ABSTRACT
Soil fertility declines following continuous cultivation along with the use of fertilizers causing concern for sustainable food productivity. This study assessed chemical properties of degraded Alfisol, and also evaluated potential of organic residues and fertilizers in improving them. Soil samples (0 to 30 cm) of degraded and fallow fields were collected and analyzed. One kilogram each of soil from degraded field was weighed into incubation cups; mixed with NPK 15-15-15 (NPK), tithonia compost, poultry manure, and Gliricidia sepium leaves, and their combinations at 120 kg N/ha incubated for six weeks. Treatment without amendment was also included. After incubation, chemical properties of the soil were determined. Thereafter, maize was planted for six weeks. Maize biomass and NPK uptake was determined. Compared with fallow field, soil pH in the continuously cultivated plot declined by 12% while total nitrogen, organic carbon, phosphorus, Zn, and effective cation exchange capacity (ECEC) declined by 44, 55, 73.5, 83 and 70%, respectively. After incubation, organic residues or their combinations with NPK had higher pH as compared with control. Incubation of tithonia increased organic carbon, total nitrogen, ECEC, Ca, Mg and Mn than NPK alone by 12, 11, 16, 20, 11 and 7%, respectively. Maize shoot weight, N and K uptakes were also improved with addition of tithonia as compared to NPK and untreated control. Continuous cultivation reduced soil fertility levels. Incorporation of tithonia compost was better than NPK in improving soil chemical properties and maize growth.
Key words: Alfisol, soil chemical properties, organic residues, maize, incubation, Nigeria.
In recent years, agricultural productivity in the sub-Saharan Africa (SSA) has declined drastically due to shortened fallow periods, increasing human population, land use intensification consequently leading to decreasing size of land available to a farmer, reducing soil fertility and crop productivity in the region (Odoemena et al., 2010).
This has made continuous cropping inevitable. As such, soil nutrient depletion on smallholder farms as observed by Sanchez and Leakey (1997) continuedthe cause of low per capital food production in Africa. Problems of declining soil fertility are wide spread in SSA, largely as a consequence of continued cultivation of crops with low levels of nutrient inputs (Zingore, 2011). With intensification of cropping system, organic matter and N in the soils are readily depleted, while phosphorus and other nutrient reserves are slowly but steadily mined (Tanimu et al., 2013). To sustain high crop yields in intensive crop production system, nitrogen fertilizer input is required.
Soil fertility and crop productivity has been largely sustained with the use of mineral fertilizer (Shen et al., 2010). Additionally, soil organic matter content in term of soil organic carbon and nitrogen diminishes as a result of conversion of natural forest into arable crop production (Osher et al., 2003).
To achieve high quality crop production and soil productivity without degrading the soil properties under long term use, appropriate management practices should be adopted. Fertilizer is required at the low levels of soil nutrients to reverse declining soil fertility (Olawale et al., 2009). Although application of mineral fertilizers have recorded increased crop yields. Research results showed that continuous cropping within organic fertilizers alone have impaired many soil properties (Yusuf and Yusuf, 2008). Therefore, the combined application of inorganic fertilizer and organic residues has been proposed as a more sustainable means of alleviating the soil fertility and crop productivity constraints of the sub-Saharan Africa.
Nigeria is the 10th largest producer of maize in the world and largest maize producer in Africa (USDA, 2010). With continuous cropping and use of fertilizers, maize yield have averaged at 1.36 tons ha-1 in Nigeria. This is about 20% of the average yield obtained in North America and other intensive maize producing regions in the world (Afolami and Fawole, 1991). Among the major reasons for this low yield is poor soil fertility. Limitation of nutrients in soils has led to a drastic decline in maize yields in most small holder farms (Vanlauwe et al., 2006).
Agricultural output is fundamentally affected by soil productivity (Liasu et al., 2008). Moreover, poor soil management practices and the nature of tropical soils account for heavy nutrient losses through soil erosion and nutrient leaching (Hossner and Juo, 1999). This decline in soil fertility has decreased farmland productivity in most small holder farming communities (Amede, 2003). The land used for the cultivation of arable crops at the Teaching and Research Farm, Ladoke Akintola University of Technology has been continuously cultivated for >20 years with input of fertilizers, mainly NPK compound fertilizer and Urea. Nevertheless, there has been reduction in yield of crops (particularly maize) over the years which may be attributed to loss of soil productivity.
Therefore, assessment of the soil chemical properties of the arable land compared with adjacent fallow becomes imperative to suggest appropriate amendments that have the potential to remediate the soil. The objectives of this study were to:
(1) Compare the soil chemical properties in continuously cultivated arable plot and adjacent fallow and
(2) Evaluate the potentials of organic residues in improving soil chemical properties of the degraded land and maize performance
Site description
The research was carried out at the Teaching and Research Farm, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria. Ogbomoso is located on latitude 8°5′ N and longitude 4°1′ E. The temperature ranges between 28 and 33°C with ~74% relative humidity all year round. Rainfall is >1000 mm per annum. The arable field had been cropped to maize/cassava intercrop for > 20 years. An adjacent fallow field was selected because it has similar slope. The soils of the two sites were Alfisol (OxicPaleustalf) derived from the residue of metamorphic rock (Basement Complex) with no coarse fragments/stones up to 50 cm depth or rock outcrops. The soils are well drained, and they are suitable for arable crop production. The dominant weed species on the arable plot were Tridax procumbens, Euphorbia heterophylla, and Chromolaena odorata. The fallow field had shrubs, scattered trees and C. odorata. Mineral fertilizers, particularly NPK 15-15-15 compound fertilizer and urea applied at the recommended rate for maize in the zone; 120 kg N, 40 kg P and 80 kg K per hectare had been used on the arable field and weeds had been controlled with herbicides such as Atrazine, Paraforce, Glyphosate, etc.
Soil fertility assessment of the arable field
A non-replicated trial was conducted to assess the effects of >20 years continuous maize/cassava cropping on soil chemical properties. Soil properties of the arable field under continuous maize/cassava cultivation were compared with those of an adjacent fallow field.
Soil sampling and analysis
Soil samples (0 to 30 cm) were randomly collected across the arable and fallow fields using soil auger. The soil samples were bulked to form composites. The samples were air dried and sieved through 2 and 0.5 mm mesh sizes, and taken to the laboratory for routine chemical analysis. Soil pH was measured with the glass-electrode pH meter on 1:1 soil:solution mixture (Mclean, 1982). The organic carbon was determined by Walkey-Black method (Black, 1965) and N was by regular macro Kjeldahl method (Bremmer and Mulvaney, 1982). Available P was determined by the Mehlich method (Mehlich, 1984) while the exchangeable cations were extracted with 1 N NH4OAc solution. Calcium, Na and K were measured with the flame photometer, and Mg and Zn were determined on the atomic absorption spectro-photometeter (IITA, 1982). Effective cation exchange capacity (ECEC) was established as the sum of the exchangeable cations, K, Na, Ca and Mg and H+ expressed in c mol(+) kg-1 of soil (Tel and Hargerty, 1984).
Incubation study
Soil and organic residue preparation
The organic residues used were Tithonia diversifolia compost (TC), Gliricidia sepium (GS) and cured poultry manure (PM). Fully expanded medium-aged leaves of gliricidia were collected, dried at 65°C for 72 h, and ground to pass through 2 mm sieve. The cured poultry manure was obtained from Poultry Unit, Teaching and Research Farm, LAUTECH, oven dried, ground and analyzed for N, P and K in the laboratory. Total P was determined by the Vanadomolybdate method, K was determined by flame photometry, N was analyzed by the micro-Kjeldahl procedure as described by IITA (1982). Soil bulk samples collected from the arable field were air dried and sieved to pass through 2 mm mesh. One kilogram of air dried soil from the arable field was weighed into each incubation cup (perforated at the bottom for free drainage and air movement).
Treatments and experimental design
There were nine treatments laid out in a completely randomized design replicated three times. 120 kg N/ha applied as inorganic NPK 15-15-15 fertilizer (NPK) or from organic residues GS, TC, PM or combinations of NPK 15-15-15 and organic residues and a control (no fertilizer). Nitrogen rate of 120 kg/ha was based upon recommendation for maize/cassava mixture in the derived savanna of Nigeria (FPDD, 1989). The ground organic residues and NPK 15-15-15 fertilizer were thoroughly mixed with the soil in the following amounts:
(1) 0.4 g NPK/kg soil (farmers' practice) (120 kg N, P and K/ha)
(2) 3.33 g TC/kg soil (120N - 47P - 58K in kg/ha)
(3) 1.36 g PM/kg soil (120N - 30P - 51K in kg/ha)
(4) 2.22 g GS/kg soil (120N - 6P - 57K in kg/ha)
(5) 0.2 g NPK + 1.67 g TC/kg soil (120N - 83P - 89K in kg/ha)
(6) 0.2 g NPK + 0.68 g PM /kg soil (120N - 75P - 85K in kg/ha)
(7) 0.2 g NPK + 0.56 g GS + 0.84 g TC /kg soil (120N - 74P - 89K in kg/ha)
(8) 0.2 g NPK + 0.56 g GS + 0.34 g PM /kg soil (120N - 70P - 87K in kg/ha)
(10) Control (No amendment)
Distilled water was added to each soil to bring it to field capacity. The incubation cups were covered with double layer 0.05 mm thick polyethylene film (to allow gas but not water exchange), and kept at room temperature. At the end of the incubation period (6 weeks), the incubated soil was sampled with the aid of an iron pipe (diameter 24 mm), air dried and taken to the laboratory for routine chemical analysis.
Maize growth on incubated soils
After the incubation period, the polyethylene films were removed and the cups were arranged on a bench in the screen house. Three maize seeds of an early maturing variety (Oba supa 2) were planted per cup, and the seedlings were thinned to one at two weeks after planting (WAP). Watering was done throughout the experiment. Emerging weeds were hand pulled and left in the cup to decompose. At 6 WAP, the shoots were cut at 2 cm above the soil surface, while the roots were washed free of adhering soil by carefully washing them under a running tap. The shoots and roots were oven-dried in well labeled paper bags at 65°C for 72 h for dry matter determination. The dry shoots were ground in micro-hammer stainless steel mill using 2 mm mesh sized sieve prior to N, P, and K chemical analyses in the laboratory. Nutrient uptake was calculated by multiplying nutrient content (%) with corresponding shoot dry weight.
Statistical analysis
Data were subjected to analysis of variance using statistical analysis system (SAS) software (SAS, 2009), and Duncan Multiple Range Test at P = 0.05 was used to separate means. Correlation analysis among shoot and root dry weights, and nutrient uptake (NPK) was performed using correlation of SAS.
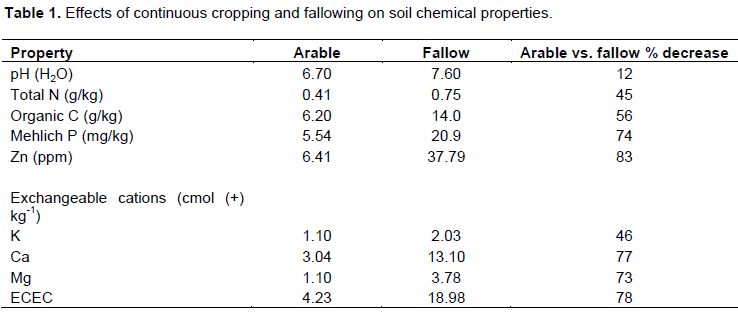
Chemical properties of arable and fallow fields
Continuous maize/cassava cultivation for >20 years even with external input of mineral fertilizers generally reduced soil chemical properties compared with fallow field. Soil pH declined by about 1 unit (11.8%). This change was not as pronounced as for other properties. Total nitrogen, organic carbon, and phosphorus declined by 45, 55 and 73.5%, respectively in arable field (Table 1). Among the micronutrients, Zn was the most affected with >80% decrease. Effective Cation Exchange Capacity decreased by 70%.
Nitrogen, phosphorus and potassium contents of organic residues used for the incubation study
Poultry manure had the highest nitrogen (44 g/kg), phosphorus (11.1 g/kg) and potassium (18.6 g/kg) contents compared with G. sepium and tithonia compost. G. sepium had the least P content while tithonia compost had the least N and K contents (Table 2).
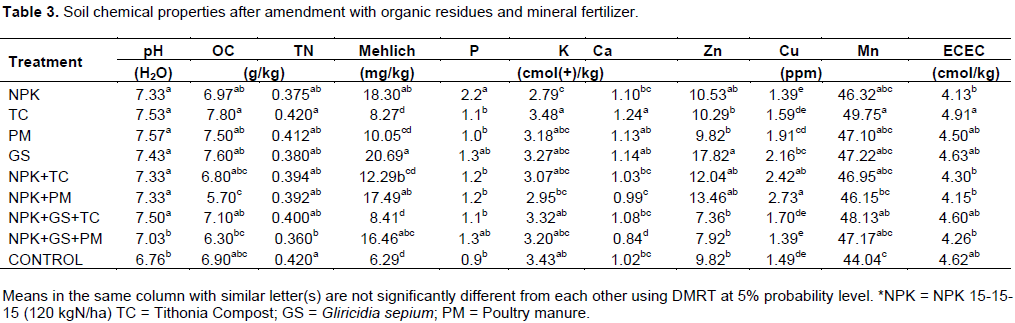
Chemical properties of arable soil after incubation with mineral fertilizer and organic residues
Incubation of sole organic residues, NPK and their various combinations (except NPK+GS+PM) significantly influenced higher pH compared with the control (Table 3). The highest SOC was observed with the application of TC (7.80 g/kg) which was significantly higher than those of NPK+GS+PM (6.30 g/kg) and NPK+PM (5.70 g/kg) which had the lowest values (Table 3).
Other treatments were not significantly different from each other. Significantly higher total nitrogen was observed with the application of TC (0.42 g/kg) compared with NPK+GS+PM (0.360 g/kg) and control (0.351 g/kg). Other treatments were not significantly different. Application of GS enhanced significantly the highest available phosphorus (20.67 mg/kg), but not significantly different from NPK, NPK+PM and NPK+GS+PM treatments. The control (6.29 mg/kg), TC (8.27 mg/kg), and NPK+GS+TC (8.41 mg/kg), had significantly the lowest available P (Table 3).
NPK significantly increased exchangeable potassium than TC, PM, NPK+TC, NKP+PM, NPK+GS+TC and control (Table 3), but not significantly so when compared with GS and NPK+GS+PM. The control had the lowest K. TC increased Ca more than NPK which had the lowest value.
Variations among other treatments were not pronounced. The application of TC led to significantly the highest soil Mg which was not significantly different from PM and GS, while NPK+GS+PM had the lowest value. Incubation of GS had the highest Zn (17.82 ppm) but not significantly different from NPK+PM (13.46 ppm), NPK+TC (12.04 ppm) and NPK (10.53 ppm), while NPK+GS+TC had the lowest value (7.36 ppm) which was not significantly different from NPK+GS+PM, control, PM, TC, NPK, NPK+TC and NPK+PM.
Copper was the highest with NPK+PM (2.73 ppm) but not significantly different from NPK+TC (2.42 ppm), while NPK and NPK+GS+PM had the lowest value (1.39 ppm). The highest level of Mn (49.75 ppm) was observed with TC than NPK+PM and control. NPK+GS+TC was significantly different from the control, others were not significantly different from each other. TC significantly increased ECEC than NPK, NPK+TC, NPK+PM and NPK+GS+PM. Others were not significantly different (Table 3).
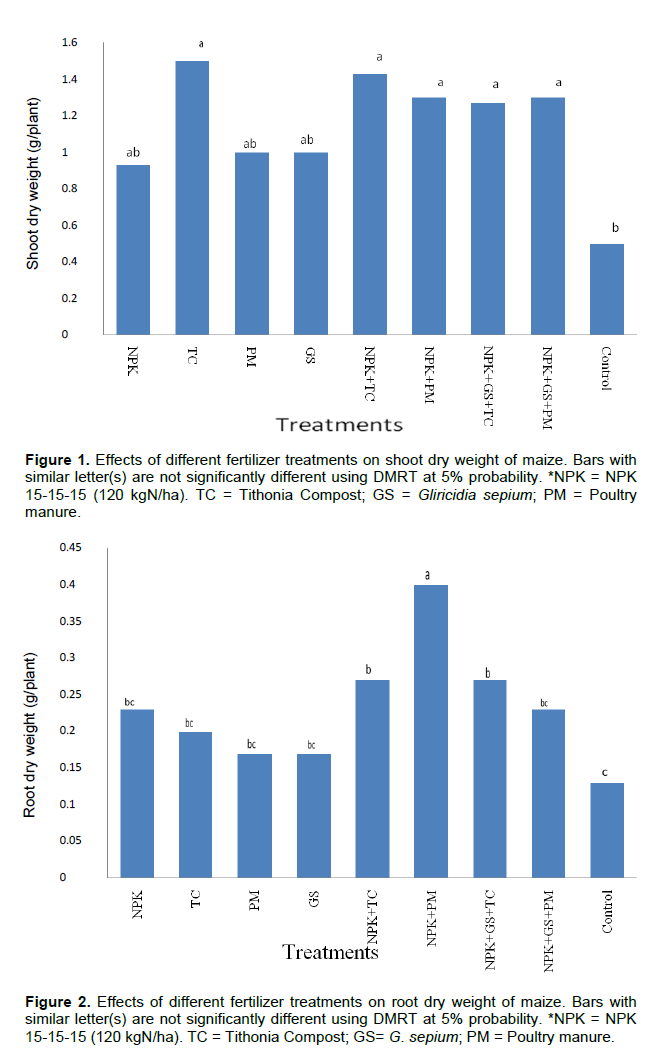
Shoot dry weight (SDW) and root dry weight (RDW)
All amendments (except NPK, PM and GS) significantly influenced higher SDW than control (Figure 1). NPK+PM significantly enhanced RDW than others which were not significantly different from each other except for NPK+TC and NPK+GS+TC which had the lowest RDW (Figure 2).
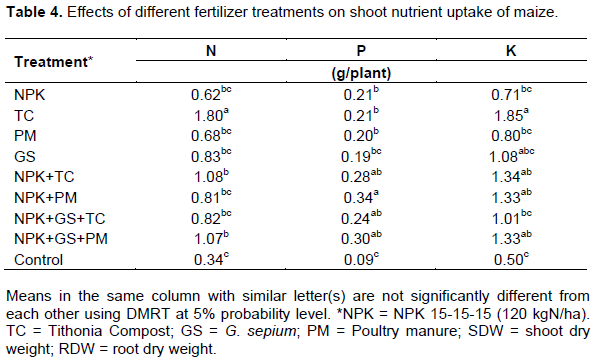
Maize plant nutrient uptake
Nitrogen content of maize planted on soil incubated with TC was significantly (P = 0.05) higher than others. Similarly, TC significantly increased K over NPK, PM, NPK+GS+TC and control (Table 4). NPK+PM had significantly the highest RDW while the control had the lowest.
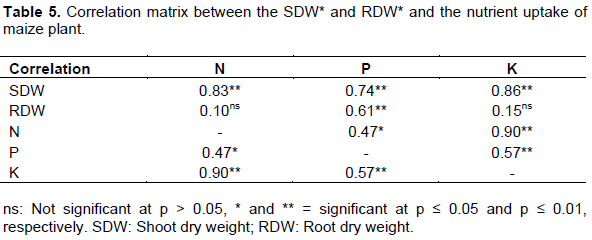
Correlation analysis
There was positive relationship between the shoot dry weight and N P and K uptake of maize. The root dry weight was positively associated (p=0.01) with the P uptake (Table 5).
Effect of continuous cultivation on soil chemical properties
The rather generally low values observed for soil nutrient contents of the continuously cultivated arable soil despite record of receiving inorganic fertilizer compared with fallow plot inferred that continuous cultivation had negative impact on soil available nutrient elements. This agrees with the findings of Sue et al. (2004) and Majaliwa et al. (2010), who observed that even short term cultivation is effect on the availability of nutrient elements in the soil. The observed low soil organic carbon and total nitrogen which are very crucial to soil quality in term of productivity corroborates the findings of Heald (2009) and Ito (2012), who obtained the lowest SOC and TN value for continuous maize cultivation, compared with under fallow. This observation further confirms the assertion of Galeb (2012), who opined that soil organic matter decreased under continuous cultivation, with or without addition of organic and/or inorganic soil amendments. Continuous cultivation as reported by Teklay (2005), leads to breakdown of soil aggregates by exposing the soil organic carbon thus, increasing microbial attack and mineralization.This may be the reasons why continuous tillage results in loss of soil organic carbon and nitrogen (Six et al., 2000). The exchangeable bases values of the fallow soil were higher than those of the cultivated arable soil; this implies that cultivation reduced the concentration of the exchangeable bases of these soils. Lemenin (2004) and Khormali and Shamsi (2009) also reported that intensive cultivation diminishes available K content of soils.
Chemical properties of soil after incubation
The results demonstrated that organic residues solely and in combination with mineral fertilizer have a high potential for soil fertility improvement. The increased soil pH observed agrees with the findings of Cong and Merckx (2005) and Opala et al. (2012) who reported that increase in soil pH with the decomposition of organic residues applied is due to nitrogen transformation and release of metal cations.
Different mechanisms have been suggested to explain the initial rise in soil pH when organic residues are applied to soils. These include oxidation of organic-acid anions present in the decomposing residues, ammonification of residue organic N, specific adsorption of organic molecule produced during residue decomposition and reduction reactions induced by anaerobiosis (Haynes and Mokolobate, 2001).
The role of the sandy textural group of this soil in conferring low buffering capacity to higher pH values cannot be underestimated. Soils with higher clay contents had been reported to resist pH changes as a result of higher pH buffering capacity (Anetor and Omueti, 2014).
Incorporation of organic residues increased SOC. This is in consonance with the observation of Kanwar et al. (2002), who reported that SOC content increased more through the application of organic manure than for mineral fertilizer. The major parameters used in evaluating soil fertility are SOC and TN which constitute the heterogeneous mixtures of organic substances (Huang et al., 2009).
There was an increase in TN with the application of TC though not significantly different from when TC was combined with NPK fertilizer; this is contrary to the findings of Kolawole (2016), who observed a general decrease of TN after incubation with tithonia compost. The contradiction in the results may be due to differences in crop residue quality and soils characteristics which may influence nutrient release pattern of organic residues added to the soil.
Phosphorus content was higher with organic residues, especially GS. The increase might be due to high microbial activity with addition of organic residues, which might facilitate phosphorus cycling (Parham et al., 2002). This agrees with the findings of Kolawole (2016) who noted increase in available P after application of organic residues. This is an indication of high percentage of soluble P in the organic residue which falls above the critical level of 0.25% required for net P mineralization (Nziguheba et al., 1998). Higher soil ECEC content observed with organic residues might be due to release of cations with the decomposition of organic matter similar to what was recorded by Yagi et al. (2003). There were increases in Mn, Cu and Zn after incubation of organic residues. These increases could be attributed to mineralization from the organic residues. This shows that organic residues are proficient source of these cations in the soil system (Ihenacho et al., 2015).
Maize growth and nutrient uptake
Understanding nutrient removal by a crop may provide information for soil fertility management by comparing the plant total accumulation to the application from all sources. In the present study, application of TC increased nitrogen uptake and dry shoot biomass. This can be attributed to its higher nitrogen content which is higher than the minimum critical value (0.11%) required for Nigerian soils (Adepetu, 1990).
Barker (1997) had earlier reported that, for compost to
be described as having fertilizing capabilities and for it to be used in agriculture, the total N content must be over 1% on dry weight basis. Improved yields with TC with respect to higher shoot dry weight and N and K uptake could be associated with the fast decomposition of TC, leading to a rapid release of nutrients to the crop (Nziguheba et al., 2000).
The positive relationship between the SDW and N, P and K uptake indicate that TC that enhanced higher SDW would similarly support uptake of N, P and K in maize plant. The positive correlation between the root dry weight and P uptake suggest that treatment that support good and healthy root growth will equally have a positive influence on P uptake. Similar findings were reported by Ali et al. (2002).
Continuous cultivation of arable crops even with the use of external inputs of mineral fertilizers results in depletion of soil nutrient elements in the long run. This makes amelioration of the soil inevitable to improve soil fertility and increase crop productivity. Application of organic residues or in combination with mineral fertilizer may have potential to restore lost nutrients. Tithonia compost (6.6 tonnes dry matter per hectare) showed better potential to ameliorate the poor soil fertility of the degraded soil and improve maize growth and nutrient uptake.
The authors have not declared any conflict of interests.
REFERENCES
|
Adepetu JA (1990). Soil test data interpretation in soil testing programme. Paper presented at National Workshop on Soil Testing Service for Efficient Fertilizer Use in Nigeria. Moore Plantation, Ibadan.
|
|
|
|
Afolami SO, Fawole B (1991). Effect of Pratylenchusse faensis on growth and yield of Zea mays L.cv FARZ-7 under continuous cropping. Plant Soil 138:133-138.
Crossref
|
|
|
|
|
Ali A, Khan AS, Asad MA (2002). Drought tolerance in wheat: Genetic variation and heritability for growth and ion relations. Asian J. Plant Sci. 1:420-422.
Crossref
|
|
|
|
|
Amede T (2003). Opportunities and Challenges in Reversing Land degradation: The Regional Experience. In: Amede, T(ed), Natural resource degradation and environmental concerns in the Amhara National Regional State: Impact on Food Security. Ethiop. Soils Sci. Soc. 3:173-183.
|
|
|
|
|
Anetor MO, Omueti JAI (2014). Organo-mineral fertilizer effects in some phosphorus unresponsive soils of south western Nigeria: 1. Effects on maize growth response and soil properties. Agric. Biol. J. North Am. 5(6):265-280.
|
|
|
|
|
Barker AV (1997). Composition and Uses of composts. In Agricultural Uses of Byproducts and Wastes. Rechcigl, JE and Mackinnon, HC Eds. American Chemical Society, Washington, DC. pp. 140-162.
Crossref
|
|
|
|
|
Black CA (1965). Method of soil analysis II. American Society of Agronomy, Madison, WI. pp. 573-590.
|
|
|
|
|
Bremner DC, Mulvaney JM (1982). Total Nitrogen. In: Methods of Soil Analysis. (A. L. Page, R. H. Miller and D. R. Keaney, eds). Am. Soc. Agron. 9:2
|
|
|
|
|
Cong PT, Merckx R (2005). Improving phosphorus availability in two upland soils of Vietnam using shape Tithonia diversifolia H. Plant Soil 269. 1(2):11-23
|
|
|
|
|
Fertilizer Procurement Development Division (FPDD) (1989). Literature review on soil fertility investigations in Nigeria. Federal Ministry of Agriculture and Natural Resources, Lagos. P 199.
|
|
|
|
|
Galeb EY (2012). The influence of planted fallows and continuous maize cultivation on the chemical properties of an acid Alfisol in southwestern Nigeria. J. Appl. Sci. 83(3):20-26.
|
|
|
|
|
Haynes RJ, Mokolobate MS (2001). Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: A critical review of the phenomenon and the Mechanisms involved. Nutrient Cycling Agroecosyst. 59:47-63
Crossref
|
|
|
|
|
Heald AN (2009). Uptake of soil nitrogen by groundnut as affected by symbiotic N – fixation. Soil Biochem. 44:1111-1118.
|
|
|
|
|
Hossner LR, Juo ASR (1999). Soil Nutrient Management for Sustained Food crop Production in Upland Farming Systems in the Tropics. Soil and Crop Sciences Department College Station Tennessee 77843. USA. Retrieved from http//www.agnet.org.
|
|
|
|
|
Huang QR, Hu F, Huang S, Li HX, Yuan YH (2009). Effect of long – term fertilization on organic carbon and nitrogen in a subtropical paddy soils. Pedosphere 19:727-734.
Crossref
|
|
|
|
|
Ihenacho LU, Okorie HA, Christo IE, Peter CAO (2015). Effect of poultry manure rates on growth and yield of turmeric (Curcuma longa L.) in Nigeria. J. Agric. Vet. Sci. (IOSR-JAVS). 8(1):34-38.
|
|
|
|
|
International Institute of Tropical Agriculture (IITA), (1982). Automated and semi-automated methods for soil and plant analysis. Manual series No. 7. Ibadan (Nigeria). International Institute of Tropical Agriculture.
|
|
|
|
|
Ito RC (2012). Carbon and nitrogen contents of tropical soils under natural fallows. J. Soil Environ. Sci. 74(2):352-358.
|
|
|
|
|
Kanwar K, Paliyal SS, Nandal TR (2002). Integrated nutrient management in cauliflower (Pusa Snow Ball K-1). Resour. Crops 3(3):579-583.
|
|
|
|
|
Khormali F, Shamsi S (2009). Micro-Morphology and quality attributes of the loss Derived Soils affected by land use change. A case study in Ghapan Watershed, Northern Iran. J. Manag. Sci. 6:197-204.
Crossref
|
|
|
|
|
Kolawole GO (2016). Nutrient Release Patterns of Tithonia Compost and Poultry Manure in Three Dominant Soils in the Southern Guinea Savanna, Nigeria. Int. J. Plant Soil Sci. 10(5):1-8.
Crossref
|
|
|
|
|
Lemenin M (2004). Effect of land use changes on soil quality and native Flora degradation and Restoration in the High lands of Ethiopia Ph. D Thesis, Swedish University of Agricultural Science Uppsala Sweden. pp. 15-21.
|
|
|
|
|
Liasu MO, Ogundare AO, Ologunde MO (2008). Effect of soil supplementation with fortified Tithoniamulch and directly applied inorganic fertilizer on growth and development of potted okra plants. American-Eurasian J. Sustain. Agric. 2(3):264-270.
|
|
|
|
|
Majaliwa JA, Twongyirwe R, Nyenje R, Oluka M, Ongom B, Sirike J, Mfitumukiza D, Azanga E, Natumanya R, Nwerera R, Barasa B (2010). The effect of land cover change on soil properties around Kibale National Park in South Western Uganda. Appl. Environ. Soil Sci. 10:1-7.
Crossref
|
|
|
|
|
Mclean EO (1982). Methods of soil Analysis. Part 1. Am. Soc. Agron. J. 9:986-994.
|
|
|
|
|
Mehlich M (1984). Mehlich 3 soil test extractant. Modification of the détermination of the Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 15:1409-1416.
Crossref
|
|
|
|
|
Nziguheba G, Palm CA, Buresh RJ, Smithson PC (1998). Soil phosphorus fractions and adsorption as affected by organic and inorganic sources. Plant Soil 198:159-168.
Crossref
|
|
|
|
|
Odoemena B, Eric E, Paul O, Geraldine U, Damian I, Augustine O, Francis O (2010). Econometric analysis of the micro-level determinants of woodland conversion to arable cropping and implications to policy. Afr. J. Agric. Res. 5(11):1168-1178.
|
|
|
|
|
Olawale E, Arega DA, Ikpi A (2009). Determinants of fertilizer use in northern Nigeria. Pak. J. Soc. Sci. 6(2):91-98.
|
|
|
|
|
Opala PA, Okalebo JR, Otheno CO (2012). Effect of organic and inorganic materials on soil acidity and phosphorus availability in a soil incubation study. International Scholarly Reseach Network ISRN Agronomy. P 10.
Crossref
|
|
|
|
|
Osher LJ, Pamela AM, Amundson R (2003). Effect of land use change on soil carbon in Hawaii. Biogeochemistry 65:213-232.
Crossref
|
|
|
|
|
Sanchez PA, Leakey RRB (1997). Land-use transformation in Africa: Three determinants for balancing food security with natural resource utilization. Eur. J. Agron. 7:15-23.
Crossref
|
|
|
|
|
Shen JP, Zhang LM, Guo JF, Ray JL, He JZ (2010). Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. Appl. Soil Ecol. 46:119-124.
Crossref
|
|
|
|
|
Sue VZ, Zhao HL, Zhang TH, Zhao XY (2004). Soil properties following cultivation and non-grazing of a semi-arid sandy grassland in Northern China. Soil Till. Res. 75:27-36.
Crossref
|
|
|
|
|
Six J, Paustian K, Elliott ET, Combrink C (2000). Soil structure and organic matter: 1.Distribution of aggregate-sized classes and aggregate associated carbon. Soil Sci. Soc. Am. J. 64:681-689.
Crossref
|
|
|
|
|
Tanimu J, Uyovbisere EO, Lyocks SWJ, Tanimu Y (2013). Effects of cowdung on the growth and development of maize crop. Greener J. Agric. Sci. 3(5):371-383.
|
|
|
|
|
Teklay T (2005). Organic Inputs from Agroforestry Trees for Improving Soil Quality and Crop Productivity in Ethiopia. Umea, Sweden: Faculty of Forest Sciences, Department of Forest Ecology.
|
|
|
|
|
Tel DA, Hargerty M (1984). Soil and Plant analyses study guide for agricultural laboratory directors and technologists working in tropical regions IITA and University of Guelph. P 227.
|
|
|
|
|
USDA (2010). Foreign Agricultural Service, GAIN Report (Global Agriculture Information Network), Nigeria Grain and Feed Annual-Nigeria's Wheat Imports Surge.
|
|
|
|
|
Vanlauwe B, Tittonell P, Mukalama J (2006). Within-farm soil fertility gradient affect response of maize to fertilizer application in western Kenya. Nutrient Cycling Agroecosyst. 76:171-182.
Crossref
|
|
|
|
|
Yagi R, Ferreira ME, Cruz MCP, Barbosa JC (2003). Organic matter fractions and soil fertility under the influence of liming, vermicompost and cattle manure. Scientia Agricola 60:549-557.
Crossref
|
|
|
|
|
Yusuf AA, Yusuf HA (2008). Evaluation of strategies for soil fertility improvement in northern Nigeria and the way forward. J. Agron. 7(1):15-24.
Crossref
|
|
|
|
|
Zingore S (2011). Maize productivity and response to fertilizer use as affected by soil fertility variability, manure application and cropping system. Better Crops 95(1):4-6.
|
|