Estimation of available phosphorus in soil by extraction with sodium bicarbonate. Circ. U.S. Dep. Agric. P. 939. |
|
|
|
Rael K, Otieno OJ, Nandy SS (2012). Importance and management of fusarium wilt (Fusarium udum Butler) of pigeonpea, Inter. J. Agro. Agri. Res. 2(1):1-14. |
|
|
|
Raquel G, Mezzalama M, Ambrosoli R, Barberis E, Barberis A, maria S, Piedade de S (2000). Fusarium oxysporum strains as biocontrol agent against fusarium wilt: Effect on soil microbial biomass activity, Pesq. Agropea. Bras., Brasillia. 35(1):93-101. |
|
|
|
Rohilla SK, Salar RK (2012). Isolation and characterization of various fungal strains from Agricultural soil contaminated with pesticides. Res. J. Recent. Sci. 1:297-303. |
|
|
|
Saxena MC, Singh KB (1987). The chickpea. Published by CAB International. ICARDA, pp. 250-252. |
|
|
|
Subbiah BV, Asifa GL (1956). A rapid procedure for the determination of available nitrogen in soils. Current Sci. 25:259-260. |
|
|
|
Tiyagi SA (1991). Pathogenecity and control of Meloidogyne incognita and Rotylenchulus reniformis in relation to Macrophomina phaseolina on green gram and Fusarium oxysporum f.sp.ciceri on chickpea. Ph.D. thesis, Aligarh Muslim University, Aligarh, India. |
|
|
Walkley A, Black IA (1934). An estimation of the degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 37:29-38.
Crossref |
|
|
Wyk PS van, Baard SW (1971). Germination of conidia of Verticillium dahliae in soil. Plant and soil. 35:601-611.
Crossref |