ABSTRACT
Vermicomposting is a process where worms are used to transform organic waste products into fertilizers for agricultural use and as soil amendments to improve soil health. Vermicompost is used in agriculture as both an organic fertilizer and a soil amendment due to its large biological component and abundant nutrient concentrations, in particular nitrogen (N). Vermicomposts promote soil microbial biodiversity by inoculating the soil with a wide array of beneficial microbes, which can enhance plant growth by the production of plant growth-regulating hormones and enzymes while also controlling plant pathogens and nematodes, all of which can minimize crop yield losses. Current and historical studies have evaluated many vermicompost types for their ability to increase crop growth, development, and fruit quality across a wide range of field and greenhouse crops. Many of these studies conclude that crop response to vermicompost can meet or exceed crop response to conventional rates of synthetic fertilizers. Vermicompost use has therefore been increasingly considered in conventional and organic agricultural systems as an alternative to synthetic fertilizers to decrease N losses. However, it is difficult to determine the short and long-term effects to soil N cycling with increased vermicompost use in agriculture. Here, we summarize the current state of knowledge regarding the influence of vermicompost on N dynamics in laboratory and agricultural settings. In particular, the physical, chemical and biological means of N cycle alteration following vermicompost use is examined, with emphasis on the ability of vermicomposts to influence N leaching. We find conflicting results regarding the influence of vermicomposts on soil N dynamics, in particular the influence of vermicompost on soil N flux over short and long time scales. We find evidence for both soil N retention and leaching with vermicompost use, largely contingent on rate of application and vermicompost type. The differences in chemical, biological and physical properties between vermicompost types along with a wide range of recommended rates of application may pose problems for large-scale adaptation of vermicompost use in commercial agriculture. It is important to determine a proper “goldilocks” rate of vermicompost amendment for crop systems that supports high crop yield while minimizing N leaching. We conclude that much research still needs to be performed in laboratory and field settings to study N dynamics with vermicomposts.
Key words: Organic fertilizer, organic matter, nitrate sorption, nitrification, nitrogen immobilization, nitrate leaching.
Nitrogen (N) pollution occurs through over application of chemical fertilizers in agriculture systems and is largely responsible for water quality degradation through the release of nutrients to surface and groundwater sources (Bargali, 1996; Good and Beatty, 2011; Singh et al., 2005; Vitousek et al., 1997; Vitousek et al., 2009). The use of organic fertilizers has been shown to decrease N pollution into terrestrial, aquatic, and atmospheric sources, while promoting high crop yields and improving soil health in crop production (Arancon et al., 2004; Gopinath et al., 2008; Hepperly et al., 2009; Maynard, 1993). Vermicompost is an organic fertilizer known to promote plant growth, crop yield, and soil fertility while also promoting disease suppression in some plants (Arancon et al., 2006, Atiyeh et al., 2000; Edwards, 1988, Joquet et al., 2011; Noble and Coventry, 2005). Vermicompost use has increased in both conventional and organic agriculture systems (Lazcano and Dominguez, 2011; Singh et al., 2007), with some conventional operations increasing organic fertilizer input to explore the economic feasibility of lowering synthetic fertilizer input (Lim et al., 2014).
It is difficult to determine the effects of increased vermicompost use in agricultural settings on soil N dynamics due to the inherent chemical, physical and biological variability in vermicomposts (Azarmi et al., 2008; Basheer and Agrawal, 2013; Campitelli and Ceppi, 2008; Lazcano et al., 2008.). The objective of this review article is to examine the current state of knowledge concerning the chemical, biological and physical effects of vermicompost use in agriculture, with emphasis on the ability of vermicomposts to affect N dynamics in soil, in particular the leaching of nitrate (NO3-).
Vermicompost description
Vermicompost is a finely-divided mature organic matter with high water-holding capacity, high porosity, aeration, and drainage, and are stabilized by interactions between earthworms and microorganisms (Edwards and Burrows, 1988). Unlike compost, vermicomposts are produced under mesophilic conditions (Atiyeh et al., 2000). Although soil microorganisms facilitate soil organic matter (SOM) degradation, the presence of earthworms is essential to vermicompost production (Lazcano et al., 2008). Earthworms aerate, condition and fragment the substrate, drastically altering microbial activity in the finished vermicompost (Hatamzadeh and Masouleh, 2011; Lazcano and Dominguez, 2011). Vermicomposting is a non-thermophilic process by which organic materials are converted by earthworms and microorganisms into rich soil amendments with greatly increased surface area,
microbial activity and nutrient availability (Arancon et al., 2005). Vermicomposts contain high quantities of plant-available nutrients, disease- suppressive microbial organisms, humic acids, and plant growth-regulating hormones (Jack et al., 2011; Noble and Coventry, 2005; Pereira et al., 2014).
The intestinal tract of earthworms contains a wide range of microorganisms, enzymes and hormones which greatly expedite the digestion of organic waste material, transforming them into vermicompost in a short time, often in times ranging from 4-8 weeks (Pathma and Sakthivel, 2012). Physical fractionation of large organic matter particles is performed in the gizzard of the earthworm, where it is ground into a fine powder, which then allows intestinal digestive enzymes including proteases, lipases, amylases, cellulases and chitinases to conduct rapid biochemical transformation of the cellulosic and the proteinaceous materials in the waste (Sinha et al., 2009; Pereira et al., 2014). Earthworm gut microorganisms further degrade the organic matter before eventually passing to the lower portion of the intestinal tract as “casts” (Lazcano and Dominguez, 2011), with an increased surface area through fragmentation available for further microbial transformations (Lazcano et al., 2008). These casts are then acted upon by earthworm gut microflora, including numerous bacteria, fungi, and actinomycetes which convert the casts to the final product, the “vermicompost” (Lazcano and Dominguez, 2011).
Vermicompost is produced through the processing of organic matter by a large variety of earthworm genera, but commonly with Eisenia fetida (‘red wiggler’) (Edwards, 1998, Mitchell, 1997). Vermicomposts can be produced from a variety of organic material from different sources. Farm wastes (Azarmi et al., 2008), animal wastes (Atiyeh et al, 2001b) garden wastes, sewage sludge from municipal wastewater and water treatment plants (Sinha et al., 2009), the wastewater sludge from paper pulp and cardboard industry (Basher and Agrawal, 2013) domestic household waste (Suthar and Singh, 2008), brewery and distillery wastes (Sanchez-Monedero et al., 2001), mushroom waste (Tajbakhsh et al., 2008) and sugarcane industry waste (Kumar et al., 2010) offer excellent feed material for vermicomposting by earthworms (Sinha et al., 2009). Vermicomposts produced from several waste sources (pig manure, cattle manure, food wastes) have been shown to significantly increase the rate of germination, growth and yield of many specialty, high-value crops (Atiyeh et al., 2000). Vermicomposts contain humic materials (Atiyeh et al., 2002; Arancon et al., 2006; Masciandaro et al., 1997; Senesi et al., 1992) and the phytohormones auxin, gibberellin and cytokinin (Tomati et al., 1987; Vajrabhiah,1986), which may be ultimately responsible for significant increases in the growth and yield of crops withvermicompost as a fertilizer (Atiyeh et al., 2002). Interestingly, the humic materials extracted from vermicomposts can promote auxin-like cell growth and nitrate metabolism in carrots (Daucus carota) (Muscolo et al. 1996) and maize (Canellas et al., 2002). The surfaces or internal structure of humic materials can retain exchangeable auxin (Atiyeh etal., 2002) possibly through adsorption (Arancon et al., 2005) which may be responsible for promoting the stability and persistence of humic materials in soils (Canellas et al. 2002).
VERMICOMPOST AND ENVIRONMENTAL QUALITY
One of the most common environmental impacts of conventional agricultural production is the over application of chemical fertilizers. From 1970 to 1995, nutrient additions in the United States were well in excess of crop nutrient removals, and hydrologic N and P losses led to eutrophication of freshwaters far from the sources of contamination (Vitousek et al., 2009). Some cropping systems in China from 2003-2005 and the United States from 1997-2006 had a net excess N fertilizer input (agronomic inputs minus harvest removals) of 227 and 10 kg ha-1 yr-1, respectively (Vitousek et al., 2009). In addition to excess fertilizer input, the mass discharge of livestock manure contributes to the pollution of agricultural ecosystems on a global level (Hepperly et al., 2009). Excess N from fertilize or manure can be stored in soil organic matter and leached into the hydrosphere for periods exceeding 25 years (Sebilo et al., 2013). N pollution of surface and groundwater continues to be a major environmental issue.
Excessive inputs of synthetic fertilizers required to maintain high crop yields have disrupted the natural cycling of nutrients through ecosystems on a global scale (Vitousek et al., 1997). Anthropogenic sources of nutrient input, largely in the form of conventional synthetic fertilizers, ultimately accumulate in soils and leach into groundwater due to the high solubility of nutrients in mineral fertilizers, especially nitrate-nitrogen (Broschat, 1995; Logsdon and Sauer, 2016). It is well known that chemical fertilizer application in conventional agricultural operations is a large source of nitrate (NO3-) contamination to downstream freshwater and marine ecosystems (Amor et al., 2008; Kamman et al., 2016; Vitousek et al., 2009).
With recent global increases in organic agricultural and horticultural production, the demand for organic fertilizers including vermicomposts is increasing on a global scale (Lim et al., 2014). Recent greenhouse and field studies have suggested that vermicomposts can provide plants with similar levels of macronutrients and micronutrients in comparison to synthetic inorganic fertilizers (Atiyeh et al., 2000a; Peyvast et al., 2008; Joquet et al., 2011; Singh et al., 2008), which can be an indicator of potential feasibility for increasing agricultural utilization of these amendments.
Other benefits from the use of compost amendments include the possible reductions of nitrate leaching into groundwater compared to those from inorganically fertilized controls (Maynard, 1993; Joquet et al., 2011). Numerous studies have evaluated the ability of various composts and vermicomposts to promote nutrient retention in soils and prevent excess nutrient leaching into surface and groundwater sources (Gerke et al., 1999; Hepperly et al., 2009; Joquet et al., 2011; Maynard, 1993), however; there are mixed conclusions regarding the ability of vermicomposts to influence N dynamics in agricultural soils, in particular soil NO3- flux (Gerke et al., 1999; Hepperly et al., 2009; Li et al., 1997; Logsdon and Sauer, 2016; Mullane et al., 2015).
IMPROVEMENT OF SOIL PHYSICAL, CHEMICAL AND BIOLOGICAL PROPERTIES WITH VERMICOMPOST USE
Physical properties
The use of chemical fertilizer alters the physicochemical properties of soils and adversely affects microbial populations within the soil and thus decreases overall soil productivity (Bargali and Shrivastava, 2002; Bargali et al, 2015). Many studies have shown that vermicomposts are able to improve the physical properties of soils (Ferreras et al., 2006; Marinari et al., 2000; Gopinath et al., 2008; Lazcano and Dominguez, 2011; Vo and Wang, 2014). Ferreras et al. (2006) performed a study on soils that were altered by historical agricultural cultivation. An addition of 20 tons ha-1 of vermicompost to an agricultural soil over a two year period significantly improved soil porosity and aggregate stability. A related field study by Marinari et al. (2000) on a soil with a 20-year conventional horticultural cropping history showed that in soil amended with vermicompost, total porosities were significantly higher than in the original soil. Soil macropores also increased significantly after one application of vermicompost equivalent to 200 kg ha-1 of inorganic N fertilizer.
The ability of vermicompost to perform alterations to soil physical properties increases the amount of plant –available air and water, further encouraging seedling emergence and root growth in plants (Lazcano and Dominguez, 2011). Other studies have demonstrated the positive effect of vermicompost addition on soil bulk density (Azami et al., 2008; Gopinath et al., 2008; Ibrahim et al., 2015; Singh et al., 2013) and water holding capacity (Ganesh et al., 2011; Parthasarathi et al. 2008).
Typically, soils with higher water -holding capacities can prevent excess nutrients from leaching into proximate ground water sources in comparison to sandier soils with higher porosity (Bargali et al., 1993; Maynard, 1993; Mukesh et al., 1997; Yao et al., 2012). Vermicomposts can be used in a wide variety of agricultural soils to improve physical properties which often leads to increased crop growth and yield, as poor soil physical properties are often a growth-limiting factor in agricultural and horticultural production. As many of the aforementioned studies found significant beneficial alterations to soil water holding capacity and plant-available water, it is reasonable to suggest that improvements to physical properties of soil with vermicompost additions may also facilitate nutrient retention in soils.
Chemical properties
Vermicomposts contain high amounts of macro and micronutrients, which are often present in high enough quantities to facilitate high crop yields when used in conjunction with or in place of synthetic mineral fertilizers (Arancon et al., 2003; Lazcano and Dominguez, 2011). The chemical properties of vermicomposts are highly variable (Table 1). The chemical properties of vermicomposts often vary by the waste material used for production (Atiyeh et al., 2001b; Bachman and Metzger, 2007). Vermicomposts that are produced from vegetative and paper wastes are significantly different in nutrient content compared to those produced from manures and municipal solid wastes, primarily in N, P, and K concentrations (Table 1).
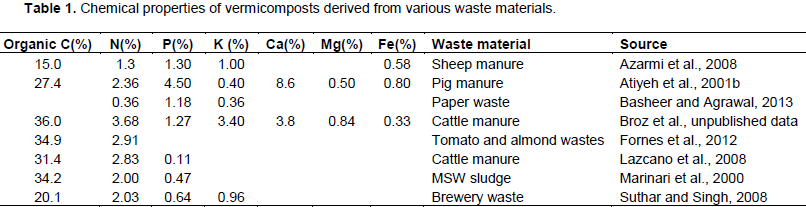
Vermicomposts typically have higher amounts of micro and macronutrients compared to composts produced from the same waste materials (Lim et al., 2014). A comparison of cow manure compost and vermicompost showed that vermicompost had a significantly lower C:N ratio as it underwent intense decomposition (Lazcano et al., 2008). Conventional cow manure compost is higher in NH4+ while vermicomposted cow manure tends to be higher in NO3-, likely due enhanced N mineralization and increased rates of conversion of NH4+ into NO3- (Atiyeh et al., 2000; Papadopolous, 1987), which is expedited in the presence of earthworms (Dominguez and Gomez-Brandon, 2013).
The ratio of NO3- to NH4+ in vermicomposts is important to understand the leaching capacity of N a reliable indicator of the maturity and stability of vermicomposts (Lim et al., 2014), with higher ratios of NO3- to NH4+ indicative of nitrification and thus compost maturity (Dominguez and Gomez-Brandon, 2013). Composts and vermicomposts with high NO3- : NH4+ ratios can leach more NO3- (Arora and Srivastava, 2013), but an increase in soil physical properties with vermicompost application can retain NO3 for plant uptake (Marinari et al., 2000). Most chemical fertilizer is applied in the form of NH4-N. The fate of NH4+ in agricultural soils is often microbial nitrification (Burger and Jackson, 2003), and thus leaching losses of N in conventional agricultural operations are high (Arora and Srivastava, 2013).
The variability of vermicompost chemical properties is also expressed in the cation exchange capacity (CEC) of vermicomposts. The cation exchange capacity (CEC) of vermicomposts has been reported as high, with values ranging from 40-300 cmolc kg-1 (Armando and Flores, 2009; Contreras-Ramos et al., 2007; Jordao et al., 2010; Nweke, 2013); however, vermicompost CEC varies significantly as a function of the waste material used for production (Pereira and Arruda, 2003). The inherent chemical variability typical between vermicompost types is important to discuss when considering an application rate and vermicompost type for use in commercial agriculture.
Biological properties
Vermicomposts are rich in microbial populations and diversity, particularly fungi, bacteria and actinomycetes (Edwards, 1998; Gopal et al., 2009; Pathma and Sakthivel, 2012; Tomati et al., 1987). Vermicomposts can contain bacteria, fungi and actinomycetes with maximum number of 126 x 106, 28 x 104 and 93 x 105 CFU g-1 (Devi et al., 2009).Total bacterial counts can exceeded 1010 organisms g-1 and include nitrobacter, azotobacter, rhizobium, phosphate solubilizers and actinomycetes (Suhane, 2007). It has been reported that vermicomposts contain populations of both nitrifying and denitrifying bacteria (Pathma and Sakthivel, 2012). The rate of itrification inside the drilosphere (earthworm-worked burrow) was found to be 1000 times higher than the denitrification rate, suggesting that nitrate created from microbial nitrification would be susceptible to leaching due to the lack of denitrification (Parkin and Berry, 1999).
Historical and current studies have attributed plant disease suppression and physiological disorders to the biological component of compost-derived soil amendments and earthworm activity (Boulter et al., 2002; Elmer, 2009; Singh et al., 2008). Interactions between earthworm gut microbes and soil microorganisms can produce copious amounts of plant growth-regulating hormones (Arancon et al., 2006; Frankenberger and Arshad, 1995), promote nutrient solubilization (Naik et al., 2008; Suhane, 2007), nitrogen fixation (Gopal et al., 2009; Han et al., 2005) and exhibit antimicrobial activity against pathogenic bacteria Staphylococcus aureus and Enterococcus faecalis (Vaz-Moreira et al. 2008). Earthworms ingest plant growth-promoting rhizospheric bacteria including Pseudomonas, Rhizobium, Bacillus, Azosprillium and Azotobacter along with rhizospheric soil which may increase the activity of these organisms due to the ideal micro-environment of the earthworm gut (Pathma and Sakthivel, 2012) and the high specific surface areas typical of vermicomposts (Shi-Wei and Fu-Zhen, 1991).
Further, earthworm activity increases the population of plant growth-promoting rhizobacteria (PGPR) (Sinha et al., 2010). Earthworm digestion of organic wastes promoted the production of plant growth-regulating hormones auxin and cytokinin (Canellas et al., 2002; Krishnamoorthy and Vajrabhiah, 1986), which are beneficial to plant growth, fruit quality and yield, and influence nutrient uptake and storage. The ability of plants to efficiently uptake and utilize available nitrogen is important to prevent excess nitrogen contamination into surface and groundwater sources.
SOIL NITROGEN RETENTION WITH VERMICOMPOST USE
Improvements to soil chemical, physical and biological properties with vermicompost use are well known. However, the ability of vermicomposts to affect soil N retention is not well understood, especially when considering vermicompost that has not been subject to irrigation or rainfall. Past studies have suggested that nitrate and phosphate contained in the structure of composts can initially leach out with a significant irrigation or rainfall event, and in some cases provide a sustained source of nutrient and metal leaching (Gerke et al., 1999; Li et al., 1997; Logsdon and Sauer, 2016; Mullane et al., 2015). However, when considering the long-term effect of composts and vermicomposts on soil N dynamics, several studies have reported increased N retention capacity (Shi-Wei and Fu-Zhen, 1991; Hepperly et al., 2009), especially when compared to N leaching from chemical fertilizers (Jouquet et al., 2011).
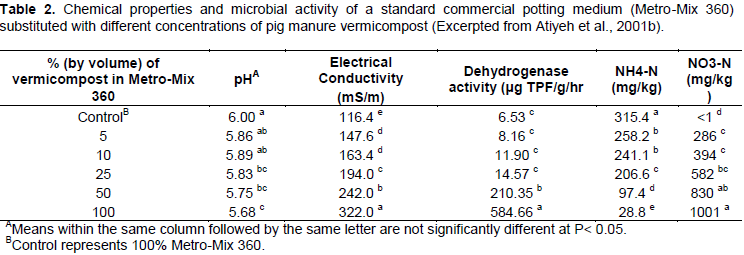
Therefore, it is important to discern whether vermicomposts can promote N retention in soils, or if the inherent amounts of N within many vermicomposts are easily leached. Shi-Wei and Fu-Zhen (1991) suggested that the large surface area of vermicompost provides many sites for continuous microbial activity and subsequently promoted retention of nutrients, either due to increased microbial NO3- immobilization or sorption to amorphous vermicompost surfaces. Hepperly et al. (2009) found that compost was superior to both conventional synthetic fertilizer and raw dairy manure in reducing nutrient losses to groundwater and surface runoff, promoting soil macronutrient levels, and increasing long-term nutrient availability to a wheat crop system. In contrast, Atiyeh et al. (2001) found that increased application of pig manure vermicompost to a potting media substrate increased levels of nitrate-N in saturated soil extracts. With levels of vermicompost amendment of 25% (v/v), the nitrate- N concentration exceeded 580 mg kg-1. This value nearly doubled to 1001 mg kg-1 nitrate-N after increasing the vermicompost amendment to 100% of the total medium (Table 2). In addition, EC and dehydrogenase activity (indicator of microbial abundance) increased with increasing amounts of vermicompost substitution. The total NH4-N content decreased with increasing amounts of vermicompost, which indicates that increasing vermicompost application may increase soil NO3- to NH4+ ratio and facilitate soil N losses through NO3- leaching (Table 2).
There are mixed results regarding the ability of composts and vermicomposts to affect nutrient leaching (Hepperly et al., 2009; Logsdon and Sauer, 2016; Mullane
et al., 2015). Thus, it is important to determine the ability of vermicomposts to promote NO3- retention in soils through biological and/or chemical mechanisms, or to promote plant uptake and transformation of NO3- within plant tissue.
Nitrate dynamics and chemical properties of vermicompost
There is abundant literature on the cation exchange capacity (CEC) of vermicomposts (Neweke, 2013; Pereira and Arruda, 2003; Theunissen et al., 2010), which is a good way to quantify improvements to soil fertility with vermicompost use. However, little is known regarding the anion exchange capacity (AEC) of vermicomposts and other SOM-rich soil amendments (Carrasquero-Duran and Flores, 2009). In particular, the ability of vermicomposts to adsorb and retain anions including nitrate (NO3-) is not well known. Vermicompost filter beds have been investigated for the treatment of domestic wastewaters as a way to remove NO3- and expedite decomposition of solid domestic household wastes (Taylor et al., 2003).
Nitrate-N is high in many vermicomposts (Atiyeh et al., 2000; Atiyeh et al., 2001b; Lazcano et al., 2008; Marinari et al., 2000; Parkin and Berry, 1999) and thus it is reasonable to suggest a release of NO3- upon water application may initially overwhelm the adsorption capacity of NO3- in a given vermicompost system. It is typical for a continuum of competitive adsorption onto soil anion exchange sites to occur in the order of phosphates > sulfates > chlorides > nitrate (Feder et al., 2015; Katou et al., 1996) which can explain the high mobility and low adsorption capacity of nitrate in many systems. However, the surface charge of the sorbent, which is pH dependent in the case of SOM (Appel and Ma, 2002; Marcano-Martinez and McBride, 1989) determines the capacity for anion sorption to occur, with positive surface charges generally promoting anion sorption. The surface charge of a sugar cane waste vermicompost with added calcite was positive at pH less than 7.5 (Carrasquero-Duran and Flores, 2009), suggesting anion exchange capacity at pH less than 7.5 (Appel et al., 2003).
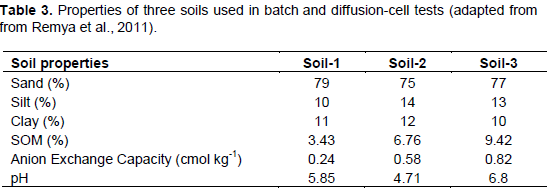
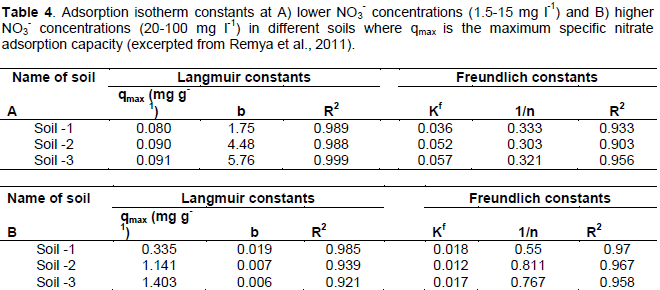
Anion exchange capacity is important to N retention in SOM and soils, as certain species of anions including NO3- are readily leached in soils in part due to low AEC and variable surface charge (Appel et al., 2003; Feder et al., 2015). Studies have been performed to measure NO3- sorption to SOM surfaces through batch and diffusion-cell tests. Remya et al. (2011) measured the maximum specific nitrate adsorption capacity (MSNAC, Qmax) of three sandy soils that were similar in all characteristics except pH and OM content (Table 3). SOM is largely responsible for nutrient retention in soils (Lehmann and Kleber, 2015), and thus increasing SOM may promote N retention. The authors observed an increase in MSNAC and AEC with increasing amounts of soil SOM, suggesting that organic matter may promote soil NO3- retention (Table 4). These data agree with Sebilo et al. (2013) where approximately 19% of NO3- from synthetic fertilizer N was retained in both the SOM pool and microbial biomass for periods of over 25 years. Thus, the organic matter content of vermicomposts may significantly contribute to N retention in soil, especially when considering the ability of SOM to retain N over long time scales where synthetic fertilizer N is applied (Sebilo et al., 2013).
NO3 adsorption is considered as one of several abiotic mechanisms of NO3- removal from solution (Dhakal, 2013); however, it is reasonable to suggest that a biotic mechanism of NO3- transformation may have also occurred due in part to the high microbial component and complex “social” microbial interactions associated with SOM (Kaiser et al., 2015). Vermicompost is fractionated SOM with high surface area (Pathma and Sakthivel, 2012), and thus may have a significant capacity to influence NO3- dynamics either directly by facilitating NO3- sorption or indirectly by harboring large numbers of nitrifying and denitrifying bacteria. Therefore it would be helpful to measure the difference between an unsterilized versus a sterilized vermicompost with added NO3- on N dynamics in laboratory conditions in order to better understand the kinetics, equilibrium and dominant mechanism of NO3- removal from solution with vermicompost. The nitrate adsorption capacity and AEC are not well known in vermicompost or vermicompost - amended soils. The AEC of soils is largely pH dependent, with lower pH generally promoting higher anion exchange capacity (Appel et al., 2003; Martinez and Mcbride, 1989; Remya et al., 2011). Thus, it is important to determine the pH where the AEC is maximized in vermicomposts in order to understand the capacity of vermicompost to retain anions through abiotic (chemical) mechanisms. Studies have been performed on tropical soils (Appel et al., 2003) and vermicomposts (Carrasquero-Duran et al., 2006) to experimentally determine the point of zero charge (PZC), a pH where the surface charge of a soil particle is zero. If a soil has a pH that is less than the PZC, anion exchange capacity (retention) readily occurs. If soil pH is greater than the PZC, cation exchange capacity increases. There is limited information regarding the surface charge typical in vermicomposts (Carrasquero-Duran and Flores, 2009). Therefore, it would be helpful to identify the pH where AEC is maximized in vermicomposts through a PZC determination and an ion adsorption study. The fate and pathways of abiotic NO3- immobilization differs from the commonly known mechanisms of NO3- reactions in SOM (Dail et al., 2001), and thus the results from NO3- adsorption studies with sterilized organic matter soil amendments may reveal the ability of these materials to promote NO3- immobilization before addressing biological and / or physical mechanisms of NO3- removal from solution.
Nitrate dynamics and biological properties of vermicomposts
The microbial content of composts and vermicomposts may be responsible for performing alterations to nitrogen dynamics in soils. Su and Puls (2007) performed an anaerobic NO3- sorption batch study on a cotton-burr compost with the objective of determining if NO3- removal from aqueous solution was dominantly a biotic (microbial NO3- reduction ) or abiotic (NO3- sorption to compost or other immobilization) process. To discern the mechanism of NO3- removal from solution, the N dynamics of a) an unmodified “pristine” cotton burr compost and b) an autoclaved cotton burr compost were studied in a series of batch tests, focusing on the kinetics and equilibrium of NO3- removal under anaerobic conditions in the unsterilized versus sterilized composts. In the unsterilized “pristine” cotton burr compost, there was a removal of nearly 100% of the added nitrate (20 mg N l-1) after approximately 100 h (Figure 1A). In contrast, the autoclaved cotton burr compost had less than 15% removal of nitrate after approximately 250 h (Figure 1B).
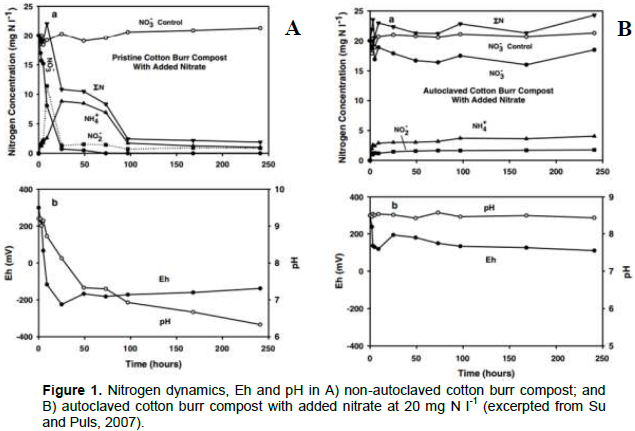
The solution redox potential (Eh) and pH of the equilibrium batch tests were studied to determine the influence of the nitrate removal mechanism on solution pH and redox potential (Su and Puls, 2007). A negative solution redox potential (Eh) and decreasing pH were observed in the unsterilized “pristine” cotton burr compost which corresponded to the fastest rate of nitrate removal, which occurred during the first 50 h (Figure 1A). There was no significant change in Eh or pH in the autoclaved cotton burr compost (Figure 1B). It is typical for Eh to become increasingly negative in response to the reduction of nitrate facilitated by anaerobic and facultative microbial activity (Bailey and Beauchamp, 1971).
These data suggest that the dominant mechanism of nitrate removal from aqueous solution with compost is a biotic process, which may include microbial denitrification of nitrate (Su and Puls, 2007). Thus, it is reasonable to suggest that the mechanism of nitrogen removal from aqueous solution with vermicomposts under anaerobic conditions may be dominantly a biotic process. However,no known studies have addressed batch NO3- dynamics with vermicompost in aerobic systems. Further, there are large differences in the biological properties of vermicomposts and composts, most specifically in bacterial and fungal community composition (Lazcano and Dominguez, 2011). The maximum specific NO3 removal capacity of vermicomposts may be greater than certain composts because of the presence of a large and diverse microbial consortium typical in many vermicomposts, where populations of nitrifying, denitrifying and N-immobilizing bacteria may comprise a significant fraction of the total microbial population (Pathma and Sakthivel, 2012). It is important to evaluate differences in microbial abundance and diversity across different types of vermicomposts, as differences in microbial species can influence the ability of vermicomposts to affect nitrogen dynamics, in particular anaerobic NO3- reduction (Su and Puls, 2007). It would be helpful to measure the biological and chemical capacity of vermicomposts to remove NO3- from solution through a series of aerobic batch and column-leaching studies. Little is known regarding NO3- adsorption kinetics or equilibrium when vermicompost is used as a sorbent material. Further, there is a lack of knowledge regarding the microbial NO3- reduction or immobilization capacity of vermicomposts. Results from these studies may better inform a proper level and /or preparation technique for vermicompost use as a way to reduce N losses in agriculture systems.
Nutrient pollution and soil degradation from conventional agricultural systems continue to be a global environmental quality issue. The use of vermicompost as both a replacement for chemical fertilizer and to improve soil properties has been shown to be effective. Much is known about the ability of vermicompost to improve soil chemical, physical and biological properties; however, the kinetics of N release or retention in the wide consortium of vermicompost types is not well understood. In particular, there is little agreement regarding a correct application rate of vermicompost that will not facilitate increases in nutrient N leaching. Further, mechanisms of NO3- retention and /or removal in agricultural soils where vermicompost is added as a long-term soil amendment is also not well understood. The above uncertainties are a demonstration of the complexity of N dynamics in agricultural systems utilizing vermicompost, and are an encouragement to continue research in this field.
The authors have not declared any conflict of interests.
REFERENCES
|
Amor FM, Navarro J, Murciano I, Investigación D, Agrario D, Aparicio PM, Csic U (2008). Isotopic Discrimination as a Tool for Organic Farming Certifi cation in Sweet Pepper. J. Environ. Qual. 37(1):182-185.
Crossref
|
|
|
|
Appel C, Ma L (2002). Concentration, pH and surface charge effects on cadmium and lead sorption in three tropical soils. J. Environ. Qual. 31:581-589.
Crossref
|
|
|
|
|
Appel C, Ma LQ, Rhue RD, Kennelley E (2003). Point of zero charge determination in soils and minerals via traditional methods and detection of electroacoustic mobility. Geoderma. 113(1-2):77-93.
Crossref
|
|
|
|
|
Arancon NQ, Edwards CA (2005). Effects of vermicomposts on plant growth. Int. Symp. Work. Vermitechnologies Dev. Ctries. 1-25. Arancon NQ, Edwards CA, Bierman P, Metzger JD, Lucht C (2005). Effects of vermicomposts produced from cattle manure, food waste and paper waste on the growth and yield of peppers in the field. Pedobiologia. 49:297-306.
Crossref
|
|
|
|
|
Arancon NQ, Edwards CA, Bierman P, Welch C, Metzger JD (2004). Influences of vermicomposts on field strawberries : 1. Effects on growth and yields. Bioresour. Technol. 93:145-153.
Crossref
|
|
|
|
|
Arancon NQ, Edwards CA, Lee S, Byrne R (2006). Effects of humic acids from vermicomposts on plant growth. Eur. J. Soil Biol. 42:65-69.
Crossref
|
|
|
|
|
Armando C, Flores I (2009). Bioresource Technology Evaluation of lead (II) immobilization by a vermicompost using adsorption isotherms and IR spectroscopy. Bioresour. Technol. 100(4):1691-1694.
Crossref
|
|
|
|
|
Arora K, Srivastava A (2013). Nitrogen Losses due to nitrification: Plant-based remedial prospects. Int. J. Bioassays. pp. 984-991.
|
|
|
|
|
Atiyeh RM, Domínguez J, Subler S, Edwards CA (2000a). Changes in biochemical properties of cow manure during processing by earthworms (Eisenia andrei, Bouchee) and the Effects on Seedling Growth. Pedobiologia 724:709-724.
Crossref
|
|
|
|
|
Atiyeh RM, Edwards CA, Subler S, Metzger JD (2001). Pig manure vermicompost as a component of a horticultural bedding plant medium: Effects on physicochemical properties and plant growth. Bioresour. Technol. 78(1):11-20.
Crossref
|
|
|
|
|
Atiyeh RM, S Lee, Edwards CA, Arancon NQ, Metzger JD (2002). The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresour. Technol. 84:7-14.
Crossref
|
|
|
|
|
Atiyeh RM, Subler S, Edwards CA, Bachman G, Metzger JD, Shuster W (2000b). Effects of vermicomposts and composts on plant growth within horticultural media and soil. Pedobiologia (Jena). 590:579-590.
Crossref
|
|
|
|
|
Azarmi R, Giglou MT, Taleshmikail RD (2008). Influence of vermicompost on soil chemical and physical properties in tomato (Lycopersicum esculentum) field. Afri. J. Biotechnol. 7(14):2397-2401.
|
|
|
|
|
Bachman GR, Metzger JD (2007). Physical and chemical characteristics of a commercial potting substrate amended with vermicompost produced from two different manure sources. Horttechnology. 17(3):336-340.
|
|
|
|
|
Bailey LD, Beauchamp EG (1971). Nitrate reduction, and redox potentials measured with permanently and temporarily placed platinum electrodes in saturated soils. Can. J. of Soil Sci. 51:51-58.
Crossref
|
|
|
|
|
Bargali SS, Singh RP, Mukesh J (1993). Changes in soil characteristics in eucalypt plantations replacing natural broad leaved forests. Vegetat. Sci. 4:25-28.
Crossref
|
|
|
|
|
Bargali SS (1996). Weight loss and nitrogen release in decomposing wood litter in an age series of eucalypt plantation. Soil Biol. Biochem. 28:699-702.
Crossref
|
|
|
|
|
Bargali SS, Shrivastava SK (2002). Exploration of valuable medicinal vegetal wealth from tribal belt of Bastar district in Chhattisgarh. The Botanica 52: 75-82.
|
|
|
|
|
Bargali SS, Shukla K, Singh L, Ghosh L (2015). Leaf litter decomposition and nutrient dynamics in four tree species of Dry Deciduous Forest. Trop. Ecol. 56(2):57-66.
|
|
|
|
|
Basheer M, Agrawal OP (2013). Management of paper waste by vermicomposting using epigeic earthworm, Eudrilus eugeniae in Gwalior, India. Int. J. Curr. Microbiol. Appl. Sci. 2(4):42-47.
|
|
|
|
|
Broschat TK (1995). Nitrate, Phosphate, and Potassium Leaching from Container-grown Plants Fertilized by Several Methods. Hortscience 30(1):74-77.
|
|
|
|
|
Burger M, Jackson LE (2003). Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol. Biochem. 35(2):29-36.
Crossref
|
|
|
|
|
Campitelli P, Ceppi S (2008). Chemical, physical and biological compost and vermicompost characterization: A chemometric study. Chemom. Intell. Lab. Syst. 90(1):64-71.
Crossref
|
|
|
|
|
Canellas LP, Olivares FL, Okorokova-Facanha AL, Facanha AR (2002). Humic Acids Isolated from Earthworm Compost Enhance Root Elongation, Lateral Root Emergence, and Plasma Membrane H -ATPase Activity in Maize Roots. Plant Physiol. 130:1951-1957.
Crossref
|
|
|
|
|
Carrasquero-Duran A, Flores I, Perozo C, Pernalete Z (2006). Immobilization of lead by a vermicompost and its effect on white bean (Vigna Sinenis var. Apure) uptake. Int. J. Environ. Sci. Technol. 3(3):203-212.
Crossref
|
|
|
|
|
Contreras-Ramos S, Alvarez-Bernal D, Dendooven L (2007). Dynamics of nitrogen in a PAHs contaminated soil amended with biosolid or vermicompost in the presence of earthworms. Chemosphere 67:2072-2081.
Crossref
|
|
|
|
|
Dail DB, Davidson EA, Chorover JON (2001). Rapid abiotic transformation of nitrate in an acid forest soil. Biogeochemistry. 54:131-146.
Crossref
|
|
|
|
|
Devi SH, Vijayalakshmi K, Jyotsna KP, Shaheen SK, Jyothi K, Rani MS (2009). Comparative assessment in enzyme activities and microbial populations during normal and vermicomposting. J. Environ. Biol. 30:1013-1017.
|
|
|
|
|
Dhakal P (2013). Abiotic nitrate and nitrite reactivity with iron oxide minerals. Theses and Dissertations - Plant and Soil Sciences. Paper 30.
|
|
|
|
|
Dominguez J (2011). The Microbiology of Vermicomposting. 53-66. In Vermiculture Technology: Earthworms, Organic Wastes, and Environmental Management, C. A. Edwards, N. Q. Arancon, R. Sherman, Eds. 2011.
|
|
|
|
|
Dominguez J, Gomez-Brandon M (2013). The influence of earthworms on nutrient dynamics during the process of vermicomposting. Waste Manag. Res. 31:859-868.
Crossref
|
|
|
|
|
Edwards CA, Burrows I (1988). The potential of earthworm composts as plant growth media. In: Earthworms in Environmental and Waste Management. C. A. Edward and J. Neuhauser. (Eds.). SPB Academic Publications. The Elmer WH, Pathology P, Connecticut T. A Experiment. Influence of Earthworm Activity on Soil Microbes and Soilborne Diseases of Vegetables. Am. Pathol. Soc. P 38.
|
|
|
|
|
Feder F, Bochu V, Findeling A, Doelsch E (2015). Science of the Total Environment Repeated pig manure applications modify nitrate and chloride competition and fl uxes in a Nitisol. Sci. Total Environ. 511:238-248.
Crossref
|
|
|
|
|
Ferreras L, Gomez E, Toresani S, Firpo I, Rotondo R (2006). Effect of organic amendments on some physical, chemical and biological properties in a horticultural soil. Bioresour. Technol. 97(4):635-640.
Crossref
|
|
|
|
|
Fornes F, Mendoza-hernández D, García-de-la-fuente R, Abad M, Belda RM (2012). Composting versus vermicomposting : A comparative study of organic matter evolution through straight and combined processes. Bioresour. Technol. 118:296-305.
Crossref
|
|
|
|
|
Gerke HH, Arning M, Stöppler-Zimmer H (1999). Modeling long-term compost application effects on nitrate leaching. Plant Soil. 213(1):75-92.
Crossref
|
|
|
|
|
Good AG, Beatty PH (2011). Fertilizing Nature: A Tragedy of Excess in the Commons. PLoS Biol. 9(8).
Crossref
|
|
|
|
|
Gopal M, Alka G, Sunil E, Thomas G (2009). Amplification of Plant Beneficial Microbial Communities During Conversion of Coconut Leaf Substrate to Vermicompost by Eudrilus sp . Curr. Microbiol. 15-20.
Crossref
|
|
|
|
|
Han J, Sun L, Dong X, Cai Z, Sun X, Yang H, Wang Y, Song W (2005). Characterization of a novel plant growth-promoting bacteria strain Delftia tsuruhatensis HR4 both as a diazotroph and a potential biocontrol agent against various plant pathogens. Syst. Appl. Microbiol. 28:66-76.
Crossref
|
|
|
|
|
Hatamzadeh A, Masouleh SSS (2011). The influence of vermicompost on the growth and productivity of cymbidiums. Casp. J. Environ. Sci. 9(2):125-132.
|
|
|
|
|
Hepperly P, Lotter D, Ulsh CZ, Seidel R, Reider C (2009). Compost, Manure and Synthetic Fertilizer Influences Crop Yields, Soil Properties, Nitrate Leaching and Crop Nutrient Content. Compost Sci. Util. 17(2):117-126.
Crossref
|
|
|
|
|
Iannotti DA, Grebus ME, Toth BL, Madden LV, Hoitink HAJ (1994). Oxygen Respirometry to Assess Stability and Maturity of Composted Municipal Solid-Waste. J. Environ. Qual. 23(6):1177-1183.
Crossref
|
|
|
|
|
Ibrahim MM, Mahmoud EK, Ibrahim DA (2015). Effects of vermicompost and water treatment residuals on soil physical properties and wheat
|
Jack ALH, Rangarajan A, Culman SW, Sooksa-nguan T, Thies JE (2011). Choice of organic amendments in tomato transplants has lasting effects on bacterial rhizosphere communities and crop performance in the field. Appl. Soil Ecol. 48(1):94-101.
Crossref
|
|
|
|
Jordão CP, Fernandes RBA, Ribeiro KL, Barros PM, Fontes MPF, Paula Souza FM (2010). A Study on Al (III) and Fe (II) ions sorption by cattle manure vermicompost. Water, Air, Soil Pollut. 210:51-61.
Crossref
|
|
|
|
|
Jouquet EP, Bloquel E, Doan TT, Ricoy M, Orange D, Rumpel C, Duc TT (2011). Do Compost and Vermicompost Improve Macronutrient Retention and Plant Growth in Degraded Tropical Soils? Compost Sci. Util. 19(1):15-24.
Crossref
|
|
|
|
|
Kaiser C, Franklin O, Richter A, Dieckmann U (2015). Social dynamics within decomposer communites lead to nitrogen retention and organic matter build-up in soils. Nat. Commun. 6:1-10.
Crossref
|
|
|
|
|
Katou H, Clothier BE, Green SR (1996). Anion Transport Involving Competitive Adsorption during Transient Water Flow in an Andisol. Soil Sci. Soc. Am. J. 60:1368-1375.
Crossref
|
|
|
|
|
Krishnamoorthy RV, Vajranabhaiah SN (1986). Biological activity of earthworm casts: An assessment of plant growth promotor levels in the casts. Proc. Indian Acad. Sci. 95(3):341-351.
Crossref
|
|
|
|
|
Kumar R, Verma D, Singh BL, Kumar U (2010). Bioresource Technology Composting of sugar-cane waste by-products through treatment with microorganisms and subsequent vermicomposting. Bioresour. Technol. 101(17):6707-6711.
Crossref
|
|
|
|
|
Lazcano C, Dominguez J (2011). The use of vermicompost in sustainable agriculture: Impact of plant growth and soil fertility. pp. 23-35. In Soil Nutrients.
|
|
|
|
|
Lazcano C, Gómez-brandón M, Domínguez J (2008). Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 72:1013-1019.
Crossref
|
|
|
|
|
Lehmann J, Kleber M (2015). Perspective The contentious nature of soil organic matter. Nature. 1-9. Available at http://dx.doi.org/10.1038/nature16069.
Crossref
|
|
|
|
|
Lim SL, Wu TY, Lim PN, Pui K, Shak Y (2014). The use of vermicompost in organic farming: overview, effects on soil and economics. J. Sci. Food Agric.
|
|
|
|
|
Li YC, Stoffella PJ, Alva AK, Calvert DV, Graetz DA (1997). Leaching of nitrate, ammonium, and phosphate from compost amended soil columns. Compost Science and Utilization 5:63-67.
Crossref
|
|
|
|
|
Logsdon SD, Sauer PA (2016). Nutrient Leaching When Compost Is Part of Plant Growth Media Nutrient Leaching When Compost Is Part of Plant Growth Media. Compost Sci. Util. 2397.
|
|
|
|
|
Marinari S, Masciandaro G, Ceccanti B, Grego S (2000). Influence of organic and mineral fertilizers on soil biological and physical properties. Bioresour. Technol. 72:9-17.
Crossref
|
|
|
|
|
Marcano-Martinez E, McBride MB (1989). Comparison of the titration and ion adsorption methods for surface charge measurements in Oxisols. Soil Sci. Soc. Am. J. 53:1040-1045.
Crossref
|
|
|
|
|
Masciandaro G, Ceccanti B, Garcia C (1997). Soil agro-ecological management: fertirrigation and vermicompost treatments. Biores. Technol. 59:199-206.
Crossref
|
|
|
|
|
Maynard AA (1993). Nitrate Leaching from Compost-Amended Soils. Compost Sci. Util. 1:65-72.
Crossref
|
|
|
|
|
Mba CC (1996). Treated-cassava peel vermicomposts enhanced earthworm activities and cowpea growth in field plots. Res. Conserv. Recycl. 17:219-226.
Crossref
|
|
|
|
|
Mitchell A (1997). Production of Eisenia fetida and vermicompost from feed-lot cattle manure. Soil Biol. Biochem. 29(3-4):763-766.
Crossref
|
|
|
|
|
Mondal T, Datta JK, Mondal NK (2015). Influence of indigenous inputs on the properties of old alluvial soil in a mustard cropping system. Arch. Agron. Soil Sci. 61(9):1319-1332.
Crossref
|
|
|
|
|
Moradi H, Fahramand M, Sobhkhizi A, Adibian M (2014). Effect of vermicompost on plant growth and its relationship with soil properties. Int. J. Farming Allied Sci. 1996-2001.
|
|
|
|
|
Mullane JM, Flury M, Iqbal H, Freeze PM, Hinman C, Cogger CG, Shi Z (2015). Science of the Total Environment Intermittent rainstorms cause pulses of nitrogen, phosphorus, and copper in leachate from compost in bioretention systems. Sci. Total Environ. 537:294-303.
Crossref
|
|
|
|
|
Mukesh J, Bargali K, Bargali SS (1997). Changes in physico- chemical properties and metabolic activity of soil in popular plantations replacing natural broad leaved forests. J. Arid Environ. 35:161-169.
Crossref
|
|
|
|
|
Muscolo A, Pannucio MR, Abenavoli MR, Concheri G, Nardi S (1996). Effect of molecular complexity and acidity of earthworm faeces humic fractions on glutamate dehydrogenase, glutamine synthetase, and phosphoenolpyruvate carboxylase in Daucus carota II cells. Biol. Fertil. Soils: 83-88.
Crossref
|
|
|
|
|
Naik PR, Raman G, Narayanan KB, Sakthivel N (2008). Assessment of genetic and functional diversity of phosphate solublizing fluorescent pseudomonads isolated from rhizospheric soil. BMC Microbiol. 14:1-14.
|
|
|
|
|
Noble R, Coventry E (2005). Suppression of soilborne plant diseases with composts: a review. Biocontrol. Sci. Technol. 15:3-20.
Crossref
|
|
|
|
|
Nweke IA (2013). Plant nutrient release composition in vermicompost as influenced by Eudrilus eugenae using different organic diets. J. Ecol. Nat. Environ. 5:346-351.
Crossref
|
|
|
|
|
Papadopoulos I (1986). Nitrogen fertigation of greenhouse-grown cucumber. Plant Soil. 93(1):87-93.
Crossref
|
|
|
|
|
Parkin TB, EC Berry (1999). Microbial nitrogen transformations in earthworm burrows. Soil Biol. Biochem. 31:1765-1771.
Crossref
|
|
|
|
|
Parthasarathi K, Balamurugan M, Ranganathan LS (2008). Influence of vermicompost on the physico-chemical and biological properties in different types of soil along with yield and quality of the pulse crop – blackgram. Iran J. Environ. Heal. Sci. Eng. 5(1):51-58.
|
|
|
|
|
Pathma J, Sakthivel N (2012). Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus: 1-19.
Crossref
|
|
|
|
|
Pereira MG, Arruda MAZ (2003). Vermicompost as a natural adsorbent material: characterization and potentialities for cadmium adsorption. J. Braz. Chem. Soc. 14(1):39-47.
Crossref
|
|
|
|
|
Peyvast G, Olfati JA, Madeni S, Forghani A (2008). Effect of vermicompost on the growth and yield of spinach (Spinacia oleracea L .). J. Food, Agric. Environ. 6:2006-2009.
|
|
|
|
|
Remya N, Kumar M, Mohan S, Azzam R (2011). Influence of organic matter and solute concentration on nitrate sorption in batch and diffusion-cell experiments. Bioresour. Technol. 102(9):5283-5289.
Crossref
|
|
|
|
|
Sanchez-Monedero MA, Roig A, Paredes C, Bernal MP (2001). Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 78:301-308.
Crossref
|
|
|
|
|
Sebilo M, Mayer B, Nicolardot B, Pinay G, Mariotti A (2013). Long-term fate of nitrate fertilizer in agricultural soils. Proc. Natl. Acad. Sci. 110(45):1-5.
Crossref
|
|
|
|
|
Senesi N, Saiz-jimenez C, Miano TM (1992). Spectroscopic characterization of metal-humic acid-like complexes of earthworm-composted organic wastes. Sci. Total Environ. 118:111-120.
Crossref
|
|
|
|
|
Sharma S, Pradhan K, Satya S, Vasudevan P (2005). Potentiality of Earthworms for Waste Management and in Other Uses – A Review. J. Am. Sci. 1(1):4-16.
|
|
|
|
|
Singh R, Sharma RR, Kumar S, Gupta RK, Patil RT (2008). Vermicompost substitution influences growth, physiological disorders, fruit yield and quality of strawberry
|
|
|
|